| |
| Names | |
|---|---|
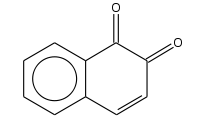
| Preferred IUPAC name Naphthalene-1,2-dione | |
| Other names
o-Naphthoquinone, β-naphthoquinone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.602 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H6O2 |
| Molar mass | 158.156 g·mol |
| Appearance | yellow solid |
| Melting point | 145 to 147 °C (293 to 297 °F; 418 to 420 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
1,2-Naphthoquinone or ortho-naphthoquinone is a polycyclic aromatic organic compound with formula C
10H
6O
2. This yellow solid is prepared by oxidation of 1-amino-2-hydroxynaphthalene with ferric chloride.
Occurrence
This diketone (an ortho-quinone) is a metabolite of naphthalene. It arises from the naphthalene-1,2-oxide.
It is also found in diesel exhaust particles. The accumulation of this toxic metabolite in rats from doses of naphthalene has been shown to cause eye damage, including the formation of cataracts.
See also
- 1,4-Naphthoquinone, an isomer of 1,2-naphthoquinone
References
- Louis F. Fieser (1937). "1,2-Naphthoquinone". Org. Synth. 17: 68. doi:10.15227/orgsyn.017.0068.
- Yoshito Kumagai; Yasuhiro Shinkai; Takashi Miura; Arthur K. Cho (2011). "The Chemical Biology of Naphthoquinones and Its Environmental Implications". Annual Review of Pharmacology and Toxicology. 52: 221–47. doi:10.1146/annurev-pharmtox-010611-134517. PMID 21942631.
- Qian, W.; Shichi, H. (2001). "Naphthoquinone-Induced Cataract in Mice: Possible Involvement of Ca Release and Calpain Activation". Journal of Ocular Pharmacology and Therapeutics. 17 (4): 383–392. doi:10.1089/108076801753162799. PMID 11572469.
External links
- Troester, M. A.; Lindstrom, A. B.; Waidyanatha, S.; Kupper, L. L.; Rappaport, S. M. (2002). "Stability of Hemoglobin and Albumin Adducts of Naphthalene Oxide, 1,2-Naphthoquinone, and 1,4-Naphthoquinone". Toxicological Sciences. 68 (2): 314–321. doi:10.1093/toxsci/68.2.314. PMID 12151627.
- Kikuno, S.; Taguchi, K.; Iwamoto, N.; et al. (2006). "1,2-Naphthoquinone Activates Vanilloid Receptor 1 through Increased Protein Tyrosine Phosphorylation, Leading to Contraction of Guinea Pig Trachea". Toxicology and Applied Pharmacology. 210 (1–2): 47–54. doi:10.1016/j.taap.2005.06.015. PMID 16039679.