 | |
| Names | |
|---|---|
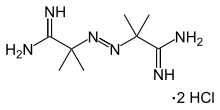
| IUPAC name (1Z,1'Z)-2,2'-bis(2-methylpropanimidamide) dihydrochloride | |
| Other names AAPH; AMPA; 2,2'-Azobisisobutyramidinium chloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H20Cl2N6 |
| Molar mass | 271.19 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
2,2'-Azobis(2-amidinopropane) dihydrochloride (abbreviated AAPH or AMPA) is a chemical compound used to study the chemistry of the oxidation of drugs.
It is a free radical-generating azo compound. It is gaining prominence as a model oxidant in small molecule and protein therapeutics for its ability to initiate oxidation reactions via both nucleophilic and free radical mechanisms.
2,2′-azobis (2-amidinopropane) dihydrochloride has been used in experiments on linoleic acid subjected to induced oxidation with different combinations of binary mixtures of natural phenolics. The experiments show that some binary mixtures can lead to a synergetic antioxidant effect while other mixtures lead to an antagonistic effect.
References
- Betigeri, S.; Thakur, A.; Raghavan, K. (2005). "Use of 2,2?-Azobis(2-Amidinopropane) Dihydrochloride as a Reagent Tool for Evaluation of Oxidative Stability of Drugs". Pharmaceutical Research. 22 (2): 310–317. doi:10.1007/s11095-004-1199-x. PMID 15783080. S2CID 38031845.
- Werber, J.; Wang, Y. J.; Milligan, M.; Li, X.; Ji, J. A. (2011). "Analysis of 2,2′-azobis (2-amidinopropane) dihydrochloride degradation and hydrolysis in aqueous solutions". Journal of Pharmaceutical Sciences. 100 (8): 3307–3315. doi:10.1002/jps.22578. PMID 21560126.
- Antioxidant activity of phenolic compounds in 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidation: Synergistic and antagonistic effects. M. N. Peyrat-Maillard, M. E. Cuvelier and C. Berset, Journal of the American Oil Chemists' Society, 2003, Volume 80, Number 10, pages 1007-1012, doi:10.1007/s11746-003-0812-z