 | |
| Clinical data | |
|---|---|
| Other names | EDMA |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
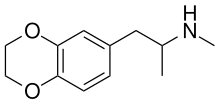
| Formula | C12H17NO2 |
| Molar mass | 207.273 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
3,4-Ethylenedioxy-N-methylamphetamine (EDMA) is an entactogen drug of the methamphetamine class. It is an analogue of MDMA where the methylenedioxy ring has been replaced by an ethylenedioxy ring. EDMA was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage is listed as 150–250 mg, and the duration listed as 3–5 hours. According to Shulgin, EDMA produces a bare threshold consisting of paresthesia, nystagmus, and hypnogogic imagery, with few to no other effects.
It has been found that EDMA acts as a non-neurotoxic serotonin releasing agent with moderately diminished potency relative to MDMA, and with negligible effects on dopamine release. However, subsequent research found that EMDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA) with EC50Tooltip half-maximal effective concentration values of 117 nM for serotonin release, 325 nM for norepinephrine release, and 597 nM for dopamine release in rat brain synaptosomes. Compared to MDMA, EDMA was about half as potent as a serotonin releaser, 4.5-fold less potent as a norepinephrine releaser, and 8-fold less potent as a dopamine releaser. The activities of the individual enantiomers of EDMA have also been assessed.
See also
- 3,4-Ethylenedioxyamphetamine (EDA)
- 3,4-Ethylenedioxymethcathinone (EDMC)
- 3,4-Ethylidenedioxyamphetamine (EIDA)
- 3,4-Isopropylidenedioxyamphetamine (IDA)
References
- ^ Shulgin A, Shulgin A (1991). Pihkal: A Chemical Love Story. Transform Press. ISBN 0-9630096-0-5.
- ^ McKenna DJ, Guan XM, Shulgin AT (March 1991). "3,4-Methylenedioxyamphetamine (MDA) analogues exhibit differential effects on synaptosomal release of 3H-dopamine and 3H-5-hydroxytryptamine". Pharmacology, Biochemistry, and Behavior. 38 (3): 505–512. doi:10.1016/0091-3057(91)90005-M. PMID 1829838. S2CID 2740262.
- ^ Del Bello F, Sakloth F, Partilla JS, Baumann MH, Glennon RA (September 2015). "Ethylenedioxy homologs of N-methyl-(3,4-methylenedioxyphenyl)-2-aminopropane (MDMA) and its corresponding cathinone analog methylenedioxymethcathinone: Interactions with transporters for serotonin, dopamine, and norepinephrine". Bioorg Med Chem. 23 (17): 5574–5579. doi:10.1016/j.bmc.2015.07.035. PMC 4562428. PMID 26233799.
External links
| Empathogens/entactogens | |
|---|---|
| Phenylalkyl- amines (other than cathinones) |
|
| Cyclized phenyl- alkylamines | |
| Cathinones | |
| Tryptamines | |
| Chemical classes | |
| Phenethylamines | |
|---|---|
| Phenethylamines |
|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|