| |
| Names | |
|---|---|
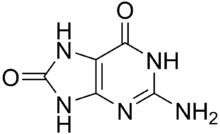
| IUPAC name 2-Amino-7,9-dihydro-1H-purine-6,8-dione | |
| Other names 7,8-Dihydro-8-oxoguanine; 8-Oxo-7,8-dihydroguanine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.024.578 |
| MeSH | 8-hydroxyguanine |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H5N5O2 |
| Molar mass | 167.128 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
8-Oxoguanine (8-hydroxyguanine, 8-oxo-Gua, or OHGua) is one of the most common DNA lesions resulting from reactive oxygen species modifying guanine, and can result in a mismatched pairing with adenine resulting in G to T and C to A substitutions in the genome. In humans, it is primarily repaired by DNA glycosylase OGG1. It can be caused by ionizing radiation, in connection with oxidative metabolism.

In body fluids
Increased concentrations of 8-oxoguanine in body fluids have been found to be associated with increased risk of mutagenesis and carcinogenesis.
Care must be taken in the assay of 8-oxoguanine due to the ease with which it can be oxidised during extraction and the assay procedure.
Cancer, aging, infertility
The role of the deoxyriboside form of 8-oxoguanine, 8-oxo-2'-deoxyguanosine (abbreviated 8-oxo-dG or 8-OHdG) in cancer and aging also applies to 8-oxoguanine. Oxoguanine glycosylase is employed in the removal of 8-oxoguanine from DNA by the process of base excision repair. As described in oxoguanine glycosylase, deficient expression of this enzyme causes 8-oxoguanine to accumulate in DNA. This accumulation may then lead upon replication of DNA to mutations including some that contribute to carcinogenesis. 8-oxoguanine is usually formed by the interaction of reactive oxygen species (ROS) with the guanine base in DNA under conditions of oxidative stress; as noted in the article about them, such species may have a role in aging and male infertility, and 8-oxoguanine can be used to measure such stress.
References
- 8-hydroxyguanine - Compound Summary, PubChem
- Kanvah, S.; et al. (2010). "Oxidation of DNA: Damage to Nucleobases". Acc. Chem. Res. 43 (2): 280–287. doi:10.1021/ar900175a. PMID 19938827.
- Cheng KC; Cahill DS; Kasai H; Nishimura S; Loeb LA (Jan 5, 1992). "8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G→T and C→A substitutions". J Biol Chem. 267 (1): 166–72. doi:10.1016/S0021-9258(18)48474-8. PMID 1730583.
- Kasai, H (December 1997). "Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis". Mutation Research. 387 (3): 147–63. doi:10.1016/s1383-5742(97)00035-5. PMID 9439711.
- Halliwell, B (December 1998). "Can oxidative DNA damage be used as a biomarker of cancer risk in humans? Problems, resolutions and preliminary results from nutritional supplementation studies". Free Radical Research. 29 (6): 469–86. doi:10.1080/10715769800300531. PMID 10098453.
- Ravanat, JL; Douki, T; Duez, P; Gremaud, E; Herbert, K; Hofer, T; Lasserre, L; Saint-Pierre, C; Favier, A; Cadet, J (November 2002). "Cellular background level of 8-oxo-7,8-dihydro-2'-deoxyguanosine: an isotope based method to evaluate artefactual oxidation of DNA during its extraction and subsequent work-up". Carcinogenesis. 23 (11): 1911–8. doi:10.1093/carcin/23.11.1911. PMID 12419840.
| Nucleic acid constituents | |||||||
|---|---|---|---|---|---|---|---|
| Nucleobase | |||||||
| Nucleoside |
| ||||||
| Nucleotide (Nucleoside monophosphate) |
| ||||||
| Nucleoside diphosphate | |||||||
| Nucleoside triphosphate | |||||||
| DNA repair | |
|---|---|
| Excision repair | |
| Homologous recombination | |
| Other pathways | |
| Regulation | |
| Other/ungrouped | |