 | |
| Names | |
|---|---|
| IUPAC name 2,6,6-Trimethylcyclohexene-1-carbaldehyde | |
| Other names 1-Formyl-2,6,6-trimethyl-1-cyclohexene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 2042086 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.439 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H16O |
| Molar mass | 152.237 g·mol |
| Boiling point | 62–63 °C (144–145 °F; 335–336 K) |
| Solubility in water | 86.14 mg/L |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H312, H315, H319, H332, H335 |
| Precautionary statements | P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P317, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
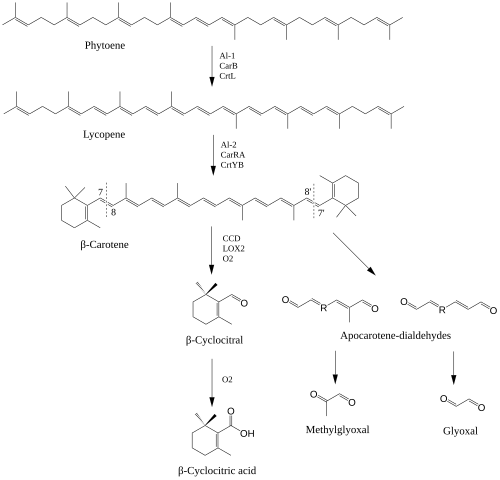
β-Cyclocitral (beta-cyclocitral) is an apocarotenoid derived from the C7 oxidation of β-carotene. This apocarotenoid has revived interest due to its roles in plant development. β-cyclocitral has been found endogenously in a variety of organisms including plants, cyanobacteria, fungi and animals. β-Cyclocitral is a volatile compound that contributes to the aroma of various fruits, vegetables and ornamental plants. In plants, β-cyclocitral was found to be an important regulator in root development.
Application
β-Cyclocitral is used as an analytical standard for the determination of volatile organic compounds in saffron due to its analog structure to safranal.
Because β-cyclocitral is associated with cyanobacteria death, it is an analyte that can be tracked in bodies of water to monitor cyanobacteria blooms.
It has also been found to promote the growth of roots in rice, prompting its consideration as a potential agricultural tool.
Biosynthesis
The biosynthesis of β-cyclocitral relies on the formation of β-carotene through the isoprenoid biosynthetic pathway underpinning carotenoid formation. Similar to other apocarotenoids, the formation of β-cyclocitral can occur via the enzymatic and non-enzymatic oxidative cleavage of double bonds in β-carotene. For β-cyclocitral to form, the cleavage of C7-C8 double bonds are needed. While no enzyme has been identified to have high specificity for the production of β-cyclocitral, a carotenoid cleavage dioxygenase (CCD4) has been identified as being capable of cleaving β-carotene at the needed position. 13-lipoxygenase (LOX2) has also been identified to cleave β-carotene at the C7 position. β-cyclocitral can also be formed from the direct oxidation of β-carotene by reactive oxygen species, especially singlet oxygen (O2). In plants, O2 is mainly produced from excited chlorophylls in the reaction center of PSII where β-carotene serves to quench the reactive oxygen species.
References
- "beta-Cyclocitral". pubchem.ncbi.nlm.nih.gov.
- Havaux, Michel (October 2020). "β-Cyclocitral and derivatives: Emerging molecular signals serving multiple biological functions". Plant Physiology and Biochemistry. 155: 35–41. doi:10.1016/j.plaphy.2020.07.032. ISSN 0981-9428. PMID 32738580. S2CID 220925143.
- Condurso, Concetta (October 2016). "Bioactive volatiles in Sicilian (South Italy) saffron: safranal and its related compounds". Journal of Essential Oil Research. 29 (3): 221–227. doi:10.1080/10412905.2016.1244115. S2CID 100505185.
- Dickinson, Alexandra (May 2019). "β-Cyclocitral is a conserved root growth regulator". Proceedings of the National Academy of Sciences. 116 (21): 10563–10567. Bibcode:2019PNAS..11610563D. doi:10.1073/pnas.1821445116. PMC 6534974. PMID 31068462.
- Huang, Heyong (2018). "Distributions of four taste and odor compounds in the sediment and overlying water at different ecology environment in Taihu Lake". Scientific Reports. 8 (8): 6179. Bibcode:2018NatSR...8.6179H. doi:10.1038/s41598-018-24564-z. PMC 5906450. PMID 29670292.
- Keeley, Jim. "A Plant Hormone that Speeds Root Growth Could Be a New Agricultural Tool". Howard Hughhes Medical Institute. Retrieved 6 June 2023.
- Havaux, Michel (2020). "β-Cyclocitral and derivatives: Emerging molecular signals serving multiple biological functions". Plant Physiology and Biochemistry. 155: 35–41. doi:10.1016/j.plaphy.2020.07.032. PMID 32738580. S2CID 220925143.
- Maria, Rodrigo (2013). "A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments". Journal of Experimental Botany. 64 (14): 4461–4478. doi:10.1093/jxb/ert260. PMC 3808326. PMID 24006419.
- Gao, Lei (2019). "The tomato pan-genome uncovers new genes and a rare allele regulating fruit flavor". Nature Genetics. 51 (6): 1044–1051. doi:10.1038/s41588-019-0410-2. PMID 31086351. S2CID 256819279.
- Triantaphylidès, Christian (2009). "Singlet oxygen in plants: production, detoxification and signaling". Trends in Plant Science. 14 (4): 219–228. doi:10.1016/j.tplants.2009.01.008. PMID 19303348.