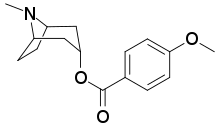
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H21NO3 |
| Molar mass | 275.348 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Datumetine is a tropane alkaloid found in leaves of Datura metel. It is said to modulate NMDA receptor and thus causes memory loss. It also causes epileptic seizures in mice. Docking studies suggest that it fits on both allosteric and orthosteric sites of NMDA receptor. It acts together with other anticholinergic tropane alkaloids of datura to cause amnesia.
See also
References
- Siddiqui S, Sultana N, Ahmed SS, Haider SI (2004). "Isolation and Structure of a New Alkaloid Datumetine from the leaves of Datura metel". Journal of Natural Products. 49 (3): 511–513. doi:10.1021/np50045a023. ISSN 0163-3864.
- ^ Ishola AO, Imam A, Ajao MS (2021). "Effects of datumetine on hippocampal NMDAR activity". Toxicology Reports. 8: 1131–1142. doi:10.1016/j.toxrep.2021.05.009. PMC 8190477. PMID 34150523.
This article about an alkaloid is a stub. You can help Misplaced Pages by expanding it. |