 | |
 | |
| Names | |
|---|---|
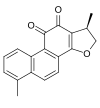
| IUPAC name 4,17β-Dimethyl-15-oxagona-1,3,5,7,9,13-hexaene-11,12-dione | |
| Systematic IUPAC name (1R)-1,6-Dimethyl-1,2-dihydrophenanthrofuran-10,11-dione | |
| Other names 15,16-dihydrotanshinone I, phenanthrofuran-10,11-dione, 1,2-dihydro-1,6-dimethyl- | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | DI |
| ChemSpider | |
| ECHA InfoCard | 100.222.905 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C18H14O3 |
| Molar mass | 278.307 g·mol |
| Appearance | Red powder |
| Density | 1.32 g/cm |
| Boiling point | 479.2 °C (894.6 °F; 752.3 K) |
| Solubility in water | 12.9 mg/L (est.) |
| Solubility in ethanol | 1 mg/mL, clear orange to red |
| log P | log Kow = 3.93 (est) |
| Vapor pressure | 3.41x10 mmHg |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H302, H400 |
| Precautionary statements | P264, P270, P273, P301+P312, P330, P391, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Dihydrotanshinone I (DI) is a naturally occurring compound extracted from Salvia miltiorrhiza Bunge, also known as Chinese sage, red sage root, and the Chinese herbal Dan Shen. It belongs to a class of lipophilic abietane diterpenoids and has been reported to have cytotoxicity to a variety of tumor cells, as well as antiviral effects in vitro. Since they were first discovered, over 40 related compounds and over 50 hydrophilic compounds have been isolated from Dan Shen.
References
- "Dihydrotanshinone I". PubChem. Retrieved 31 August 2015.
- ^ "HSDB: DIHYDROTANSHINONE I". NIH. Retrieved 1 September 2015.
- Bian W, Chen F, Bai L, Zhang P, Qin W (January 2008). "Dihydrotanshinone I inhibits angiogenesis both in vitro and in vivo". Acta Biochimica et Biophysica Sinica. 40 (1): 1–6. doi:10.1111/j.1745-7270.2008.00370.x. PMID 18180848.
- Lim CT, Tan KW, Wu M, Ulferts R, Armstrong LA, Ozono E, Drury LS, Milligan JC, Zeisner TU, Zeng J, Weissmann F, Canal B, Bineva-Todd G, Howell M, O'Reilly N, Beale R, Kulathu Y, Labib K, Diffley JF (July 2021). "Identifying SARS-CoV-2 antiviral compounds by screening for small molecule inhibitors of Nsp3 papain-like protease". The Biochemical Journal. 478 (13): 2517–2531. doi:10.1042/BCJ20210244. PMC 8286840. PMID 34198325.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |