| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 1,1′-Oxydibenzene | |
| Systematic IUPAC name Phenoxybenzene | |
| Other names
Oxydibenzene Diphenyl ether Diphenyl oxide 1,1′-Oxybisbenzene Phenoxybenzene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1364620 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.711 |
| EC Number |
|
| Gmelin Reference | 165477 |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3077 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H10O |
| Molar mass | 170.211 g·mol |
| Appearance | Colorless solid or liquid |
| Odor | geranium-like |
| Density | 1.08 g/cm (20 °C) |
| Melting point | 25 to 26 °C (77 to 79 °F; 298 to 299 K) |
| Boiling point | 258.55 °C (497.39 °F; 531.70 K) at 100 kPa (1 bar), 121 °C at 1.34 kPa (10.05 mm Hg) |
| Solubility in water | Insoluble |
| Vapor pressure | 0.02 mmHg (25 °C) |
| Magnetic susceptibility (χ) | -108.1·10 cm/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H319, H360Fd, H400, H411 |
| Precautionary statements | P264, P273, P280, P305+P351+P338, P337+P313, P391, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 115 °C (239 °F; 388 K) |
| Explosive limits | 0.7%–6.0% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 3370 mg/kg (rat, oral) 4000 mg/kg (rat, oral) 4000 mg/kg (guinea pig, oral) |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA 1 ppm (7 mg/m) |
| REL (Recommended) | TWA 1 ppm (7 mg/m) |
| IDLH (Immediate danger) | 100 ppm |
| Safety data sheet (SDS) | Aldrich MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
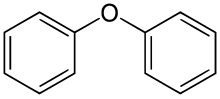
Diphenyl ether is the organic compound with the formula (C6H5)2O. It is a colorless, low-melting solid. This, the simplest diaryl ether, has a variety of niche applications.
Synthesis and reactions
Diphenyl ether was discovered by Heinrich Limpricht and Karl List in 1855, when they reproduced Carl Ettling's destructive distillation of copper benzoate and separated it from the low-melting oily distillate components ignored by previous researchers. They named the compound phenyl oxide (German: Phenyloxyd) and studied some of its derivatives.
Now it is synthesized by a modification of the Williamson ether synthesis, here the reaction of phenol and bromobenzene in the presence of base and a catalytic amount of copper:
- PhOH + PhBr → PhOPh + HBr
Involving similar reactions, diphenyl ether is a significant side product in the high-pressure hydrolysis of chlorobenzene in the production of phenol.
Related compounds are prepared by Ullmann reactions.
The compound undergoes reactions typical of other phenyl rings, including hydroxylation, nitration, halogenation, sulfonation, and Friedel–Crafts alkylation or acylation.
Uses
The main application of diphenyl ether is as a eutectic mixture with biphenyl, used as a heat transfer fluid. Such a mixture is well-suited for heat transfer applications because of the relatively large temperature range of its liquid state. A eutectic mixture (commercially, Dowtherm A) is 73.5% diphenyl ether and 26.5% biphenyl.
Diphenyl ether is a starting material in the production of phenoxathiin via the Ferrario reaction. Phenoxathiin is used in polyamide and polyimide production.
Because of its odor reminiscent of scented geranium, as well as its stability and low price, diphenyl ether is used widely in soap perfumes. Diphenyl ether is also used as a processing aid in the production of polyesters.
Related compounds
It is a component of important hormone T3 or triiodothyronine.
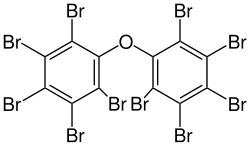
Several polybrominated diphenyl ethers (PBDEs) are useful flame retardants. Of penta-, octa-, and decaBDE, the three most common PBDEs, only decaBDE is still in widespread use since its ban in the European Union in 2003. DecaBDE, also known as decabromodiphenyl oxide, is a high-volume industrial chemical with over 450,000 kilograms produced annually in the United States. Decabromodiphenyl oxide is sold under the trade name Saytex 102 as a flame retardant in the manufacture of paints and reinforced plastics.
References
- ^ "CHAPTER P-6. Applications to Specific Classes of Compounds". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 705. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0496". National Institute for Occupational Safety and Health (NIOSH).
- Byers, Charles H.; Williams, David F. (July 1987). "Viscosities of pure polyaromatic hydrocarbons". Journal of Chemical & Engineering Data. 32 (3): 344–348. doi:10.1021/je00049a018. ISSN 0021-9568.
- "Phenyl ether". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Fiege, H.; Voges, H.-M.; Hamamoto, T; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313. ISBN 978-3527306732.
- K. List; H. Limpricht (1854). "Ueber das sogenannte Benzoëoxyd und einige andere gepaarte Verbindungen". Annalen der Chemie und Pharmacie (in German). 90 (2): 190–210. doi:10.1002/JLAC.18540900212. ISSN 0075-4617. Wikidata Q56658706.
- Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. (2003). "Flavor and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_141. ISBN 978-3-527-30673-2.
- Ungnade, H. E.; Orwoll, E. F. (1946). "2-Methoxy Diphenyl Ether". Org. Synth. 26: 50. doi:10.15227/orgsyn.026.0050.
- Patent Appeal No. 7555 United States Court of Customs and Patent Appeals 7 April 1966 http://openjurist.org/358/f2d/750/application-of-edward-s-blake-and-william-c-hammann
- "Dowtherm A 44570".
- Suter, C. M.; Maxwell, C. E. (1943). "Phenoxthin". Organic Syntheses; Collected Volumes, vol. 2, p. 485.
- Mitsuru Ueoda; Tatsuo Aizawa; Yoshio Imai (1977). "Preparation and properties of polyamides and polyimides containing phenoxathiin units". Journal of Polymer Science: Polymer Chemistry Edition. 15 (11): 2739–2747. doi:10.1002/pol.1977.170151119.
- DIRECTIVE 2003/11/EC of the European Parliament and of the Council
- Sutker, B. J. (2005). "Flame Retardants". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_123. ISBN 978-3-527-30673-2.