| |
| Names | |
|---|---|
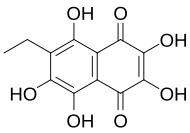
| Preferred IUPAC name 6-ethyl-2,3,5,7,8-pentahydroxynaphthalene-1,4-dione | |
| Other names 7-ethyl-2,3,5,6,8-pentahydroxy-1,4-nafphtoquinone; 6-Ethyl-2,7-trihydroxynaphthazarin; 6-ethyl-2,3,5,7,8-pentahydroxy-2-Ethyl-3,5,6,7,8-pentahydroxy-naphthoquinone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H10O7 |
| Molar mass | 266.205 g·mol |
| Appearance | Dark red crystalline powder |
| Melting point | −219 to 221.5 °C (−362.2 to 430.7 °F; 54.1 to 494.6 K) |
| Solubility in water | Practically insoluble |
| Solubility | Moderately soluble in ethanol; very slightly soluble in chloroform |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Echinochrome A (7-ethyl-2,3,5,6,8-pentahydroxy-1,4-naphthoquinone) is a polyhydroxylated 1,4-naphthoquinone, a type of pigments commonly found in sea urchin shell ("test"), spine, gonads, coelomic fluid, and eggs, of sea urchin. These type of pigments are commonly known as spinochromes and are natural marine phenolic compounds with potential pharmacological effects and modes of action.
First extracted from the sea urchin Scaphechinus mirabilis, it is the active substance of histochrome and Echino-A. Histochrome is used for ophthalmic diseases and ischemic heart disease. Echino-A has been used in nutraceutical form to diminish glucose levels, cholesterol and tryglicerides. The properties and the absence of adverse effects of echinochrome A as an antioxidant has made it the subject of scientific and clinical studies for more than 30 years.
The several hydroxyl groups have the ability to diminish reactive oxygen species (ROS) in cells, preventing redox imbalance. Echinochrome A has been found to target ophtalmologic, cardiovascular, cerebrovascular, inflammatory and metabolic diseases through its biological functions by targeting specific molecular signals. The regulation effects produced by echinochrome A in the cells makes this molecule a candidate to improve health. Sea urchins are known for their putative health properties for centuries, for example in the Materia medica of the Ming Dynasty authored by Li Zhongli in 1647.
References
- CID 135457951 from PubChem Retrieved April 13, 2022
- Anderson, H.A.; Mathieson, J.W.; Thomson, R.H. (January 1969). "Distribution of spinochrome pigments in echinoids". Comparative Biochemistry and Physiology. 28 (1): 333–345. doi:10.1016/0010-406X(69)91347-4. PMID 5777380.
- Jeong, Seung; Kim, Hyoung; Song, In-Sung; Lee, Seon; Ko, Kyung; Rhee, Byoung; Kim, Nari; Mishchenko, Natalia; Fedoryev, Sergey; Stonik, Valentin; Han, Jin (13 May 2014). "Echinochrome A Protects Mitochondrial Function in Cardiomyocytes against Cardiotoxic Drugs". Marine Drugs. 12 (5): 2922–2936. doi:10.3390/md12052922. PMC 4052324. PMID 24828295.
- Hou, Yakun; Vasileva, Elena A.; Carne, Alan; McConnell, Michelle; El-Din A. Bekhit, Alaa; Mishchenko, Natalia P. (2018). "Naphthoquinones of the spinochrome class: occurrence, isolation, biosynthesis and biomedical applications". RSC Advances. 8 (57): 32637–32650. Bibcode:2018RSCAd...832637H. doi:10.1039/C8RA04777D. PMC 9086473. PMID 35547692. S2CID 105576241.
- Rubilar, Tamara; Barbieri, Elena S.; Gazquez, Ayelén; Avaro, Marisa (May 2021). "Sea Urchin Pigments: Echinochrome A and Its Potential Implication in the Cytokine Storm Syndrome". Marine Drugs. 19 (5): 267. doi:10.3390/md19050267. PMC 8151293. PMID 34064550.
- ^ Kim, Hyoung Kyu; Vasileva, Elena A.; Mishchenko, Natalia P.; Fedoreyev, Sergey A.; Han, Jin (August 2021). "Multifaceted Clinical Effects of Echinochrome". Marine Drugs. 19 (8): 412. doi:10.3390/md19080412. PMC 8400489. PMID 34436251.
- Artyukov, Aleksandr A.; Zelepuga, Elena A.; Bogdanovich, Larisa N.; Lupach, Natalia M.; Novikov, Vyacheslav L.; Rutckova, Tatyana A.; Kozlovskaya, Emma P. (May 2020). "Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets". Journal of Clinical Medicine. 9 (5): 1494. doi:10.3390/jcm9051494. PMC 7291202. PMID 32429179.
- Barbieri, Elena Susana; Rubilar, Tamara; Gázquez, Ayelén; Avaro, Marisa; Seiler, Erina Noé; Vera-Piombo, Mercedes; Gittardi, Agustín; Chaar, Florencia; Fernandez, Jimena Pía; Sepulveda, Lucas (29 June 2020). "Sea Urchin Pigments as Potential Therapeutic Agents Against the Spike Protein of SARS-CoV-2 Based on in Silico Analysis". doi:10.26434/chemrxiv.12568595.v1. hdl:11336/109300. S2CID 225845754.
{{cite journal}}: Cite journal requires|journal=(help) This content is a preprint and has not been peer-reviewed