| This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this article by introducing citations to additional sources. Find sources: "Fanetizole" – news · newspapers · books · scholar · JSTOR (July 2018) |
 | |
| Pharmacokinetic data | |
|---|---|
| Protein binding | % |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H16N2S |
| Molar mass | 280.39 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Fanetizole is a drug that has immunoregulating activity.
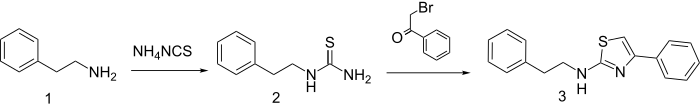
Synthesis
Reaction of β-phenethylamine with ammonium thiocyanate gives the thiourea (2). Treatment of that product with phenacyl bromide produces fanetizole (3).
References
- Lombardino, J. G.; 1981, U.S. patent 4,307,106
| Drugs for peptic ulcer and GERD/GORD (A02B) | |
|---|---|
| H2 antagonists ("-tidine") | |
| Prostaglandins (E)/ analogues ("-prost-") | |
| Proton-pump inhibitors ("-prazole") | |
| Potassium-competitive acid blockers ("-prazan") | |
| Others | |
| Combinations | |
| |
| Phenethylamines | |
|---|---|
| Phenethylamines |
|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|