 | |
| Clinical data | |
|---|---|
| Other names | HM-61713, BI-1482694 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
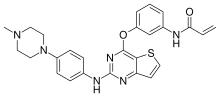
| Formula | C26H26N6O2S |
| Molar mass | 486.59 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Olmutinib (INN) is an investigational anti-cancer drug. It acts by covalently bonding to a cysteine residue near the kinase domain of epidermal growth factor receptor (EGFR).
In the US, it was given a breakthrough therapy designation in non-small cell lung cancer (NSCLC) in December 2015, and In South Korea, the drug was approved in May 2016 for the second-line treatment of NSCLC with the T790M mutation of EGFR. Resistance to olmutinib has been reported; a person's cancer started progressing after they developed a C797S mutation in EGFR.
Olmutinib was discovered by Hanmi Pharmaceutical and licensed to Boehringer Ingelheim in 2015 in an agreement with a $50 million up front payment and up $680 million in milestones. In November 2015 Hanmi granted an exclusive license to sell olmutinib in China to the Chinese company ZAI Labs.
On September 30, 2016, Korean regulatory authorities issued a safety alert about olmutinib in which it described two cases of toxic epidermal necrolysis, one of which was fatal, and a case of Stevens–Johnson syndrome; Boeheringer announced the termination its deal with Hanmi the same day, citing that the decision came after a review of "all available clinical data" on the drug, and also referring to competing drugs.
References
- "Olmutinib". AdisInsight. Springer Nature Switzerland AG. Retrieved 28 February 2017.
- ^ Liao BC, Lin CC, Lee JH, Yang JC (December 2016). "Update on recent preclinical and clinical studies of T790M mutant-specific irreversible epidermal growth factor receptor tyrosine kinase inhibitors". Journal of Biomedical Science. 23 (1): 86. doi:10.1186/s12929-016-0305-9. PMC 5135794. PMID 27912760.
- Passaro A, Guerini-Rocco E, Pochesci A, Vacirca D, Spitaleri G, Catania CM, et al. (March 2017). "Targeting EGFR T790M mutation in NSCLC: From biology to evaluation and treatment". Pharmacological Research. 117: 406–415. doi:10.1016/j.phrs.2017.01.003. PMID 28089942. S2CID 45855336.
- Garde D (July 29, 2015). "Boehringer bets up to $730M on a new lung cancer drug". FierceBiotech.
- Keenan J (April 14, 2016). "South Korea's Hanmi to spend $200M in China expansion". FiercePharma.
- Carroll J (October 1, 2016). "Following lethal tox report, Boehringer scraps plans for high-speed development, kills $730M Hanmi deal". Endpoints.
| Growth factor receptor modulators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiopoietin |
| ||||||||||
| CNTF |
| ||||||||||
| EGF (ErbB) |
| ||||||||||
| FGF |
| ||||||||||
| HGF (c-Met) |
| ||||||||||
| IGF |
| ||||||||||
| LNGF (p75) |
| ||||||||||
| PDGF |
| ||||||||||
| RET (GFL) |
| ||||||||||
| SCF (c-Kit) |
| ||||||||||
| TGFβ |
| ||||||||||
| Trk |
| ||||||||||
| VEGF |
| ||||||||||
| Others |
| ||||||||||
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |