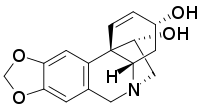
| |
| Names | |
|---|---|
| Systematic IUPAC name (3α,11R,13β)-1,2-Didehydrocrinan-3,11-diol | |
| Other names Bulbispermine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H17NO4 |
| Molar mass | 287.315 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Hamayne is an alkaloid present in plants of the family Amaryllidaceae, including Iberian Narcissus species and two Nigerian Crinum species, reported to have acetylcholinesterase inhibitory activity. The product has been made via total synthesis as well.
References
- López, Susana; Bastida, Jaume; Viladomat, Francesc; Codina, Carles (2002). "Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts". Life Sciences. 71 (21): 2521–2529. doi:10.1016/S0024-3205(02)02034-9. PMID 12270757.
- Petit, Laurent; Banwell, Martin G.; Willis, Anthony C. (2011). "The Total Synthesis of the Crinine Alkaloid Hamayne via a Pd-Catalyzed Intramolecular Alder-Ene Reaction". Organic Letters. 13 (21): 5800–5803. doi:10.1021/ol2023938. PMID 21970722.
This article about an alkaloid is a stub. You can help Misplaced Pages by expanding it. |