| |
| Names | |
|---|---|
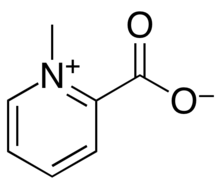
| Preferred IUPAC name 1-Methylpyridin-1-ium-2-carboxylate | |
| Other names N-methyl picolinic acid betaine, Betaine homarine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H7NO2 |
| Molar mass | 137.138 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Homarine (N-methyl picolinic acid betaine) is an organic compound with the chemical formula C7H7NO2. It is commonly found in aquatic organisms from phytoplankton to crustaceans, although it is not found in vertebrates.
Biological function
Homarine functions as an osmolyte by affecting the ionic strength of the cytosol and thereby maintaining osmotic pressure within the cell.
Homarine may also act as a methyl group donor in the biosynthesis of various other N-methylated chemicals, such as glycine betaine and choline. The process of methyl donation converts homarine into picolinic acid and is reversible.
Etymology
The name of this chemical comes from the initial discovery of the molecule in 1933 in lobster tissue: the word homarine as an adjective means "of, or relating to, lobsters" (i.e. genus Homarus).
References
- "Homarine". pubchem.ncbi.nlm.nih.gov. Retrieved 1 October 2020.
- Hoppe-Seyler, F. A. (January 1933). "Über das Homarin, eine bisher unbekannte tierische Base". Hoppe-Seyler's Zeitschrift für physiologische Chemie. 222 (3–4): 105–115. doi:10.1515/bchm2.1933.222.3-4.105.
- Dickson, D. M. J.; Kirst, G. O. (August 1987). "Osmotic Adjustment in Marine Eukaryotic Algae: The Role of Inorganic Ions, Quaternary Ammonium, Tertiary Sulphonium and Carbohydrate Solutes. I. Diatoms and a Rhodophyte". New Phytologist. 106 (4): 645–655. doi:10.1111/j.1469-8137.1987.tb00165.x. PMID 33874080.
- ^ Gasteiger, E. L.; Haake, P. C.; Gergen, J. A. (15 December 2006). "An Investigation of the Distribution and Function of Homarine (N-Methyl Picolinic Acid)". Annals of the New York Academy of Sciences. 90 (3): 622–636. doi:10.1111/j.1749-6632.1960.tb26410.x. PMID 13703887.
- Gebser, Björn; Pohnert, Georg (17 June 2013). "Synchronized Regulation of Different Zwitterionic Metabolites in the Osmoadaption of Phytoplankton". Marine Drugs. 11 (6): 2168–2182. doi:10.3390/md11062168. PMC 3721227. PMID 23774888.
- Netherton, James; Gurin, Samuel (1982). "Biosynthesis and Physiological Role of Homarine in Marine Shrimp". Journal of Biological Chemistry. 257 (20): 11971–11975. doi:10.1016/S0021-9258(18)33662-7. PMID 7118923. Retrieved 1 October 2020.