| |
| Names | |
|---|---|
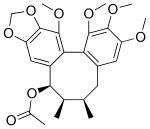
| Preferred IUPAC name (6R,7R,8R)-1,2,3,13-Tetramethoxy-6,7-dimethyl-5,6,7,8-tetrahydro-11H-benzocyclooctabenzodioxol-8-yl acetate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C25H30O8 |
| Molar mass | 458.507 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Kadsurin is a bioactive isolate of Kadsura.
References
- Yang, X. W.; Hattori, M; Namba, T; Chen, D. F.; Xu, G. J. (1992). "Anti-lipid peroxidative effect of an extract of the stems of Kadsura heteroclita and its major constituent, kadsurin, in mice". Chemical & Pharmaceutical Bulletin. 40 (2): 406–9. doi:10.1248/cpb.40.406. PMID 1606637.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |