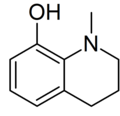
| |
| Names | |
|---|---|
| Preferred IUPAC name 1-Methyl-1,2,3,4-tetrahydroquinolin-8-ol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H13NO |
| Molar mass | 163.220 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Kairine is a derivative of tetrahydroquinoline which was first described by Wilhelm Fischer in 1883. Its name comes from the Greek kairos, meaning "the right time". It is an antipyretic, formerly used against typhoid fever, but now largely obsolete due to severe side effects. Both kairine and its N-ethyl homolog show similar antipyretic activity.
See also
References
- W.E.Flood (1963). The Origins of Chemical Names. Oldbourne Book Co Ltd. p. 126.
- Fischer, Wilhelm (1883). "On Kairine and Kairoline". New Remedies. 12 (2): 41.
- Fruitnight, J. Henry (1886). "Kairine and Antipyrine". Medical Record. 29 (23): 646–648.
- Bockmuhl M, Dorzbach E. Antipyretics of the tetrahydroquinoline series. Med. u. Chem. (1942) 4: 179-212.
- Slater, Leo Barney (2009). War and Disease: Biomedical Research on Malaria in the Twentieth Century. Rutgers University Press. p. 26. ISBN 978-0-8135-4438-0.
This article about a heterocyclic compound is a stub. You can help Misplaced Pages by expanding it. |