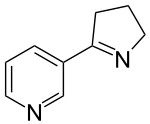
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 3-(3,4-Dihydro-2H-pyrrol-5-yl)pyridine | |
| Other names 3-(1-Pyrrolin-2-yl)pyridine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.165.015 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H10N2 |
| Molar mass | 146.193 g·mol |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 |
| Related compounds | |
| Related compounds | Isomyosamine |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Myosmine is an alkaloid found in tobacco and other plants. Chemically, it is closely related to nicotine. It inhibits aromatase sevenfold more potently than nicotine. It also releases dopamine in adult but not adolescent rats.
See also
References
- "Myosmine". pubchem.ncbi.nlm.nih.gov. Retrieved 14 August 2023.
- Laszlo C, Kaminski K, Guan H, Fatarova M, Wei J, Bergounioux A, Schlage WK, Schorderet-Weber S, Guy PA, Ivanov NV, Lamottke K, Hoeng J (November 2022). "Fractionation and Extraction Optimization of Potentially Valuable Compounds and Their Profiling in Six Varieties of Two Nicotiana Species". Molecules. 27 (22): 8105. doi:10.3390/molecules27228105. PMC 9694777. PMID 36432206.
- Tyroller, Stefan; Zwickenpflug, Wolfgang; Richter, Elmar (2002). "New Sources of Dietary Myosmine Uptake from Cereals, Fruits, Vegetables, and Milk". Journal of Agricultural and Food Chemistry. 50 (17): 4909–15. doi:10.1021/jf020281p. PMID 12166981.
- Doering IL, Richter E (April 2009). "Inhibition of human aromatase by myosmine". Drug Metabolism Letters. 3 (2): 83–6. doi:10.2174/187231209788654045. PMID 19601869.
- Marusich JA, Darna M, Wilson AG, Denehy ED, Ebben A, Deaciuc AG, Dwoskin LP, Bardo MT, Lefever TW, Wiley JL, Reissig CJ, Jackson KJ (November 2017). "Tobacco's minor alkaloids: Effects on place conditioning and nucleus accumbens dopamine release in adult and adolescent rats". European Journal of Pharmacology. 814: 196–206. doi:10.1016/j.ejphar.2017.08.029. PMC 6563910. PMID 28844873.
This article about a heterocyclic compound is a stub. You can help Misplaced Pages by expanding it. |