| |||
| Names | |||
|---|---|---|---|
| IUPAC name Nitrosourea | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | CH3N3O2 | ||
| Molar mass | 89.054 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
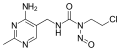
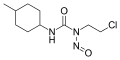
Nitrosourea is both the name of a molecule, and a class of compounds that include a nitroso (R-NO) group and a urea.
Examples
Examples include:
- Arabinopyranosyl-N-methyl-N-nitrosourea (Aranose)
- Carmustine (BCNU, BiCNU)
- Chlorozotocin
- Ethylnitrosourea (ENU)
- Fotemustine
- Lomustine (CCNU)
- Nimustine
- N-Nitroso-N-methylurea (NMU)
- Ranimustine (MCNU)
- Semustine
- Streptozocin (Streptozotocin)
Nitrosourea compounds are DNA alkylating agents and are often used in chemotherapy. They are lipophilic and thus can cross the blood–brain barrier, making them useful in the treatment of brain tumors such as glioblastoma multiforme.
-
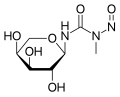 Arabinopyranosyl-N-methyl-N-nitrosourea
Arabinopyranosyl-N-methyl-N-nitrosourea
-
 Carmustine
Carmustine
-
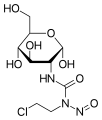 Chlorozotocin
Chlorozotocin
-
 Ethylnitrosourea
Ethylnitrosourea
-
 Fotemustine
Fotemustine
-
 Lomustine
Lomustine
Side effects
Some nitrosoureas (e.g. lomustine) have been associated with the development of interstitial lung disease.
References
- "Antineop". Archived from the original on 2009-03-07. Retrieved 2009-01-24.
- Takimoto CH, Calvo E. "Principles of oncologic pharmacotherapy". in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer management: a multidisciplinary approach. 11 ed. 2008.
- Tucci E, Verdiani P, Di Carlo S, Sforza V (1986). "Lomustine (CCNU)-induced pulmonary fibrosis". Tumori. 72 (1): 95–8. doi:10.1177/030089168607200114. PMID 3952821. S2CID 33327504.
External links
- Nitrosourea+Compounds at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Diseases Database (DDB): 9052
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |