| This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this article by introducing citations to additional sources. Find sources: "Ortho-DOT" – news · newspapers · books · scholar · JSTOR (November 2022) |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 1-propan-2-amine | |
| Other names (±)-1-propan-2-amine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
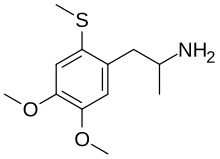
| Chemical formula | C12H19NO2S |
| Molar mass | 241.350 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ortho-DOT, or 4,5-dimethoxy-2-methylthioamphetamine, is a lesser-known psychedelic drug. Ortho-DOT was first synthesized by Alexander Shulgin. In his book PiHKAL (Phenethylamines i Have Known And Loved), neither the dosage nor the duration are known. Ortho-DOT produces few to no effects. Very little data exists about the pharmacological properties, metabolism, and toxicity of Ortho-DOT.
See also
References
This psychoactive drug-related article is a stub. You can help Misplaced Pages by expanding it. |