 | |
| Names | |
|---|---|
| Preferred IUPAC name 1,4-Dioxan-2-one | |
| Other names para-Dioxanone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.130.057 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H6O3 |
| Molar mass | 102.089 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
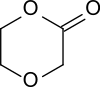
p-Dioxanone (1,4-dioxan-2-one) is the lactone of 2-(2-hydroxyethoxy)acetic acid. It is a monomer that can undergo ring-opening polymerization to give polydioxanone, a biodegradable implant material. It is isomeric to trimethylene carbonate (1,3-dioxan-2-one).
Preparation
The common synthetic process for p-dioxanone is continuous gas-phase dehydrogenation of diethylene glycol on a copper or copper chromite catalyst at 280 °C.
This gives yields of up to 86%. Removal of excess diethylene glycol is crucial to the stability of the product as a monomer. Further purification with recrystallization, vacuum distillation, or melt crystallization allows purities of >99.5% to be achieved.
Properties
Pure p-dioxanone is a white crystalline solid with a melting point of 28 °C.
Uses
The oxidation of p-dioxanone with nitric acid or dinitrogen tetroxide gives diglycolic acid at 75% yield.
p-Dioxanone can undergo ring-opening polymerization catalyzed by organic compounds of tin, such as tin(II) octoate or dibutyltin dilaurate, or by basic alkoxides such as aluminium isopropoxide. This affords polydioxanone, a biodegradable, semicrystalline and thermally labile polymer with uses in industry and medicine. Depolymerization back to the monomer is triggered at 100 °C.
References
- Sangamesh Kumbar, Cato Laurencin and Meng Deng, ed. (20 February 2014). Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine. Elsevier Science. ISBN 978-0-12-396983-5.
{{cite book}}:|work=ignored (help) - ^ US 5675022, "Recovery of dioxanone by melt crystallization", published 1995-08-23, issued 1997-10-07
- US 2142033, "Process for the production of 2-p-dioxanone", published 1936-07-01, issued 1938-10-27
- Lee, Sang-Won; Kim, Sung-Il; Park, So-Jin (2008). "Solubility and density of p-dioxanone in organic solvent systems" (PDF). J. Korean Oil Chem. Soc. 25 (4): 429–437.
- US 3952054, Shen, C.Y., "Process for preparing diglycolic acid", issued 1976-04-20
- US 3645941, "Method of preparing 2-p-dioxanone polymers", issued 1972-02-09
- Bezwada, R.S.; Jamiolkowski, D.D.; Cooper, K. (1997). "Poly(p-dioxane) and its copolymers". Handbook of biodegradable polymers. A. J. Domb, Joseph Kost, David M. Wiseman. Australia: Harwood Academic Publishers. pp. 29–61. ISBN 90-5702-153-6. OCLC 38861271.
This article about a ketone is a stub. You can help Misplaced Pages by expanding it. |

