| |
| |
| Names | |
|---|---|
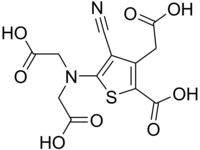
| IUPAC name 3-(Carboxymethyl)-5--4-cyanothiophene-2-carboxylic acid | |
| Systematic IUPAC name 5--3-(carboxymethyl)-4-cyanothiophene-2-carboxylic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H10N2O8S |
| Molar mass | 342.28 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Ranelic acid is an organic acid capable of chelating metal cations.
It forms the ranelate ion, C12H6N2O8S. Strontium ranelate, the strontium salt of ranelic acid, is a drug used to treat osteoporosis and increase bone mineral density (BMD).
References
- CID 3052774 from PubChem
- EP 415850, Wierzbicki, Michel; Bonnet, Jacqueline & Brisset, Martine et al., "Bivalent metal salts of 2-N,N-di(carboxymethyl)amino,3-cyano,4-carboxymethyl,5-carboxy-thiophene-acid, process for their preparation and pharmaceutical compositions containing them", published 1991-03-06, assigned to Adir & Co.
This article about a heterocyclic compound is a stub. You can help Misplaced Pages by expanding it. |