 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.315 |
| Chemical and physical data | |
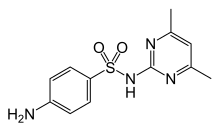
| Formula | C12H14N4O2S |
| Molar mass | 278.33 g·mol |
| 3D model (JSmol) | |
| Melting point | 176 °C (349 °F) |
SMILES
| |
InChI
| |
| (verify) | |
Sulfadimidine or sulfamethazine is a sulfonamide antibacterial.
There are non-standardized abbreviations for it as "sulfadimidine" (abbreviated SDI and more commonly but less reliably SDD) and as "sulfamethazine" (abbreviated SMT and more commonly but less reliably SMZ). Other names include sulfadimerazine, sulfadimezine, and sulphadimethylpyrimidine.
References
- Romváry A, Simon F (1992). "Sulfonamide residues in eggs". Acta Veterinaria Hungarica. 40 (1–2): 99–106. PMID 1476095.
- Reddy KS, Jain SK, Uppal RP (1988). "Pharmacokinetic studies of sulphonamides in poultry". Indian Journal of Animal Sciences.
- Kamakura K, Hasegawa M, Koiguchi S, Miyata M, Okamoto K, Narita M, et al. (1993). "". Eisei Shikenjo Hokoku. Bulletin of National Institute of Hygienic Sciences (111): 61–5. PMID 7920569.
- Garg SK, Ghosh SS, Mathur VS (January 1986). "Comparative pharmacokinetic study of four different sulfonamides in combination with trimethoprim in human volunteers". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 24 (1): 23–5. PMID 3485584.
- Peña MS, Salinas F, Mahedero MC, Aaron JJ (February 1994). "Solvent effect on the determination of sulfamethazine by room-temperature photochemically induced fluorescence". Talanta. 41 (2): 233–6. doi:10.1016/0039-9140(94)80113-4. PMID 18965913.
- Kaniou S, Pitarakis K, Barlagianni I, Poulios I (July 2005). "Photocatalytic oxidation of sulfamethazine". Chemosphere. 60 (3): 372–80. Bibcode:2005Chmsp..60..372K. doi:10.1016/j.chemosphere.2004.11.069. PMID 15924956.
- Calvo R, Sarabia S, Carlos R, Du Souich P (Mar 1987). "Sulfamethazine absorption and disposition: effect of surgical procedures for gastroduodenal ulcers". Biopharmaceutics & Drug Disposition. 8 (2): 115–24. doi:10.1002/bdd.2510080203. PMID 3593892.
- De Liguoro M, Fioretto B, Poltronieri C, Gallina G (June 2009). "The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim". Chemosphere. 75 (11): 1519–24. Bibcode:2009Chmsp..75.1519D. doi:10.1016/j.chemosphere.2009.02.002. PMID 19269673.
Further reading
- ChemDB. "Sulfamethazine", ChemDB, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH)
| Antibacterials that inhibit nucleic acid (J01E, J01M) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antifolates (inhibit bacterial purine metabolism, thereby inhibiting DNA and RNA synthesis) |
| ||||||||||||||||
| Quinolones (inhibit bacterial topoisomerase and/or DNA gyrase, thereby inhibiting DNA replication) |
| ||||||||||||||||
| Anaerobic DNA inhibitors |
| ||||||||||||||||
| RNA synthesis |
| ||||||||||||||||
| |||||||||||||||||
This systemic antibiotic-related article is a stub. You can help Misplaced Pages by expanding it. |