| |||
| Names | |||
|---|---|---|---|
| IUPAC name Pentafluoroorthotelluric acid | |||
| Other names Teflic acid | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.161.534 | ||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
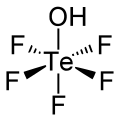
| Chemical formula | HOTeF5 | ||
| Molar mass | 239.60 g·mol | ||
| Appearance | colorless solid | ||
| Melting point | 39.1 °C (102.4 °F; 312.2 K) | ||
| Boiling point | 59.7 °C (139.5 °F; 332.8 K) | ||
| Acidity (pKa) | 8.8 (in Ac2O) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | corrosive, toxic | ||
| GHS labelling: | |||
| Pictograms | 
| ||
| Signal word | Danger | ||
| Hazard statements | H314 | ||
| Precautionary statements | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Teflic acid is a chemical compound with the formula HOTeF5. This strong acid is related to orthotelluric acid, Te(OH)6. Teflic acid has a slightly distorted octahedral molecular geometry.
Preparation
Teflic acid was accidentally discovered by Engelbrecht and Sladky. Their synthesis did not yield the anticipated telluryl fluoride TeO2F2, but a mixture of volatile telluric compounds, containing HOTeF5:
- BaTeO4 + 10 FSO2OH → HOTeF5 (25%)
Teflic acid can also be prepared from fluorosulfonic acid and barium tellurate:
- 5 FSO2OH + Ba[TeO2(OH)4] → HOTeF5 + 4 H2SO4 + BaSO4
It is also the first hydrolysis product of tellurium hexafluoride:
- TeF6 + H2O → HOTeF5 + HF
Teflates

The conjugate base of teflic acid is called the teflate anion, F5TeO (not to be confused with triflate). Many teflates are known, one example being B(OTeF5)3, that can be pyrolysed to give acid anhydride O(TeF5)2.
- 2 B(OTeF5)3 → 2 B(OTeF5)2F + O(TeF5)2
The teflate anion is known to resist oxidation. This property has allowed the preparation several highly unusual species such as the hexateflates M(OTeF5)−6 (in which M = As, Sb, Bi). Xenon forms the cation Xe(OTeF5).
References
- Perrin, D. D., ed. (1982) . Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution. IUPAC Chemical Data (2nd ed.). Oxford: Pergamon (published 1984). Entry 220. ISBN 0-08-029214-3. LCCN 82-16524.
- Engelbrecht, A.; Sladky, F. "Pentafluoro-orthotellursaure, HOTeF5" Angewandte Chemie 1964. 76(9), 379-380, doi:10.1002/ange.19640760912.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Mercier, H. P.A.; Sanders, J. C. P.; Schrobilgen, G. J. "The Hexakis(pentafluorooxotellurato)pnictate(V) Anions, M(OTeF5)−6 (M = As, Sb, Bi): A Series of Very Weakly Coordinating Anions" Journal of the American Chemical Society, volume 116, 2921, (1994). doi:10.1021/ja00086a025.
Further reading
- R.B. King; Inorganic Chemistry of Main Group Elements, VCH Publishers, New York,1994.
| Tellurium compounds | |
|---|---|