| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Trichloroacetic acid | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 970119 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.844 | ||
| Gmelin Reference | 2842 | ||
| KEGG | |||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
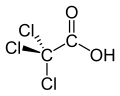
| Chemical formula | C2HCl3O2 | ||
| Molar mass | 163.38 g·mol | ||
| Appearance | Colorless to white, crystalline solid | ||
| Odor | Sharp, pungent | ||
| Density | 1.63 g/cm | ||
| Melting point | 57 to 58 °C (135 to 136 °F; 330 to 331 K) | ||
| Boiling point | 196 to 197 °C (385 to 387 °F; 469 to 470 K) | ||
| Solubility in water | 1000 g/100 mL | ||
| Vapor pressure | 1 mmHg (51.1 °C) | ||
| Acidity (pKa) | 0.66 | ||
| Magnetic susceptibility (χ) | −73.0·10 cm/mol | ||
| Structure | |||
| Dipole moment | 3.23 D | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |  
| ||
| Signal word | Danger | ||
| Hazard statements | H314, H410 | ||
| Precautionary statements | P260, P264, P273, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P391, P405, P501 | ||
| NFPA 704 (fire diamond) |
 | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 5000 mg/kg orally in rats | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | None | ||
| REL (Recommended) | TWA 1 ppm (7 mg/m) | ||
| IDLH (Immediate danger) | N.D. | ||
| Related compounds | |||
| Related chloroacetic acids | Chloroacetic acid Dichloroacetic acid | ||
| Related compounds | Acetic acid Trifluoroacetic acid Tribromoacetic acid | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Trichloroacetic acid (TCA; TCAA; also known as trichloroethanoic acid) is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms. Salts and esters of trichloroacetic acid are called trichloroacetates.
Synthesis
Trichloroacetic acid was discovered by Jean-Baptiste Dumas in 1830.
It is prepared by the reaction of chlorine with acetic acid in the presence of a suitable catalyst such as red phosphorus. This reaction is Hell–Volhard–Zelinsky halogenation.
- CH
3COOH + 3 Cl
2 → CCl
3COOH + 3 HCl
Another route to trichloroacetic acid is the oxidation of trichloroacetaldehyde.
Use
It is widely used in biochemistry for the precipitation of macromolecules, such as proteins, DNA, and RNA. TCA and DCA are both used in cosmetic treatments (such as chemical peels and tattoo removal) and as topical medication for chemoablation of warts, including genital warts. It can kill normal cells as well. It is considered safe for use for this purpose during pregnancy.
The sodium salt (sodium trichloroacetate) was used as an herbicide starting in the 1950s but regulators removed it from the market in the late 1980s and early 1990s.
Environmental and health concerns
According to the European Chemicals Agency, "This substance causes severe skin burns and eye damage, is very toxic to aquatic life and has long lasting toxic effects."
History
The discovery of trichloroacetic acid by Jean-Baptiste Dumas in 1839 delivered a striking example to the slowly evolving theory of organic radicals and valences. The theory was contrary to the beliefs of Jöns Jakob Berzelius, starting a long dispute between Dumas and Berzelius.
Popular culture
In the 1958 film The Blob, a bottle of trichloroacetic acid is tossed at the Blob in a futile attempt to fend it off.
See also
References
- ^ NIOSH Pocket Guide to Chemical Hazards #0626 National Institute for Occupational Safety and Health (NIOSH)
- ^ Budavari, Susan, ed. (1996). The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (12th ed.). Merck. ISBN 0911910123.
- Databog fysik kemi, F&K Forlaget 11. udgave 2009
- Terchloracetic Acid in Gmelin, L., Hand-book of Chemistry: Organic chemistry
- Jones, Kirtly (June 21, 2012). Marshall, Sarah (ed.). "Trichloroacetic Acid or Bichloroacetic Acid for Genital Warts (Human Papillomavirus)". WebMD. HealthWise. Archived from the original on 16 July 2015.
- Wiley DJ, Douglas J, Beutner K, Cox T, Fife K, Moscicki AB, Fukumoto L (2002). "External genital warts: Diagnosis, treatment, and prevention". Clinical Infectious Diseases. 35 (Suppl 2): S210 – S224. doi:10.1086/342109. PMID 12353208.
- TCA-sodium in the Pesticide Properties DataBase (PPDB), accessed June 20, 2014
- G. S. Rai and C. L. Hamner. "Persistence of Sodium Trichloroacetate in Different Soil Types." Weeds 2(4) Oct. 1953: 271-279. JSTOR 4040104. DOI 10.2307/4040104.
- United Nations Environment Programme. "Trichloroacetic Acid CAS N°: 76-03-9" (OECD SIDS). Accessed June 20, 2014. Archived from the original on 15 August 2018.
- Heier, Al (December 1991). "Trichloroacetic Acid (TCA)". EPA. Accessed June 20, 2014 — via Cornell PMEP Pesticide Active Ingredient Information database. Archived from the original on 15 Aug 2020.
- "Trichloroacetic acid". C&L Inventory. European Chemical Agency. Retrieved 14 March 2022.
- Dumas (1839). "Trichloroacetic acid". Annalen der Pharmacie. 32: 101–119. doi:10.1002/jlac.18390320109.
- William Albert Noyes (1927). "Valence". Proceedings of the American Philosophical Society. 66: 287–308. JSTOR 3301070.