| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.587 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
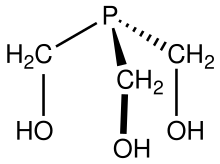
| Chemical formula | C3H9O3P |
| Molar mass | 124.076 g·mol |
| Appearance | white solid |
| Density | 1.16 g/cm |
| Melting point | 51–53 °C (124–127 °F; 324–326 K) |
| Boiling point | decomposes |
| Solubility in water | alcohols, dmf |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H301, H315, H318, H335 |
| Precautionary statements | P261, P264, P264+P265, P270, P271, P280, P301+P316, P302+P352, P304+P340, P305+P354+P338, P317, P319, P321, P330, P332+P317, P362+P364, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Tris(hydroxymethyl)phosphine is the organophosphorus compound with the formula P(CH2OH)3. It is a white solid. The compound is multifunctional, consisting of three alcohol functional groups and a tertiary phosphine. It is prepared by treating tetrakis(hydroxymethyl)phosphonium chloride with strong base:
- Cl + NaOH → P(CH2OH)3 + H2O + H2C=O + NaCl
The compound can be prepared on a large scale using triethylamine as base and as solvent.
Reactions
The compound forms complexes with a variety of metals. These complexes display some solubility in water but more so in methanol. The compound decomposes violently to phosphine and formaldehyde upon attempted distillation. In air, it oxidizes to the oxide.
Upon heating with hexamethylenetetramine, it converts to triazaphosphaadamantane.
References
- "Tris(hydroxymethyl)phosphine". pubchem.ncbi.nlm.nih.gov.
- Ferguson, Marcelle L.; O’Leary, Daniel J.; Grubbs, Robert H. (2003). "Ring-Closing Metathesis Synthesis of N-BOC-3-Pyrroline". Organic Syntheses. 80: 85. doi:10.15227/orgsyn.080.0085
{{cite journal}}: CS1 maint: multiple names: authors list (link). - M. Caporali, L. Gonsalvi, F. Zanobini, M. Peruzzini (2010). "Functional Ligands and Complexes". Inorganic Syntheses. Vol. 35. pp. 92–108. doi:10.1002/9780470651568.ch5. ISBN 978-0-471-68255-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Ellis, James W.; Harrison, Karl N.; Hoye, Peter A. T.; Orpen, A. Guy; Pringle, Paul G.; Smith, Martin B. (1992). "Water-Soluble Tris(hydroxymethyl)phosphine Complexes with Nickel, Palladium, and Platinum. Crystal Structure of Pd4CH3OH". Inorganic Chemistry. 31 (14): 3026–3033. doi:10.1021/ic00040a009.
- Daigle, D. J.; Pepperman, A. B.; Vail, Sidney L. (1974). "Synthesis of a Monophosphorus Analog of Hexamethylenetetramine". Journal of Heterocyclic Chemistry. 11 (3): 407–408. doi:10.1002/jhet.5570110326.