| Revision as of 12:46, 17 February 2007 editAlnoktaBOT (talk | contribs)19,917 editsm robot Adding: ar:تناسخ الحمض النووي الريبي منقوص الأكسجين← Previous edit | Revision as of 03:24, 21 February 2007 edit undo74.97.31.113 (talk)No edit summaryNext edit → | ||
| (One intermediate revision by the same user not shown) | |||
| Line 4: | Line 4: | ||
| ] | ] | ||
| '''DNA replication''' or '''DNA synthesis''' is the process of copying a double-stranded ] molecule. This process is paramount to all life as we know it |
'''DNA replication''' or '''DNA synthesis''' is the process of copying a double-stranded ] molecule. This process is paramount to all life as we know it and the general mechanisms of DNA replication is conserved among prokaryotic and eukaryotic organisms. DNA replication involves copying the genetic material and passing it on to daughter cells, therefore the process is important in continuation of life. | ||
| A DNA molecule is a long ] |
A DNA molecule is a long ] built from nucelotides. Both strands in DNA run anti-parallel to eachother and are complementary to eachother. One of the DNA strands is built in the 5' → 3' direction, while the complementary strand is built in the 3' → 5' direction (5' and 3' each mark one end of a strand). The nucleotides which make up DNA pair with each other and form base pairs such as ]::] and ]:::] (two dots between A and T indicate that they are bound by two ]s, and three dots between G and C indicate the presence of three hydrogen bonds). This means that the strand running in the 5'→ 3' direction will have base A that will pair with base T on the opposite strand running in 3'→ 5' direction. | ||
| Since DNA strands are antiparallel and complementary, each strand can serve as a template for the reproduction of the opposite strand. The template strand is preserved as a whole piece and the new strand is assembled from nucleotide triphosphates. This process is called '']''. Ideally, the two resulting strands are identical, although in reality there are always errors, though proofreading and error-checking mechanisms exist to ensure a very high level of fidelity. | Since DNA strands are antiparallel and complementary, each strand can serve as a template for the reproduction of the opposite strand. The template strand is preserved as a whole piece and the new strand is assembled from nucleotide triphosphates. This process is called '']''. Ideally, the two resulting strands are identical, although in reality there are always errors, though proofreading and error-checking mechanisms exist to ensure a very high level of fidelity. | ||
| In a ], |
In a ], DNA replicaton is obligatory prior to ]. ]s persistently replicate their DNA. In ]s, timings are highly regulated and this occurs during the ] of the ], preceding ] or ] I. The process may also be performed ''in vitro'' using reconstituted or completely artificial components, in a process known as ]. | ||
| DNA may be synthesized artificially, but this process is not fundamentally a replicative process, and only produces a single strand of DNA. | DNA may be synthesized artificially, but this process is not fundamentally a replicative process, and only produces a single strand of DNA. | ||
| Line 22: | Line 22: | ||
| The process may also be performed in-vitro using reconstituted or completely artificial components, in a process known as PCR. DNA may be synthesized artificially, but this process is not fundamentally a replicative process, and only produces one strand of DNA. | The process may also be performed in-vitro using reconstituted or completely artificial components, in a process known as PCR. DNA may be synthesized artificially, but this process is not fundamentally a replicative process, and only produces one strand of DNA. | ||
| Although the general mechanisms of DNA replication are conserved among all species, differences do exist between prokaryotic and eukaryotic DNA replication. The model organisms used to study DNA replicaton is E. coli (for prokaryotes) and yeast - specifically S. pombe and S. cerevisiae) for eukaryotes. The following explinations will take each case seperatly. | |||
| == 3 Steps for DNA polymerization == | |||
| == Prokaryotic DNA Replicaton == | |||
| This is a description of DNA polymerization using an enzyme. This is not the synthetic, purely chemical, laboratory method of artificially synthesizing oligos in a laboratory or oligo factory. Artificially synthesized oligos are a key aspect of the Polymerase Chain Reaction (PCR). | |||
| DNA replicaton in E. coli is bi-directional and originates at a single origin of replicaton (OriC). The initiation of replication is mediated by DnaA, a protein that binds to a region of the origin known as the DnaA box. In E. coli, there are 5 DnaA boxes, each of which contains a highly conserved 9bp consensous sequence 5' - TTATCCACA - 3'. Binding of DnaA to this region causes it to become negativly supercoiled. Following this, a region of OriC upstream of the DnaA boxes (known as DnaB boxes) become melted. There are three of these regions, and each are 13bp long, and AT rich (which obviously facilitates melting because less energy is required to break the two hydrogen bonds that form between A and T nucleotides). This region has the consensous sequence 5' - GATCTNTTNTTTT - 3. Melting of the DnaB boxes requires ATP (which is hydrolyzed by DnaA). Following melting, DnaA recuits a hexameric helicase (six DnaB proteins) to oppisate ends of the melted DNA. This is where the replication fork will form. Recruitment of helicase requires six DnaC proteins, each of which is attached to one subunit of helicase. Once this complex is formed, an additional five DnaA proteins bind to the original five DnaA proteins to form five DnaA dimers. DnaC is then released, and the prepriming complex is complete. In order for DNA replication to continue, SSB is needed to prevent the single strands of DNA from forming any secondary structures and to prevent them from reannealing, and DNA gyrase is needed to relieves the stress (by creating negative supercoils) created by the action of DnaB helicase. The unwinding of DNA by DnaB helicase allows for primase (DnaG) and RNA polymerase to prime each DNA template so that DNA sysnthesis can begin. | |||
| === Initiation === | |||
| The specific site at which DNA unwinding and initiation of replication occur is called ]. DNA synthesis, although on a very general level is the same, differs between species and organisms. Having said that, depending on the organism, there may be a single origin of replication or as many as thousands per chromosome. Because bacteria (]) on molecular level are simpler organisms than humans (]) the initiation of DNA synthesis was first fully understood in bacteria, and model organism used was ]. In ], ], ] and ] defined all of DNA replicated from a particular origin in E. Coli as a '''replicon'''. They further proposed that this replicon must have two components that initiate replication of DNA: the '''replicator''' (an entire set of cis-acting DNA sequences that is sufficient to direct the initiation of DNA synthesis) and the '''initiator''' (a protein that specifically recognizes a particular DNA element in the replicator and activates the initiation process). | |||
| Once priming is complete, DNA polymerase III holoenzyme is loaded into the DNA and replication begins. The catalytic mechanism of DNA polymerase III involves the use of two metal ions in the active site, and a region in the active site that can discriminate between deoxynucleotides and ribonucleotides. The metal ions are general divalent cations that help the 3' OH initiate a nucleophilic attack onto the alpha phosphate of the deoxyribonucleotide and orient and stabalize the negatively charged triphosphate on the deoxyribonucleotide. Nucleophillic attack by the 3' OH on the alpha phosphate releases pyrophosphate, which is then subsequently hydrolyzed (by inorganic phosphatase) into two phophates. This hydrolysis drives DNA sysnthesis to completion. | |||
| Initiator proteins have three roles: | |||
| # They bind to a specific DNA sequence and event in the replicator. | |||
| # Once bound they unwind short region of DNA adjacent to their binding site. | |||
| # They recruit additional initiation factors to the replicator. | |||
| Furthermore, DNA polymerase III must be able to distinguish between correctly paired bases and incorrectly paired bases. This is accomplished by distinguishing Watson-Crick base pairs through the use of an active site pocket that that is complementary in shape to the structure of correctly paired nucleotides. This pocket has a tyrosine residue that is able to form van der Waals interactions with the correctly paired nucleotide. In addition, dsDNA in the active site has a wider and shallower minor groove that permits the formation of hydrogen bonds with the third nitrogen of purine bases and the second oxygen of pyrimidine bases. Finally, the active site makes extensive hydrogen bonds with the DNA backbone. These interactions result in the DNA polymerase III closing around a correctly paired base. If a base is inserted and incorrectly paired, these interactions could not occur due to disruptions in hydrogen bonding and van der Waals interactions. | |||
| ** In E. Coli (a ]) the initiator protein is '''DnaA''', which binds to a specific sequence defining prokaryotic origin of replication ''ori''C and starts interaction with ]. This initial DnaA - ATP interaction results in unwinding of short region, no more than 20 base pairs long, and provides a single - stranded DNA (abbr. ssDNA) template for additional replication proteins to begin DNA synthesis. These additional replication proteins are '''DnaB''' (a ]) and a '''DnaC''' (a helicase loader). | |||
| DNA is synthesized in one direction only, 5' -> 3'. However, one of the parent strands of DNA is 3' -> 5' and the other is 5' -> 3'. At the replication fork, one strand is synthesized continuously using the aformentioned mechanisms, while the other is synthesized in a series of short fragments known as Okazaki fragments. The 3' -> 5' strand is synthesized continuously, and is known as the leading strand. The RNA primers of Okazaki fragments are subsequently degraded by RNAseH and DNA Polymerase I, and the gap is filled with deoxyribonucleotides and sealed by the enzyme ligase. | |||
| ** In higher organisms (]) the initiator is a protein complex called origin of recognition complex (abbr. '''ORC'''), which analogous to DnaA in prokaryotes, recognizes a specific DNA sequence and binds to it. This is followed by DNA replicating machinery assembling. | |||
| Termination of DNA replication in E. coli is completed through the use of termination sequences and the Tus protein. These sequences allow the two replicaton forks to pass through in only one direction, but not the other. However, these sequences are not required for termination of replication. | |||
| This origin of replication is unwound (i.e., the two strands are pulled apart at that site) and the partially unwound strands form a "replication bubble", with one ] on either end. Each group of enzymes at the replication fork moves away from the origin, unwinding and replicating the original DNA strands as they proceed. ] mark the individual sequences and the starting points to be replicated. | |||
| Regulation of DNA replication is achieved through several mechanisms. Mechanisms involve the ratio of ATP to ADP, of DnaA to the number of DnaA boxes and the hemimethylation and sequestering of OriC. The ratio of ATP to ADP indicates that the cell has reached a specific size and is ready to divide. This "signal" occurs becuase in a rich medium, the cell will grow quickly and will have a lot of excess DNA. Futhermore, DnaA binds equally well to ATP or ADP, and only the DnaA-ATP complex is able to initate replication. Thus, in a fast growing cell, there will be more DnaA-ATP than DnaA-ADP. Because the levels of DnaA are strictly regulated, and 5 DnaA-DnaA dimers are needed to initiate replication, the ratio of DnaA to the number of DnaA boxes in the cell is important. After DNA replicaton is complete, this number is halved, thus DNA replication cannot occur until the levels of DnaA protein increases. Finally, DNA is sequestered to a membrane binding protein called SeqA. This protein binds to hemimethylated GATC DNA sequences. This four bp sequences occurs 11 times in OriC, and newly synthesized DNA only has its parent strand methylated. DAM methyltrasferase methylates the newly synthesized strand of DNA ONLY if it is not bound to SeqA. The importance of hemimethylation is twofold. Firstly, OriC becomes inaccessable to DnaA, and secondly, DnaA binds better to fully methylated DNA than hemimethylated DNA. | |||
| The factors involved are collectively called the ]. It consists of the following: | |||
| * A '''],''' which introduces negative ]s into the DNA in order to minimize torsional strain induced by the unwinding of the DNA by helicase ahead of the replicational complex. The topoisomerase reversibly breaks the DNA strand, allowing the DNA to swivel, preventing the DNA from knotting up. | |||
| * A ], which unwinds and splits the DNA ahead of the fork. Thereafter, ]s (SSB) swiftly bind to the separated DNA, preventing the strands from reuniting. | |||
| * A ] (] or ] in ]s, ] in ]s), which generates an ] primer to be used in DNA replication. | |||
| * A ], which in reality is a complex of proteins that together perform the "actual" replication, i.e., the polymerization of nucleotides complementary to the template strand. | |||
| **] in prokaryotes | |||
| **] and ] in eukaryotes. | |||
| === Elongation=== | |||
| == Eukaryotic DNA Replicaton == | |||
| ] _ _ _ _ _ _ _ _ _ _ _] <--] ] | |||
| ]-]-]-] <--]s | |||
| ] _ _ _ _ _ _ _ _ _ _ _] <--] ] ] | |||
| Although the mechanisms of DNA synthesis is both Eukaryotes and prokaryotes is similar, eukaryotes have a much more complicated method of DNA replication. Even though DNA synthesis in E. coli is regulated, DNA replication is always initiated before the end of the cell cycle, this cannot occur in eukaryotes or a host of diseases could manifest themselves. | |||
| At the beginning of elongation, an enzyme called ] binds to the DNA and synthesizes DNA from the RNA primer, which indicates the starting point for the elongation. DNA polymerases can only synthesize the new DNA in the ] to ] direction. Because of this these enzymes can only travel on one side of the original strand without any interruption. This new strand, which proceeds from 5’ to 3’, is the ]. The other new strand, which proceeds from 3’ to 5’, is the ]. | |||
| DNA replication in eukaryotes occurs only in the S phase of the cell cycle. However, pre-initiation occurs in G1. Due to the sheer size of chromosomes in eukaryotes, eukaryotic chromosomes contain multiple origins of replication. Some origins are well characterized, such as the autonomously replicating sequences (ARS) of yeast while other eukaryotic origins, particularly those in metazoa, can be found in spans of thousands of basepairs. However, the assembly and initiation of replicaton is similar in both the protozoa and metazoa. | |||
| In prokaryotes RNA primers are removed by DNA polymerase I, which also synthesizes DNA in their place, in eukaryotes they are removed by ] or ] and replaced with DNA by DNA polymerase δ or ε. | |||
| The first step in DNA replication is the formation of the pre-initiation replication complex (the pre-RC). The formation of this complex occurs in two stages. The first stage requires that there is no CDK activity. This can only occur in early G1. The formation of the pre-RC is known as licensing, but a licensed pre-RC cannot initaite replicaton. Initiation of replication can only occur during the S-phase. Thus, the seperation of licensing and activation ensures that the origin can only fire once per cell cycle. | |||
| Since DNA synthesis only occurs in the 5' to 3' direction, the DNA of the lagging strand is replicated in pieces known as ]. Each time DNA polymerase reaches the 5' end of the RNA primer for the next Okazaki fragment, it dissociates and reassociates at the 3' end of the primer. Another enzyme, DNA ligase, is necessary to connect the Okazaki fragments. | |||
| DNA replication in eukaryotes is not very well characterized. However, researchers believe that it begins with the binding of the origin recognition complex (ORC) to the origin. This complex is a hexamer of related proteins and remains bound to the origin, even after DNA replication occurs. Furthermore, ORC is the functional analogue of DnaA. Following the binding of ORC to the origin, Cdc6/Cdc18 and Cdt1 coordinate the loading of the MCM (minichromosome maitenance functions) complex to the origin by first binding to ORC and then binding to the MCM complex. The MCM complex is thought to be the major DNA helicase in eukaryotic organisms, and is a hexamer (mcm2-7). Once binding of MCM occurs, a fully licensed pre-RC exists. | |||
| In prokaryotes, leading strand and lagging strand synthesis are coupled by the action of the ]. One complex replicates the leading and lagging strands simultaneously. During this stage ] continues to unwind the DNA into two single strands while ]s relieves the ] caused by this. | |||
| Activation of the complex occurs in S-phase and requires Cdk2-Cyclin E and Ddk. The activation process begins with the addition of Mcm10 to the pre-RC, which displaces Cdt1. Following this, Ddk phosphorylates Mcm3-7, which activates the helicase. It is believed that ORC and Cdc6/18 are phosphorylated by Cdk2-Cyclin E. Ddk and the the Cdk complex then recruits another protein called Cdc45, which then recruits all of the DNA replicaton proteins to the replicaton fork. At this stage the origin fires and DNA synthesis begins. | |||
| Both prokaryotes and eukaryotes have DNA polymerases with proof-reading and 3' exonuclease activities. These functions increase the fidelity of replication. Prokaryotes tend to have fewer or weaker proof-reading mechanisms due to the nature of their natural selection of their gene pools. | |||
| Activation of a new round of replicaton is prevented through the actions of the cyclin dependant kinases and a protein known as geminin. Geminin binds to Cdt1 and sequesters it. It is a periodic protein that first appears in S-phase and is degraded in late M-phase, possibly through the action of the anaphase promoting complex (APC). In addition, phosphorylation of Cdc6/18 prevent it from binding to the ORC (thus inhibiting loading of the MCM complex) while the phosphorylation of ORC remains unclear. Cells in the G0 stage of the cell cycle are prevented from initiating a round of replication because the Mcm proteins are not expressed. | |||
| === Termination === | |||
| Termination occurs when DNA replication forks meet one another or run to the end of a linear DNA molecule. Also, termination may occur when a replication fork is deliberately stopped by a special protein, called a replication terminator protein, that binds to specific sites on a DNA molecule. | |||
| Numerous polymerases can repicate DNA in eukaryotic cells. Currently, six families of polymerases (A, B, C, D, X, Y) have been discovered. At least four different types of DNA polymerases are involved in the replication of DNA in animal cells (POLA, POLG, POLD1 and POLE). POL1 functions by extending the primer in the 5' -> 3' direction and tightly associates with primase. However, it lacks the ability to proofread DNA. POLD1 has a proofreading ability and is able to replicate the entire length of a template only when associated with PCNA. POLE is able to replicate the entire length of a template in teh absence of PCNA and is able to proofread DNA while POLG replicates mitochondrial DNA via the D-Loop mechanism of DNA replicaton. All primers are removed by RNaseH1 and Flap Endonuclease I. The general mechanisms of DNA replication on the leading and lagging strand, however, are the same as to those found in prokaryotic cells. | |||
| When the polymerase reaches the end of a linear DNA molecule, there is a potential problem due to the antiparallel structure of DNA. Because an RNA primer must be regularly laid down on the lagging strand, the last section of the lagging-strand DNA cannot be replicated because there is no DNA template for the primer to be synthesized on. To solve this problem, the ends of most chromosomes consist of ] that contains repeat sequences. The end of a linear chromosome is called the ]. Cells can endure the shortening of the chromosome at the telomere to a degree, since it's necessary for chromosome stability. Many cells use an enzyme called ] that adds the repeat units to the end of the chromosome so the ends do not become too short after multiple rounds of DNA replication. Many simple, single-celled organisms overcome the whole problem by having circular chromosomes. | |||
| ⚫ | == References == | ||
| Before the DNA replication is finally complete, enzymes are used to proofread the sequences to make sure the nucleotides are paired up correctly in a process called ]. If mistake or damage occurs, enzymes such as a ] will remove the incorrect DNA. DNA polymerase will then fill in the gap. | |||
| * Voet and Voet. ''Biochemistry'', Third Edition (2004). ISBN 0-471-19350-X. Wiley International Edition. | |||
| ===Equation=== | |||
| A ] can be written that represents the process: | |||
| <blockquote> (DNA)<sub>n</sub> + dNTP → (DNA)<sub>n+1</sub> + PP<sub>i</sub></blockquote> | |||
| Nucleotides (dNTP) used by DNA replications contain three phosphates attached to the sugar, like ] and are named accordingly ], ], and ]. However, in contrast to most other processes of the cell in which only one phosphate group (P<sub>i</sub>), the last two phosphate groups (PP<sub>i</sub>) (pyrophosphate group) are detached. | |||
| ==Organization of multiple replication sites== | |||
| A diploid human cell contains 6 billion nucleotide pairs (contained in 46 linear chromosomes) that are copied at about 50 ]s per second by each replication fork. Yet in a typical cell the entire replication process takes only about 8 hours. This is because there are many ]s, called origins of replication, on a eukaryotic chromosome. Replication can begin at some origins earlier than at others. As replication nears completion, "bubbles" of newly replicated DNA meet and fuse, forming two new DNA molecules. | |||
| There must be some form of regulation and organization of these multiple replication sites to prevent re-initiation of replication at origins that have already initiated replication (re-initiation would result in too many copies of the DNA). To date, two replication control mechanisms have been identified: one positive and one negative. Each replication origin site must be bound by a set of proteins called the ''origin recognition complex''. These remain attached to the DNA at each origin throughout the replication process. In addition to the origin recognition complex, specific accessory proteins called ]s, must also be present for initiation of replication. Altogether, these proteins promote initiation and they represent the positive control mechanism for initiation. After the cell has signaled to the origins to initiate DNA replication, specific destruction of certain licensing factors prevents further replication cycles from occurring. Licensing factors are only replenished in cells during the cell division process, so they cannot act to reinitiate replication inappropriately before division. | |||
| == See also == | |||
| * ] | |||
| * ] (chemical DNA synthesis) | |||
| ⚫ | == References == | ||
| * Watson, Baker, Bell, Gann, Levine, Losick. ''Molecular Biology of the Gene'', Fifth Edition (2003). ISBN 0-8053-4635-X. Pearson/Benjamin Cummings Publishing. | * Watson, Baker, Bell, Gann, Levine, Losick. ''Molecular Biology of the Gene'', Fifth Edition (2003). ISBN 0-8053-4635-X. Pearson/Benjamin Cummings Publishing. | ||
Revision as of 03:24, 21 February 2007
| This article needs attention from an expert in Biology. Please add a reason or a talk parameter to this template to explain the issue with the article. WikiProject Biology may be able to help recruit an expert. |
| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "DNA replication" – news · newspapers · books · scholar · JSTOR (November 2006) (Learn how and when to remove this message) |

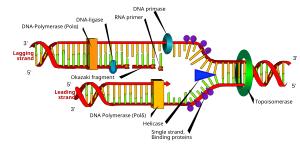
DNA replication or DNA synthesis is the process of copying a double-stranded DNA molecule. This process is paramount to all life as we know it and the general mechanisms of DNA replication is conserved among prokaryotic and eukaryotic organisms. DNA replication involves copying the genetic material and passing it on to daughter cells, therefore the process is important in continuation of life.
A DNA molecule is a long polymer built from nucelotides. Both strands in DNA run anti-parallel to eachother and are complementary to eachother. One of the DNA strands is built in the 5' → 3' direction, while the complementary strand is built in the 3' → 5' direction (5' and 3' each mark one end of a strand). The nucleotides which make up DNA pair with each other and form base pairs such as A::T and C:::G (two dots between A and T indicate that they are bound by two hydrogen bonds, and three dots between G and C indicate the presence of three hydrogen bonds). This means that the strand running in the 5'→ 3' direction will have base A that will pair with base T on the opposite strand running in 3'→ 5' direction.
Since DNA strands are antiparallel and complementary, each strand can serve as a template for the reproduction of the opposite strand. The template strand is preserved as a whole piece and the new strand is assembled from nucleotide triphosphates. This process is called semiconservative replication. Ideally, the two resulting strands are identical, although in reality there are always errors, though proofreading and error-checking mechanisms exist to ensure a very high level of fidelity.
In a cell, DNA replicaton is obligatory prior to cell division. Prokaryotes persistently replicate their DNA. In eukaryotes, timings are highly regulated and this occurs during the S phase of the cell cycle, preceding mitosis or meiosis I. The process may also be performed in vitro using reconstituted or completely artificial components, in a process known as PCR.
DNA may be synthesized artificially, but this process is not fundamentally a replicative process, and only produces a single strand of DNA.
Introduction

DNA replication or DNA synthesis is the process of copying a double-stranded DNA strand. Since DNA strands are antiparallel and complementary, each strand can serve as a template for the reproduction of the opposite strand. The template strand is preserved as a whole piece and the new strand is assembled from nucleotide triphosphates. This process is called semiconservative replication. Ideally, the two resulting strands are identical, although in reality there are always errors, though proofreading and error-checking mechanisms exist to ensure a very high level of fidelity.
In a cell, this step is obligatory prior to cell division. Prokaryotes persistently replicate their DNA and creating a whole, new chromosome is a limiting step in cell division.The large size of eukaryotic chromosomes and the limits of nucleotide incorporation during DNA synthesis, make it necessary for multiple origins of replication to exist in order to complete replication in a reasonable period of time. However, it is clear that at a replication origin the strands of DNA must dissociate and unwind in order to allow access to DNA polymerase.
The process may also be performed in-vitro using reconstituted or completely artificial components, in a process known as PCR. DNA may be synthesized artificially, but this process is not fundamentally a replicative process, and only produces one strand of DNA.
Although the general mechanisms of DNA replication are conserved among all species, differences do exist between prokaryotic and eukaryotic DNA replication. The model organisms used to study DNA replicaton is E. coli (for prokaryotes) and yeast - specifically S. pombe and S. cerevisiae) for eukaryotes. The following explinations will take each case seperatly.
Prokaryotic DNA Replicaton
DNA replicaton in E. coli is bi-directional and originates at a single origin of replicaton (OriC). The initiation of replication is mediated by DnaA, a protein that binds to a region of the origin known as the DnaA box. In E. coli, there are 5 DnaA boxes, each of which contains a highly conserved 9bp consensous sequence 5' - TTATCCACA - 3'. Binding of DnaA to this region causes it to become negativly supercoiled. Following this, a region of OriC upstream of the DnaA boxes (known as DnaB boxes) become melted. There are three of these regions, and each are 13bp long, and AT rich (which obviously facilitates melting because less energy is required to break the two hydrogen bonds that form between A and T nucleotides). This region has the consensous sequence 5' - GATCTNTTNTTTT - 3. Melting of the DnaB boxes requires ATP (which is hydrolyzed by DnaA). Following melting, DnaA recuits a hexameric helicase (six DnaB proteins) to oppisate ends of the melted DNA. This is where the replication fork will form. Recruitment of helicase requires six DnaC proteins, each of which is attached to one subunit of helicase. Once this complex is formed, an additional five DnaA proteins bind to the original five DnaA proteins to form five DnaA dimers. DnaC is then released, and the prepriming complex is complete. In order for DNA replication to continue, SSB is needed to prevent the single strands of DNA from forming any secondary structures and to prevent them from reannealing, and DNA gyrase is needed to relieves the stress (by creating negative supercoils) created by the action of DnaB helicase. The unwinding of DNA by DnaB helicase allows for primase (DnaG) and RNA polymerase to prime each DNA template so that DNA sysnthesis can begin.
Once priming is complete, DNA polymerase III holoenzyme is loaded into the DNA and replication begins. The catalytic mechanism of DNA polymerase III involves the use of two metal ions in the active site, and a region in the active site that can discriminate between deoxynucleotides and ribonucleotides. The metal ions are general divalent cations that help the 3' OH initiate a nucleophilic attack onto the alpha phosphate of the deoxyribonucleotide and orient and stabalize the negatively charged triphosphate on the deoxyribonucleotide. Nucleophillic attack by the 3' OH on the alpha phosphate releases pyrophosphate, which is then subsequently hydrolyzed (by inorganic phosphatase) into two phophates. This hydrolysis drives DNA sysnthesis to completion.
Furthermore, DNA polymerase III must be able to distinguish between correctly paired bases and incorrectly paired bases. This is accomplished by distinguishing Watson-Crick base pairs through the use of an active site pocket that that is complementary in shape to the structure of correctly paired nucleotides. This pocket has a tyrosine residue that is able to form van der Waals interactions with the correctly paired nucleotide. In addition, dsDNA in the active site has a wider and shallower minor groove that permits the formation of hydrogen bonds with the third nitrogen of purine bases and the second oxygen of pyrimidine bases. Finally, the active site makes extensive hydrogen bonds with the DNA backbone. These interactions result in the DNA polymerase III closing around a correctly paired base. If a base is inserted and incorrectly paired, these interactions could not occur due to disruptions in hydrogen bonding and van der Waals interactions.
DNA is synthesized in one direction only, 5' -> 3'. However, one of the parent strands of DNA is 3' -> 5' and the other is 5' -> 3'. At the replication fork, one strand is synthesized continuously using the aformentioned mechanisms, while the other is synthesized in a series of short fragments known as Okazaki fragments. The 3' -> 5' strand is synthesized continuously, and is known as the leading strand. The RNA primers of Okazaki fragments are subsequently degraded by RNAseH and DNA Polymerase I, and the gap is filled with deoxyribonucleotides and sealed by the enzyme ligase.
Termination of DNA replication in E. coli is completed through the use of termination sequences and the Tus protein. These sequences allow the two replicaton forks to pass through in only one direction, but not the other. However, these sequences are not required for termination of replication.
Regulation of DNA replication is achieved through several mechanisms. Mechanisms involve the ratio of ATP to ADP, of DnaA to the number of DnaA boxes and the hemimethylation and sequestering of OriC. The ratio of ATP to ADP indicates that the cell has reached a specific size and is ready to divide. This "signal" occurs becuase in a rich medium, the cell will grow quickly and will have a lot of excess DNA. Futhermore, DnaA binds equally well to ATP or ADP, and only the DnaA-ATP complex is able to initate replication. Thus, in a fast growing cell, there will be more DnaA-ATP than DnaA-ADP. Because the levels of DnaA are strictly regulated, and 5 DnaA-DnaA dimers are needed to initiate replication, the ratio of DnaA to the number of DnaA boxes in the cell is important. After DNA replicaton is complete, this number is halved, thus DNA replication cannot occur until the levels of DnaA protein increases. Finally, DNA is sequestered to a membrane binding protein called SeqA. This protein binds to hemimethylated GATC DNA sequences. This four bp sequences occurs 11 times in OriC, and newly synthesized DNA only has its parent strand methylated. DAM methyltrasferase methylates the newly synthesized strand of DNA ONLY if it is not bound to SeqA. The importance of hemimethylation is twofold. Firstly, OriC becomes inaccessable to DnaA, and secondly, DnaA binds better to fully methylated DNA than hemimethylated DNA.
Eukaryotic DNA Replicaton
Although the mechanisms of DNA synthesis is both Eukaryotes and prokaryotes is similar, eukaryotes have a much more complicated method of DNA replication. Even though DNA synthesis in E. coli is regulated, DNA replication is always initiated before the end of the cell cycle, this cannot occur in eukaryotes or a host of diseases could manifest themselves.
DNA replication in eukaryotes occurs only in the S phase of the cell cycle. However, pre-initiation occurs in G1. Due to the sheer size of chromosomes in eukaryotes, eukaryotic chromosomes contain multiple origins of replication. Some origins are well characterized, such as the autonomously replicating sequences (ARS) of yeast while other eukaryotic origins, particularly those in metazoa, can be found in spans of thousands of basepairs. However, the assembly and initiation of replicaton is similar in both the protozoa and metazoa.
The first step in DNA replication is the formation of the pre-initiation replication complex (the pre-RC). The formation of this complex occurs in two stages. The first stage requires that there is no CDK activity. This can only occur in early G1. The formation of the pre-RC is known as licensing, but a licensed pre-RC cannot initaite replicaton. Initiation of replication can only occur during the S-phase. Thus, the seperation of licensing and activation ensures that the origin can only fire once per cell cycle.
DNA replication in eukaryotes is not very well characterized. However, researchers believe that it begins with the binding of the origin recognition complex (ORC) to the origin. This complex is a hexamer of related proteins and remains bound to the origin, even after DNA replication occurs. Furthermore, ORC is the functional analogue of DnaA. Following the binding of ORC to the origin, Cdc6/Cdc18 and Cdt1 coordinate the loading of the MCM (minichromosome maitenance functions) complex to the origin by first binding to ORC and then binding to the MCM complex. The MCM complex is thought to be the major DNA helicase in eukaryotic organisms, and is a hexamer (mcm2-7). Once binding of MCM occurs, a fully licensed pre-RC exists.
Activation of the complex occurs in S-phase and requires Cdk2-Cyclin E and Ddk. The activation process begins with the addition of Mcm10 to the pre-RC, which displaces Cdt1. Following this, Ddk phosphorylates Mcm3-7, which activates the helicase. It is believed that ORC and Cdc6/18 are phosphorylated by Cdk2-Cyclin E. Ddk and the the Cdk complex then recruits another protein called Cdc45, which then recruits all of the DNA replicaton proteins to the replicaton fork. At this stage the origin fires and DNA synthesis begins.
Activation of a new round of replicaton is prevented through the actions of the cyclin dependant kinases and a protein known as geminin. Geminin binds to Cdt1 and sequesters it. It is a periodic protein that first appears in S-phase and is degraded in late M-phase, possibly through the action of the anaphase promoting complex (APC). In addition, phosphorylation of Cdc6/18 prevent it from binding to the ORC (thus inhibiting loading of the MCM complex) while the phosphorylation of ORC remains unclear. Cells in the G0 stage of the cell cycle are prevented from initiating a round of replication because the Mcm proteins are not expressed.
Numerous polymerases can repicate DNA in eukaryotic cells. Currently, six families of polymerases (A, B, C, D, X, Y) have been discovered. At least four different types of DNA polymerases are involved in the replication of DNA in animal cells (POLA, POLG, POLD1 and POLE). POL1 functions by extending the primer in the 5' -> 3' direction and tightly associates with primase. However, it lacks the ability to proofread DNA. POLD1 has a proofreading ability and is able to replicate the entire length of a template only when associated with PCNA. POLE is able to replicate the entire length of a template in teh absence of PCNA and is able to proofread DNA while POLG replicates mitochondrial DNA via the D-Loop mechanism of DNA replicaton. All primers are removed by RNaseH1 and Flap Endonuclease I. The general mechanisms of DNA replication on the leading and lagging strand, however, are the same as to those found in prokaryotic cells.
References
- Voet and Voet. Biochemistry, Third Edition (2004). ISBN 0-471-19350-X. Wiley International Edition.
- Watson, Baker, Bell, Gann, Levine, Losick. Molecular Biology of the Gene, Fifth Edition (2003). ISBN 0-8053-4635-X. Pearson/Benjamin Cummings Publishing.
External links
- DNA Workshop
- WEHI-TV - DNA Replication video- Detailed DNA replication animation from different angles with description below.
- Breakfast of Champions Does Replication- creative primer on the process from the Science Creative Quarterly
- Template:McGrawHillAnimation
- Basic Polymerase Chain Reaction Protocol
- Animated Biology
- DNA makes DNA (Flash Animation)
- DNA replication info pageby George Kakaris, Biologist MSc in Applied Genetics and Biotechnology
| DNA replication (comparing prokaryotic to eukaryotic) | |||||||
|---|---|---|---|---|---|---|---|
| Initiation |
| ||||||
| Replication |
| ||||||
| Termination | |||||||