| Revision as of 20:27, 10 August 2018 editAnomalocaris (talk | contribs)Extended confirmed users, Pending changes reviewers88,054 editsm rm bogus image option ("Figure 1", "Figure 2"); wiki bullets and numbered points; avoid all caps; genus and species in italics; journal in italics; spell out journal names; article titles in quotes; spaced initials; external links bulleted; dashes; headings in sentence capitalization; nonbreaking space before unit abbrev (kDa, Å)← Previous edit | Latest revision as of 20:07, 14 August 2024 edit undoArjayay (talk | contribs)Autopatrolled, Extended confirmed users, Page movers, Pending changes reviewers, Rollbackers627,303 editsm Reword | ||
| (75 intermediate revisions by 23 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|Toxin found in earthworms}} | |||
| {{AFC submission|||ts=20180810103526|u=Munguira|ns=118}} | |||
| '''Lysenin''' is a ] (PFT) present in the ]ic fluid of the ] '']''. Pore-forming toxins are a group of ]s that act as virulence factors of several ]ic ]. Lysenin proteins are chiefly involved in the defense against cellular pathogens.<ref>{{cite journal |last1=Bruhn |first1=Heike |last2=Winkelmann |first2=Julia |last3=Andersen |first3=Christian |last4=Andrä |first4=Jörg |last5=Leippe |first5=Matthias |title=Dissection of the mechanisms of cytolytic and antibacterial activity of lysenin, a defence protein of the annelid Eisenia fetida |url=https://pubmed.ncbi.nlm.nih.gov/16386304/ |journal=Developmental and Comparative Immunology |pages=597–606 |doi=10.1016/j.dci.2005.09.002 |date=2006|volume=30 |issue=7 |pmid=16386304 }}</ref> Following the general mechanism of action of PFTs lysenin is segregated as a soluble monomer that binds specifically to a ], ] in the case of lysenin. After attaching to the membrane, the oligomerization begins, resulting in a nonamer on top of membrane, known as a prepore. After a conformational change, which could be triggered by a decrease of ], the oligomer is inserted into the membrane in the so-called pore state. | |||
| Lysenin is a ] in the coelomic fluid of the earthworm '']''. {{New unreviewed article | |||
| | source = ArticleWizard | |||
| | date = August 2018 | |||
| }} | |||
| <!-- Write the text of your article below this line. The first sentence should begin with the subject of your article surrounded by three apostrophes (for example: '''Article name''' is...) --> | |||
| == |
==Monomer== | ||
| ] | |||
| Lysenin is a ] produced in the ]-] of the earthworm '']''.<ref>{{cite journal|last1=Yilmaz|first1=N.|last2=Yamaji-Hasegawa|first2=A.|last3=Hullin-Matsuda|first3=F.|last4=Kobayashi|first4=T.|date=2018|title=Molecular mechanisms of action of sphingomyelin-specific pore-forming toxin, lysenin|journal=Seminars in Cell & Developmental Biology|language=English|volume=73|pages=188–198|doi=10.1016/j.semcdb.2017.07.036|pmid=28751253}}</ref> This protein was first isolated from the coelomic fluid in 1996 and named lysenin (from lysis and ''Eisenia'').<ref>{{cite journal|last1=Sekizawa|first1=Y.|last2=Hagiwara|first2=K.|last3=Nakajima|first3=T.|last4=Kobayashi|first4=H.|date=1996|title=A Novel Protein, Lysenin, that Causes Contraction of the Isolate Rat Aorta: Its Purification from the Coelomic Fluid of the Earthworm, ''Eisenia foetida''|journal=Biomedical Research|language=English|volume=17|issue=3|pages=197–203|doi=10.2220/biomedres.17.197|doi-access=free}}</ref> Lysenin is a relatively small water-soluble molecule with a molecular weight of 33 kDa. Using ], lysenin was classified as a member of the ] protein family by structure and function.<ref name="Colibus2">{{cite journal|last1=De Colibus|first1=L.|last2=Sonnen|first2=A. F.-P.|last3=Morris|first3=K. J.|last4=Siebert|first4=C. A.|last5=Abrusci|first5=P.|last6=Plitzko|first6=J.|last7=Hodnik|first7=V.|last8=Leippe|first8=M.|last9=Volpi|first9=E.|date=2012|title=Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins And Its Mode of Sphingomyelin Recognition|journal=Structure|language=English|volume=20|issue=9|pages=1498–1507|doi=10.1016/j.str.2012.06.011|pmid=22819216|last10=Anderluh|first10=G.|last11=Gilbert|first11=R. J. C.|pmc=3526787|doi-access=free}}</ref> Structurally, each lysenin monomer consists of a receptor binding domain (grey globular part on right of Figure 1) and a Pore Forming Module (PFM); domains shared throughout the aerolysin family.<ref name="Colibus2" /> The lysenin receptor binding domain shows three ] binding motifs. The Pore Forming Module contains the regions that undergo large conformational changes to become the β-barrel in the pore.<ref name="Bokori2">{{cite journal|last1=Bokori-Brown|first1=M.|last2=Martin|first2=T. G.|last3=Naylor|first3=C. E.|last4=Basak|first4=A. K.|last5=Titball|first5=R. W.|last6=Savva|first6=C. G.|date=2016|title=Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein|journal=Nature Communications|language=English|volume=7|issue=1|page=11293|doi=10.1038/ncomms11293|pmid=27048994|pmc=4823867|bibcode=2016NatCo...711293B|doi-access=free}}</ref> | |||
| Lysenin,] is a protein produced in the coelomocytes-Leucocyte of animals with coelom-of ''Eisenia fetida.''] The protein was first isolated from the ] by Yoshiyuki Sekizawa from Kobayashi group in 1996. They also named the protein Lysenin (lysis+Eisenia). Lysenin is a relatively small protein with a molecular weight of 33 kDa (Figure 2). The X-ray structure was first determined by de Colibus ''et al.''], and hence classified as a member of Aerolysin family,] with which shares structure and function. Lysenin monomers, from a structural point of view, comprises the receptor binding domain and Pore Forming Module (PFM). Actually, this classification is shared with the rest of the ] family.] De Colibus ''et al''. obtained the first X-ray structure of the monomer, which showed that PFM is the motive that specifically interacts with sphingomyelin. Two recently published works revealed that the receptor binding domain is the part in contact with sphingomyelin], where it was even shown the fit of the phosphatidylcholine headgroups of sphingomyelin.] | |||
| ==Membrane receptors== | |||
| The definition of PFM changed after extracting the Lysenin pore structure. The domain that forms the β-barrel, the so-called β-hairpin, was alleged to be a small region in the vicinity of the PFM (shown in green in Figure 2). Nowadays, it is known that the β-barrel is formed by a larger region, also including regions in the middle of the PFM (illustrated in yellow in Figure 1). | |||
| The natural ] target of lysenin is an animal plasma membrane ] called ] located mainly in its outer leaflet, involving at least three of its ] (PC) groups.<ref name=":0">{{cite journal|last1=Ishitsuka|first1=R.|last2=Kobayashi|first2=T.|date=2007|title=Cholesterol and Lipid/Protein Ratio Control the Oligomerization of a Sphingomyelin-Specific Toxin, Lysenin|journal=Biochemistry|language=English|volume=46|issue=6|pages=1495–1502|doi=10.1021/bi061290k|pmid=17243772|s2cid=22016219}}</ref> Sphingomyelin is usually found associated with ] in ].<ref>{{cite journal|last1=Simons|first1=K.|last2=Gerl|first2=M. J.|date=2010|title=Revitalizing membrane rafts: new tools and insights|journal=Nature Reviews Molecular Cell Biology|language=English|volume=11|issue=10|pages=688–699|doi=10.1038/nrm2977|pmid=20861879|s2cid=1866391}}</ref> Cholesterol, which enhances ], provides a stable platform with high lateral mobility where monomer-monomer encounters are more probable.<ref name=":0" /> PFTs have shown to be able to remodel the membrane structure,<ref name="More2">{{cite journal|last1=Ros|first1=U.|last2=García-Sáez|first2=A. J.|date=2015|title=More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes|journal=The Journal of Membrane Biology|language=English|volume=248|issue=3|pages=545–561|doi=10.1007/s00232-015-9820-y|pmid=26087906|s2cid=16305100}}</ref> sometimes even mixing lipid phases.<ref>{{cite journal|last1=Yilmaz|first1=N.|last2=Kobayashi|first2=T.|date=2015|title=Visualization of Lipid Membrane Reorganization Induced by a Pore-Forming Toxin Using High-Speed Atomic Force Microscopy|journal=ACS Nano|language=English|volume=9|issue=8|pages=7960–7967|doi=10.1021/acsnano.5b01041|pmid=26222645|url=https://figshare.com/articles/journal_contribution/2138179}}</ref> | |||
| ] | |||
| The region of the lysenin pore β-barrel expected to be immersed in the hydrophobic region of the membrane is the 'detergent belt', the 3.2 nm high region occupied by detergent in ] (Cryo-EM) studies of the pore.<ref name="Bokori3">{{cite journal|last1=Bokori-Brown|first1=M.|last2=Martin|first2=T. G.|last3=Naylor|first3=C. E.|last4=Basak|first4=A. K.|last5=Titball|first5=R. W.|last6=Savva|first6=C. G.|date=2016|title=Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein|journal=Nature Communications|language=English|volume=7|issue=1|page=11293|doi=10.1038/ncomms11293|pmid=27048994|pmc=4823867|bibcode=2016NatCo...711293B|doi-access=free}}</ref> On the other hand, sphingomyelin/Cholesterol bilayers are about 4.5 nm height.<ref>{{cite journal|last1=Quinn|first1=P. J.|date=2013|title=Structure of Sphingomyelin Bilayers and Complexes with Cholesterol Forming Membrane Rafts|journal=Langmuir|language=English|volume=29|issue=30|pages=9447–9456|doi=10.1021/la4018129|pmid=23863113}}</ref> This difference in height between the detergent belt and the sphingomyelin/cholesterol bilayer implies a bend of the membrane in the region surrounding the pore, called negative mismatch.<ref>{{cite journal|last1=Guigas|first1=G.|last2=Weiss|first2=M.|date=2016|title=Effects of protein crowding on membrane systems|journal=Biochimica et Biophysica Acta (BBA) - Biomembranes|language=English|volume=1858|issue=10|pages=2441–2450|doi=10.1016/j.bbamem.2015.12.021|pmid=26724385|doi-access=free}}</ref> This bending results in a net attraction between pores that induce pores aggregation. | |||
| ==Lysenin membrane receptors== | |||
| ==Binding, oligomerization and insertion== | |||
| The natural target of Lysenin is an animal plasma membrane lipid called ].] Sphingomyelin is synthesized in the ], and is mostly present in the outer leaflet of the animal plasma membrane, where it plays an important role as a secondary messenger.]<sup>,]</sup> | |||
| ]Membrane binding is a requisite to initiate PFT oligomerization. Lysenin monomers bind specifically to sphingomyelin via the receptor binding domain.<ref name="Colibus3">{{cite journal|last1=De Colibus|first1=L.|last2=Sonnen|first2=A. F.-P.|last3=Morris|first3=K. J.|last4=Siebert|first4=C. A.|last5=Abrusci|first5=P.|last6=Plitzko|first6=J.|last7=Hodnik|first7=V.|last8=Leippe|first8=M.|last9=Volpi|first9=E.|date=2012|title=Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins And Its Mode of Sphingomyelin Recognition|journal=Structure|language=English|volume=20|issue=9|pages=1498–1507|doi=10.1016/j.str.2012.06.011|pmid=22819216|last10=Anderluh|first10=G.|last11=Gilbert|first11=R. J. C.|pmc=3526787|doi-access=free}}</ref> The final lysenin oligomer is constituted by nine monomers without quantified deviations.<ref>{{cite journal|last1=Munguira|first1=I.|last2=Casuso|first2=I.|last3=Takahashi|first3=H.|last4=Rico|first4=F.|last5=Miyagi|first5=A.|last6=Chami|first6=M.|last7=Scheuring|first7=S.|date=2016|title=Glasslike Membrane Protein Diffusion in a Crowded Membrane|journal=ACS Nano|language=English|volume=10|issue=2|pages=2584–2590|doi=10.1021/acsnano.5b07595|pmid=26859708|s2cid=206699095 |url=https://www.hal.inserm.fr/inserm-01285787/file/Munguira_et_al_ACS-Nano-Manuscript_Corrected.pdf}}</ref> When lysenin monomers bind to sphingomyelin-enriched membrane regions, they provide a stable platform with a high lateral mobility, hence favouring the oligomerization.<ref name=":02">{{cite journal|last1=Ishitsuka|first1=R.|last2=Kobayashi|first2=T.|date=2007|title=Cholesterol and Lipid/Protein Ratio Control the Oligomerization of a Sphingomyelin-Specific Toxin, Lysenin|journal=Biochemistry|language=English|volume=46|issue=6|pages=1495–1502|doi=10.1021/bi061290k|pmid=17243772|s2cid=22016219}}</ref> As with most PFTs, lysenin oligomerization occurs in a two-step process, as was recently imaged. | |||
| The process begins with monomers being adsorbed into the membrane by specific interactions, resulting in an increased concentration of monomers. This increase is promoted by the small area where the membrane receptor accumulates since the majority of PFT membrane receptors are associated with lipid rafts.<ref>{{cite journal|last1=Lafont|first1=F.|last2=Van Der Goot|first2=F. G.|date=2005|title=Bacterial invasion via lipid rafts|journal=Cellular Microbiology|language=English|volume=7|issue=5|pages=613–620|doi=10.1111/j.1462-5822.2005.00515.x|pmid=15839890|s2cid=26547616|doi-access=free}}</ref> Another side effect, aside from the increase of monomer concentration, is the monomer-monomer interaction. This interaction increases lysenin oligomerization. After a critical threshold concentration is reached, several oligomers are formed simultaneously, although sometimes these are incomplete.<ref name="yfirst2">{{cite journal|last1=Yilmaz|first1=N.|last2=Yamada|first2=T.|last3=Greimel|first3=P.|last4=Uchihashi|first4=T.|last5=Ando|first5=T.|last6=Kobayashi|first6=T.|date=2013|title=Real-Time Visualization of Assembling of a Sphingomyelin-Specific Toxin on Planar Lipid Membranes|journal=Biophysical Journal|language=English|volume=105|issue=6|pages=1397–1405|doi=10.1016/j.bpj.2013.07.052|pmid=24047991|pmc=3785888|bibcode=2013BpJ...105.1397Y|doi-access=free}}</ref> In contrast to PFTs of the ] family,<ref>{{cite journal|last1=Mulvihill|first1=E.|last2=van Pee|first2=K.|last3=Mari|first3=S. A.|last4=Müller|first4=D. J.|last5=Yildiz|first5=Ö.|date=2015|title=Directly Observing the Lipid-Dependent Self-Assembly and Pore-Forming Mechanism of the Cytolytic Toxin Listeriolysin O|journal=Nano Letters|language=English|volume=15|issue=10|pages=6965–6973|doi=10.1021/acs.nanolett.5b02963|pmid=26302195|bibcode=2015NanoL..15.6965M}}</ref> the transition from incomplete lysenin oligomers to complete oligomers has not been observed. | |||
| In the raft, lipids are in liquid-ordered phase, meaning more lateral dense packed, rigid, low chain motion and high lateral mobility. This rigidity is in part due to the high transition temperature of sphingolipids, as well as the interactions of these lipids with ].] Cholesterol is a relatively small, amphipathic molecule that can accommodate between the large acyl chains of sphingolipids, resulting in the so-called liquid ordered phase. | |||
| A complete oligomerization results in the so-called prepore state, a structure on the membrane. Determining the prepore's structure by X-ray or Cryo-EM is a challenging process that so far has not produced any results. The only available information about the prepore structure was provided by ] (AFM). The measured prepore height was 90 Å; and the width 118 Å, with an inner pore of 50 Å.<ref name="yfirst2" /> A model of the prepore was built aligning the monomer structure ({{PDB|3ZXD}}) with the pore structure ({{PDB|5GAQ}}) by their receptor-binding domains (residues 160 to 297). A recent study in aerolysin suggests that the currently accepted model for the lysenin prepore should be revisited, according to the new available data on the aerolysin insertion.<ref>{{cite journal|last1=Iacovache|first1=Ioan|last2=De Carlo|first2=Sacha|last3=Cirauqui|first3=Nuria|last4=Dal Peraro|first4=Matteo|last5=van der Goot|first5=F. Gisou|last6=Zuber|first6=Benoît|title=Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process|journal=Nature Communications|volume=7|doi=10.1038/ncomms12062|year=2016|page=12062|pmid=27405240|pmc=4947156|bibcode=2016NatCo...712062I|doi-access=free}}</ref> | |||
| Cholesterol plays an important role in the oligomerization of Lysenin.] This sterol provides a stable platform with high lateral mobility were the monomer-monomer encounters are more probable.]<sup>,]</sup> | |||
| A ] transforms the PFM into the transmembrane ], leading to the pore state.<ref name="Bokori4">{{cite journal|last1=Bokori-Brown|first1=M.|last2=Martin|first2=T. G.|last3=Naylor|first3=C. E.|last4=Basak|first4=A. K.|last5=Titball|first5=R. W.|last6=Savva|first6=C. G.|date=2016|title=Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein|journal=Nature Communications|language=English|volume=7|issue=1|page=11293|doi=10.1038/ncomms11293|pmid=27048994|pmc=4823867|bibcode=2016NatCo...711293B|doi-access=free}}</ref> The trigger mechanism for the prepore-to-pore transition in lysenin depends on three glutamic acid residues (E92, E94 and E97), and is activated by a decrease in pH,<ref>{{cite journal|last1=Munguira|first1=I. L. B.|last2=Takahashi|first2=H.|last3=Casuso|first3=I.|last4=Scheuring|first4=S.|date=2017|title=Lysenin Toxin Membrane Insertion Is pH-Dependent but Independent of Neighboring Lysenins|journal=Biophysical Journal|language=English|volume=113|issue=9|pages=2029–2036|doi=10.1016/j.bpj.2017.08.056|pmid=29117526|pmc=5685674|bibcode=2017BpJ...113.2029M|doi-access=free}}</ref> from physiological conditions to the acidic conditions reached after endocytosis, or an increase in calcium extracellular concentration.<ref>{{cite journal |last1=Munguira |first1=I.L.B. |title=Lysenin toxin insertion mechanism is Calcium-dependent |journal=bioRxiv |doi=10.1101/771725|year=2019 |doi-access=free }}</ref> These three glutamic acids are located in an α-helix that forms part of the PFM, and glutamic acids are found in aerolysin family members in its PFMs. Such a conformational change produces a decrease in the oligomer height of 2.5 nm according to AFM measurements.<ref name="yfirst2" /> The main dimensions, using lysenin pore X-ray structure, are height 97 Å, width 115 Å and the inner pore of 30 Å.<ref name="Bokori4" /> However, complete oligomerization into the nonamer is not a requisite for the insertion, since incomplete oligomers in the pore state can be found.<ref name="yfirst2" /> The prepore to pore transition can be blocked in crowded conditions, a mechanism that could be general to all β-PFTs. The first hint of crowding effect on prepore to pore transition was given by congestion effects in electrophysiology experiments.<ref>{{cite journal|last1=Krueger|first1=E.|last2=Bryant|first2=S.|last3=Shrestha|first3=N.|last4=Clark|first4=T.|last5=Hanna|first5=C.|last6=Pink|first6=D.|last7=Fologea|first7=D.|date=2015|title=Intramembrane congestion effects on lysenin channel voltage-induced gating|journal=European Biophysics Journal|language=English|volume=45|issue=2|pages=187–194|doi=10.1007/s00249-015-1104-z|pmid=26695013|pmc=4803513}}</ref> | |||
| PFT have been shown to be able to remodel the membrane structure, even mixing lipid phases.] The detergent belt-the part of the β-barrel occupied by detergent in the Cryogenic Electron Microscopy (Cryo-EM) study- of Lysenin pore is 32 Å in height.] This height implies a bend of the membrane in the region surrounding the pore, call negative mismatch.] | |||
| ==Insertion consequences== | |||
| ==Lysenin binding, oligomerization and insertion== | |||
| The ultimate consequences of lysenin pore formation are not well documented; however, it is thought to induce ] via three possible hypotheses: | |||
| * Breaking the sphingomyelin asymmetry between the two leaflets of the lipid bilayer by punching holes in the membrane<ref>{{cite journal|last1=Green|first1=D. R.|date=2000|title=Apoptosis and Sphingomyelin Hydrolysis|journal=The Journal of Cell Biology|language=English|volume=150|issue=1|pages=F5–F8|doi=10.1083/jcb.150.1.F5|pmid=10893276|pmc=2185551}}</ref> and inducing ] (reorientation of a lipid from one leaflet of a membrane bilayer to the other).<ref name="More4">{{cite journal|last1=Ros|first1=U.|last2=García-Sáez|first2=A. J.|date=2015|title=More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes|journal=The Journal of Membrane Biology|language=English|volume=248|issue=3|pages=545–561|doi=10.1007/s00232-015-9820-y|pmid=26087906|s2cid=16305100}}</ref> | |||
| Membrane attachment is a requisite to initiate oligomerization. Lysenin monomers bind specifically to sphingomyelin via the receptor binding domain. How the oligomerization process is remains as an open question, due to the current technical limitations for recording the monomer diffusion. | |||
| * Increasing the calcium concentration in the cytoplasm.<ref>{{cite journal|last1=Orrenius|first1=S.|last2=Zhivotovsky|first2=B.|last3=Nicotera|first3=P.|date=2003|title=Regulation of cell death: the calcium–apoptosis link|journal=Nature Reviews Molecular Cell Biology|language=English|volume=4|issue=7|pages=552–565|doi=10.1038/nrm1150|pmid=12838338|s2cid=19079491}}</ref> | |||
| * Decreasing the potassium concentration in the cytoplasm.<ref>{{cite journal|last1=Yu|first1=S. P.|date=2003|title=Regulation and critical role of potassium homeostasis in apoptosis|journal=Progress in Neurobiology|language=English|volume=70|issue=4|pages=363–386|doi=10.1016/s0301-0082(03)00090-x|pmid=12963093|s2cid=13893235}}</ref> | |||
| ==Biological role== | |||
| The final Lysenin oligomer is constituted by nine monomers without quantified deviations, as shown for the first time by Munguira ''et al''.] | |||
| The biological role of lysenin remains unknown. It has been suggested that lysenin may play a role as a ] against attackers such as ], ] or small ].<ref>{{cite book|title=Lessons in immunity: from single-cell organisms to mammals|last1=Ballarin|first1=L.|last2=Cammarata|first2=M.|date=2016|publisher=Academic Press|isbn=9780128032527}}</ref> However, lysenin's activity is dependent upon binding to sphingomyelin, which is not present in the membranes of bacteria, fungi or most invertebrates. Rather, sphingomyelin is mainly present in the plasma membrane of ].<ref>{{cite journal|last1=Kobayashi|first1=H.|last2=Sekizawa|first2=Y.|last3=Aizu|first3=M.|last4=Umeda|first4=M.|date=2000|title=Lethal and non-lethal responses of spermatozoa from a wide variety of vertebrates and invertebrates to lysenin, a protein from the coelomic fluid of the earthworm ''Eisenia foetida''|journal=Journal of Experimental Zoology|language=English|volume=286|issue=5|pages=538–549|doi=10.1002/(sici)1097-010x(20000401)286:5<538::aid-jez12>3.0.co;2-w|pmid=10684578|bibcode=2000JEZ...286..538K }}</ref> Another hypothesis is that the earthworm, which is able to expel coelomic fluid under stress,<ref>{{cite journal|last1=Sukumwang|first1=N.|last2=Umezawa|first2=K.|date=2013|title=Earthworm-Derived Pore-Forming Toxin Lysenin and Screening of Its Inhibitors|journal=Toxins|language=English|volume=5|issue=8|pages=1392–1401|doi=10.3390/toxins5081392|pmid=23965430|pmc=3760042|doi-access=free}}</ref><ref>{{Cite journal|last1=Kobayashi|first1=H.|last2=Ohta|first2=N.|last3=Umeda|first3=M.|date=2004|title=Biology of lysenin, a protein in the coelomic fluid of the earthworm ''Eisenia foetida''|journal=International Review of Cytology|volume=236|pages=45–99|doi=10.1016/S0074-7696(04)36002-X|pmid=15261736|isbn=9780123646408}}</ref> generates an avoidance behaviour to its ] predators (such as birds, ] or ]).<ref>{{cite journal|last1=Swiderska|first1=B.|last2=Kedracka-Krok|first2=S.|last3=Panz|first3=T.|last4=Morgan|first4=A. J.|last5=Falniowski|first5=A.|last6=Grzmil|first6=P.|last7=Plytycz|first7=B.|date=2017|title=Lysenin family proteins in earthworm coelomocytes – Comparative approach|journal=Developmental & Comparative Immunology|language=English|volume=67|pages=404–412|doi=10.1016/j.dci.2016.08.011|pmid=27567602|s2cid=19895826}}</ref> If that is the case, the expelled lysenin might be more effective if the coelomic fluid reaches the eye, where the concentration of sphingomyelin is ten times higher than in other body organs.<ref>{{cite book|title=Biochemistry of the Eye|last1=Berman|first1=E. R.|date=1991|publisher=Springer|isbn=978-1-4757-9441-0|language=English|doi=10.1007/978-1-4757-9441-0|s2cid=41192657}}</ref> A complementary hypothesis is that the pungent smell of the coelomic fluid - giving the earthworm its specific epithet ''foetida'' - is an ]. However, it remains unknown whether lysenin contributes to avoidance of ''Eisenia'' by predators.<ref>{{cite book|title=Biology and Ecology of Earthworms|last1=Edwards|first1=C. A.|last2=Bohlen|first2=P. J.|date=1996|publisher=Springer Science & Business Media|isbn=978-0-412-56160-3|language=English}}</ref> | |||
| Lysenin monomers bind to sphingomyelin-enriched domains, which provide a stable platform with a high lateral mobility, hence favouring the oligomerization. Lysenin oligomerization occurs in a two-step process, as for proteins in general.]<sup>,]</sup> | |||
| ==Applications== | |||
| First, the monomers adsorb to the membrane by specific interactions, resulting in an increased concentration. Lipid rafts are believed to play a key role contributing to the increasing of the monomer concentration. After a critical concentration is reached, several oligomers are formed simultaneously. The second step consists in the growth of new oligomers. It takes places in the borders of the oligomers cluster. | |||
| Lysenin's conductive properties have been studied for years.<ref>{{cite journal|last1=Bryant|first1=S.|last2=Clark|first2=T.|last3=Thomas|first3=C.|last4=Ware|first4=K.|last5=Bogard|first5=A.|last6=Calzacorta|first6=C.|last7=Prather|first7=D.|last8=Fologea|first8=D.|date=2018|title=Insights into the Voltage Regulation Mechanism of the Pore-Forming Toxin Lysenin|journal=Toxins|language=English|volume=10|issue=8|pages=334|doi=10.3390/toxins10080334|pmid=30126104|pmc=6115918|doi-access=free}}</ref> Like most pore-forming toxins, lysenin forms a non-specific channel that is permeable to ions, small molecules, and small peptides.<ref>{{cite journal|last1=Shrestha|first1=N.|last2=Bryant|first2=S. L.|last3=Thomas|first3=C.|last4=Richtsmeier|first4=D.|last5=Pu|first5=X.|last6=Tinker|first6=J.|last7=Fologea|first7=D.|date=2017|title=Stochastic sensing of Angiotensin II with lysenin channels|journal=Scientific Reports|language=English|volume=7|issue=1|page=2448|doi=10.1038/s41598-017-02438-0|pmid=28550293|pmc=5446423|bibcode=2017NatSR...7.2448S|doi-access=free}}</ref> There have also been over three decades of studies into finding suitable pores for converting into ] that can have their conductive properties tuned by point mutation.<ref>{{cite journal|last1=Deamer|first1=D.|last2=Akeson|first2=M.|last3=Branton|first3=D.|date=2016|title=Three decades of nanopore sequencing|journal=Nature Biotechnology|language=English|volume=34|issue=5|pages=518–524|doi=10.1038/nbt.3423|pmid=27153285|pmc=6733523}}</ref> Owing to its binding affinity for sphingomyelin, lysenin (or just the receptor binding domain) has been used as a fluorescence marker to detect the sphingomyelin domain in membranes.<ref>{{cite journal|last1=Ishitsuka|first1=R.|last2=Kobayashi|first2=T.|date=2004|title=Lysenin: A new tool for investigating membrane lipid organization|journal=Anatomical Science International|language=English|volume=79|issue=4|pages=184–190|doi=10.1111/j.1447-073x.2004.00086.x|pmid=15633456|s2cid=1558393}}</ref> | |||
| A complete oligomerization results in a prepore. To determine the structure by X-ray or Cryo-EM of the prepore is a challenging process that till the date did not produce any result. The only available information about the prepore structure was provided by Atomic Force Microscopy (AFM).] The measured prepore height was 90 Å]<sup>,]</sup> and the width 118 Å, with an inner pore of 50 Å (Figure 3).<sup>40,56</sup> Interestingly, a recent study in Aerolysin points out that the currently accepted model for the Lysenin prepore should be revisited, according to the new available data about the Aerolysin insertion.] | |||
| ] | |||
| A conformational change transforms the PFM in the transmembrane β-barrel, leading to the pore state. Such a conformational change produces a decrease of the oligomer height of , according to AFM measurements.] Using X-ray structure, they were measured the main dimensions, being height 97 Å, width 115 Å and the inner pore of 30 Å (Figure 2).] Interestingly, the complete oligomerization is not a requisite for the insertion. It is usual to observe incomplete oligomers in prepore and pore states ]<sup>,]</sup>. However, the incomplete pores did not evolve to complete, a phenomenon that was observed in CDCs family.] The triggering mechanism for the prepore to pore transition in Lysenin depend in three glutamic acids (E92, E94 and E97), and is activate by a decreased of pH<sup>25</sup>, from physiological conditions. This residues are located in an α-helix that form part of the PFM. | |||
| A conformational change transforms the PFM in the transmembrane β-barrel, leading to the pore state. Such a conformational change produces a decrease of the oligomer height of , according to AFM measurements.] Using X-ray structure, they were measured the main dimensions, being height 97 Å, width 115 Å and the inner pore of 30 Å (Figure 2).] Interestingly, the complete oligomerization is not a requisite for the insertion. It is usual to observe incomplete oligomers in prepore and pore states ]<sup>,]</sup>. However, the incomplete pores did not evolve to complete, a phenomenon that was observed in CDCs family.] The triggering mechanism for the prepore to pore transition in Lysenin depend in three glutamic acids (E92, E94 and E97), and is activate by a decreased of pH<sup>25</sup>, from physiological conditions. This residues are located in an α-helix that form part of the PFM. | |||
| ==Lysenin insertion consequences== | |||
| The ultimate consequences caused by the formation of the Lysenin pore are not well documented, however three are the plausible consequences after the formation of the Lysenin pore: | |||
| * To punch the membrane breaks the sphingomyelin asymmetry between the two leaflets of the lipid bilayer, which leads to apoptosis.]<sup>6</sup> PFT are supposed to induce lipid flip-flop that can also break the sphingomyelin asymmetry.] | |||
| * The presence of the pore increases the calcium concentration in the cytoplasm, which drives to apoptosis. <sup>]</sup> | |||
| * A decrease in the potassium concentration in the cytoplasm causes apoptosis.] | |||
| ==Biological role of Lysenin== | |||
| The biological role of Lysenin still remains unknown. It was suggested that Lysenin can play a role as a defeat mechanism against attackers such as bacteria, fungi or small invertebrates.] Nevertheless, sphingomyelin starts to appear in the plasma membrane of chordates.] Therefore, Lysenin, being dependent on sphingomyelin as a binding factor, cannot affect bacteria, fungi or in general invertebrates. Another hypothesis is that the earthworm, which is able to expel coelomic fluid under stress,]<sup>,]</sup> generates an avoidance behaviour to its vertebrate predators (like birds, hedgehogs or moles).] In that case, the expelled Lysenin could be more effective if the coelomic fluid reaches the eye, where the concentration of sphingomyelin is ten times higher than in other body organs.] | |||
| Related to this, coelomic fluid, exuded under stressful situations by the earthworm, has a pungent smell -that gives name to the earthworm- was suggested to be an antipredator adaptation.] In any case, if Lysenin plays a role generating an avoiding behaviour, remains unknown. | |||
| == References == | == References == | ||
| {{Academic peer reviewed|Q76846397|doi-access=free}} | |||
| <!--- See ] on how to create references using <ref></ref> tags which will then appear here automatically --> | |||
| 1. Sekizawa, Y., Hagiwara, K., Nakajima, T. & Kobayashi, H. "A Novel Protein, Lysenin, That Causes Contraction of the Isolated Rat Aorta : Its Puriification from the Coleomic Fluid of the Earthworm, ''Eisenia foetida''". ''Biomedical research'' 17, 197–203 (1996). | |||
| 2. De Colibus, L. et al. "Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins And Its Mode of Sphingomyelin Recognition" . ''Structure'' 20, 1498–1507 (2012). | |||
| 3. Bernheimer, A. W. & Avigad, L. S. "Partial Characterization of Aerolysin, a Lytic Exotoxin from ''Aeromonas hydrophila''". ''Infection and Immunity'', 1016–1021 (1974). | |||
| 4. Parker, M.W. et al. "Structure of the ''Aeromonas'' toxin in its water-soluble and membrane-channel states". ''Nature'' 367, 292–295 (1994). | |||
| 5. Szczesny, P. et al. "Extending the Aerolysin Family: From Bacteria to Vertebrates". ''PLoS ONE'' 6, 1–10 (2011). | |||
| 6. Bokori-Brown, M. et al. "Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein". ''Nature Communications'' 7, 11293 (2016). | |||
| 7. Podobnik, M. et al. "Crystal structure of an invertebrate cytolysin pore reveals unique properties and mechanism of assembly". ''Nature Communications'' 7, 11598 (2016). | |||
| 8. Yamaji, A. et al. "Lysenin, a Novel Sphingomyelin-specific Binding Protein". ''The Journal of Biological Chemistry'' 273, 5300–5306 (1998). | |||
| 9. Murate, M. & Kobayashi, T. "Revisiting transbilayer distribution of lipids in the plasma membrane". ''Chemistry and Physics of Lipids'' 194, 58–71 (2016). | |||
| 10. "Sphingomyelin breakdown and cell fate". ''Trends in Biochemical Sciences'' 21, 468–471 (1996). | |||
| 11. Slotte, J. P. "The importance of hydrogen bonding in sphingomyelin's membrane interactions with co-lipids". ''Biochimica et Biophysica Acta'' 1858, 304–10 (2016). | |||
| 12. Ishitsuka, R. & Kobayashi, T. "Cholesterol and lipid/protein ratio control the oligomerization of a sphingomyelin-specific toxin, lysenin". ''Biochemistry'' 46, 1495–1502 (2007). | |||
| 13. Yamaji-Hasegawa, A. et al. "Oligomerization and Pore Formation of a Sphingomyelin-specific Toxin, Lysenin". ''Journal of Biological Chemistry'' 278, 22762–22770 (2003). | |||
| 14. Rojko, N. & Anderluh, G. "How Lipid Membranes Affect Pore Forming Toxin Activity". ''Accounts of Chemical Research'' 48, 3073–9 (2015). | |||
| 15. Ros, U. & Garcia-Saez, A.J. "More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes". ''The Journal of Membrane Biology'' 248, 545–61 (2015). | |||
| 16. Guigas, G. & Weiss, M. "Effects of protein crowding on membrane systems". ''Biochimica et Biophysica Acta'' 1858, 2441–50 (2016). | |||
| 17. Phillips, R., Ursell, T., Wiggins, P. & Sens, P. "Emerging roles for lipids in shaping membrane-protein function". ''Nature'' 459, 379–385 (2009). | |||
| 18. Munguira, I. et al. "Glasslike Membrane Protein Diffusion in a Crowded Membrane". ''ACS Nano'' 10, 2584–90 (2016). | |||
| 19. Wolde, P. R. t. "Enhancement of Protein Crystal Nucleation by Critical Density Fluctuations". ''Science'' 277, 1975–1978 (1997). | |||
| 20. Chunga, S., Shin, S.-H., Bertozzi, C. R. & Yoreo, J. J. D. 'Self-catalyzed growth of S layers via an amorphousto-crystalline transition limited by folding kinetics". ''Proceedings of the National Academy of Sciences of the United States of America'' 107, 16536–16541 (2010). | |||
| 21. Yilmaz, N. et al. "Real-Time Visualization of Assembling of a Sphingomyelin-Specific Toxin on Planar Lipid Membranes". ''Biophysical Journal'' 105, 1397–1405 (2013). | |||
| 22. Iacovache, I. et al. "Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process". ''Nature Communications'' 7, 12062 (2016). | |||
| 23. Kyte, J. & Doolittle, R. F. "A simple method for displaying the hydropathic character of a protein". ''Journal of Molecular Biology'' 157, 105–132 (1982). | |||
| 24. Mulvihill, E., van Pee, K., Mari, S.A., Muller, D. J. & Yildiz, O. "Directly Observing the Lipid-Dependent Self-Assembly and Pore-Forming Mechanism of the Cytolytic Toxin Listeriolysin" O. ''Nano Letters'' 15, 6965–73 (2015). | |||
| 25. Munguira, I. L. B., Takahashi, H., Casuso, I. & Scheuring, S. "Lysenin Toxin Membrane Insertion Is pH-Dependent but Independent of Neighboring Lysenins". ''Biophysical Journal'' 113, 2029–2036 (2017). | |||
| 26. Green, D. R. "Apoptosis and Sphingomyelin Hydrolysis: The Flip Side". ''The Journal of Cell Biology'' 150, F5–F7 (2000). | |||
| 27. Orrenius, S., Zhivotovsky, B. & Nicotera, P. "Regulation of cell death: the calcium-apoptosis link". ''Nature Reviews Molecular Cell Biology'' 4, 552–65 (2003). | |||
| 28. Yu, S.P. "Regulation and critical role of potassium homeostasis in apoptosis". ''Progress in Neurobiology'' 70, 363–386 (2003). | |||
| 29. Ballarin, L. & Cammarata, M. ''Lessons in Immunity: From Single-cell Organisms to Mammals''. (Academic Press, 2016). | |||
| 30. Kobayashi, H., Sekizawa, Y., Aizu, M. & Umeda, M. "Lethal and Non-Lethal Responses of Spermatozoa From a Wide Variety of Vertebrates and Invertebrates to Lysenin, a Protein From the Coelomic Fluid of the Earthworm ''Eisenia foetida''". ''Journal of Experimental Zoology'' 286, 538–549 (2000). | |||
| 31. Sukumwang, N. & Umezawa, K. "Earthworm-derived pore-forming toxin lysenin and screening of its inhibitors". ''Toxins (Basel)'' 5, 1392–401 (2013). | |||
| 32. Kobayashi, H., Ohta, N. & Umeda, M. "Biology of Lysenin, a Protein in the Coelomic Fluid of the Earthworm ''Eisenia foetida''". ''International Review of Cytology'' 236, 45–99 (2004). | |||
| 33. Swiderska, B. et al. "Lysenin family proteins in earthworm coelomocytes - Comparative approach". ''Developmental & Comparative Immunology'' 67, 404–412 (2017). | |||
| 34. Berman, E. R. ''Biochemistry of the eye''. (1991). | |||
| 35. Edwards, C. A. & Bohlen, P. J. ''Biology and Ecology of Earthworms'', Volume 3. ''Springer Science & Business Media'', 426 (1996). | |||
| {{reflist}} | {{reflist}} | ||
| Line 134: | Line 44: | ||
| * https://www.theses.fr/2017AIXM0124 | * https://www.theses.fr/2017AIXM0124 | ||
| ] | |||
| <!--- Categories ---> | |||
| <!-- ] does not exist --> | |||
| ] | |||
Latest revision as of 20:07, 14 August 2024
Toxin found in earthwormsLysenin is a pore-forming toxin (PFT) present in the coelomic fluid of the earthworm Eisenia fetida. Pore-forming toxins are a group of proteins that act as virulence factors of several pathogenic bacteria. Lysenin proteins are chiefly involved in the defense against cellular pathogens. Following the general mechanism of action of PFTs lysenin is segregated as a soluble monomer that binds specifically to a membrane receptor, sphingomyelin in the case of lysenin. After attaching to the membrane, the oligomerization begins, resulting in a nonamer on top of membrane, known as a prepore. After a conformational change, which could be triggered by a decrease of pH, the oligomer is inserted into the membrane in the so-called pore state.
Monomer
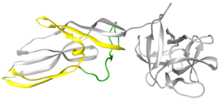
Lysenin is a protein produced in the coelomocyte-leucocytes of the earthworm Eisenia fetida. This protein was first isolated from the coelomic fluid in 1996 and named lysenin (from lysis and Eisenia). Lysenin is a relatively small water-soluble molecule with a molecular weight of 33 kDa. Using X-ray crystallography, lysenin was classified as a member of the Aerolysin protein family by structure and function. Structurally, each lysenin monomer consists of a receptor binding domain (grey globular part on right of Figure 1) and a Pore Forming Module (PFM); domains shared throughout the aerolysin family. The lysenin receptor binding domain shows three sphingomyelin binding motifs. The Pore Forming Module contains the regions that undergo large conformational changes to become the β-barrel in the pore.
Membrane receptors
The natural membrane target of lysenin is an animal plasma membrane lipid called sphingomyelin located mainly in its outer leaflet, involving at least three of its phosphatidylcholines (PC) groups. Sphingomyelin is usually found associated with cholesterol in lipid rafts. Cholesterol, which enhances oligomerization, provides a stable platform with high lateral mobility where monomer-monomer encounters are more probable. PFTs have shown to be able to remodel the membrane structure, sometimes even mixing lipid phases.
The region of the lysenin pore β-barrel expected to be immersed in the hydrophobic region of the membrane is the 'detergent belt', the 3.2 nm high region occupied by detergent in Cryogenic Electron Microscopy (Cryo-EM) studies of the pore. On the other hand, sphingomyelin/Cholesterol bilayers are about 4.5 nm height. This difference in height between the detergent belt and the sphingomyelin/cholesterol bilayer implies a bend of the membrane in the region surrounding the pore, called negative mismatch. This bending results in a net attraction between pores that induce pores aggregation.
Binding, oligomerization and insertion

Membrane binding is a requisite to initiate PFT oligomerization. Lysenin monomers bind specifically to sphingomyelin via the receptor binding domain. The final lysenin oligomer is constituted by nine monomers without quantified deviations. When lysenin monomers bind to sphingomyelin-enriched membrane regions, they provide a stable platform with a high lateral mobility, hence favouring the oligomerization. As with most PFTs, lysenin oligomerization occurs in a two-step process, as was recently imaged.
The process begins with monomers being adsorbed into the membrane by specific interactions, resulting in an increased concentration of monomers. This increase is promoted by the small area where the membrane receptor accumulates since the majority of PFT membrane receptors are associated with lipid rafts. Another side effect, aside from the increase of monomer concentration, is the monomer-monomer interaction. This interaction increases lysenin oligomerization. After a critical threshold concentration is reached, several oligomers are formed simultaneously, although sometimes these are incomplete. In contrast to PFTs of the cholesterol-dependent cytolysin family, the transition from incomplete lysenin oligomers to complete oligomers has not been observed.
A complete oligomerization results in the so-called prepore state, a structure on the membrane. Determining the prepore's structure by X-ray or Cryo-EM is a challenging process that so far has not produced any results. The only available information about the prepore structure was provided by Atomic Force Microscopy (AFM). The measured prepore height was 90 Å; and the width 118 Å, with an inner pore of 50 Å. A model of the prepore was built aligning the monomer structure (PDB: 3ZXD) with the pore structure (PDB: 5GAQ) by their receptor-binding domains (residues 160 to 297). A recent study in aerolysin suggests that the currently accepted model for the lysenin prepore should be revisited, according to the new available data on the aerolysin insertion.
A conformational change transforms the PFM into the transmembrane β-barrel, leading to the pore state. The trigger mechanism for the prepore-to-pore transition in lysenin depends on three glutamic acid residues (E92, E94 and E97), and is activated by a decrease in pH, from physiological conditions to the acidic conditions reached after endocytosis, or an increase in calcium extracellular concentration. These three glutamic acids are located in an α-helix that forms part of the PFM, and glutamic acids are found in aerolysin family members in its PFMs. Such a conformational change produces a decrease in the oligomer height of 2.5 nm according to AFM measurements. The main dimensions, using lysenin pore X-ray structure, are height 97 Å, width 115 Å and the inner pore of 30 Å. However, complete oligomerization into the nonamer is not a requisite for the insertion, since incomplete oligomers in the pore state can be found. The prepore to pore transition can be blocked in crowded conditions, a mechanism that could be general to all β-PFTs. The first hint of crowding effect on prepore to pore transition was given by congestion effects in electrophysiology experiments.
Insertion consequences
The ultimate consequences of lysenin pore formation are not well documented; however, it is thought to induce apoptosis via three possible hypotheses:
- Breaking the sphingomyelin asymmetry between the two leaflets of the lipid bilayer by punching holes in the membrane and inducing lipid flip-flop (reorientation of a lipid from one leaflet of a membrane bilayer to the other).
- Increasing the calcium concentration in the cytoplasm.
- Decreasing the potassium concentration in the cytoplasm.
Biological role
The biological role of lysenin remains unknown. It has been suggested that lysenin may play a role as a defence mechanism against attackers such as bacteria, fungi or small invertebrates. However, lysenin's activity is dependent upon binding to sphingomyelin, which is not present in the membranes of bacteria, fungi or most invertebrates. Rather, sphingomyelin is mainly present in the plasma membrane of chordates. Another hypothesis is that the earthworm, which is able to expel coelomic fluid under stress, generates an avoidance behaviour to its vertebrate predators (such as birds, hedgehogs or moles). If that is the case, the expelled lysenin might be more effective if the coelomic fluid reaches the eye, where the concentration of sphingomyelin is ten times higher than in other body organs. A complementary hypothesis is that the pungent smell of the coelomic fluid - giving the earthworm its specific epithet foetida - is an anti-predator adaptation. However, it remains unknown whether lysenin contributes to avoidance of Eisenia by predators.
Applications
Lysenin's conductive properties have been studied for years. Like most pore-forming toxins, lysenin forms a non-specific channel that is permeable to ions, small molecules, and small peptides. There have also been over three decades of studies into finding suitable pores for converting into nanopore sequencing systems that can have their conductive properties tuned by point mutation. Owing to its binding affinity for sphingomyelin, lysenin (or just the receptor binding domain) has been used as a fluorescence marker to detect the sphingomyelin domain in membranes.
References
![]() This article was submitted to WikiJournal of Science for external academic peer review in 2019 (reviewer reports). The updated content was reintegrated into the Misplaced Pages page under a CC-BY-SA-3.0 license (2019). The version of record as reviewed is:
Ignacio L. B. Munguira; et al. (17 August 2019). "Lysenin" (PDF). WikiJournal of Science. 2 (1): 6. doi:10.15347/WJS/2019.006. ISSN 2470-6345. Wikidata Q76846397.
This article was submitted to WikiJournal of Science for external academic peer review in 2019 (reviewer reports). The updated content was reintegrated into the Misplaced Pages page under a CC-BY-SA-3.0 license (2019). The version of record as reviewed is:
Ignacio L. B. Munguira; et al. (17 August 2019). "Lysenin" (PDF). WikiJournal of Science. 2 (1): 6. doi:10.15347/WJS/2019.006. ISSN 2470-6345. Wikidata Q76846397.
- Bruhn, Heike; Winkelmann, Julia; Andersen, Christian; Andrä, Jörg; Leippe, Matthias (2006). "Dissection of the mechanisms of cytolytic and antibacterial activity of lysenin, a defence protein of the annelid Eisenia fetida". Developmental and Comparative Immunology. 30 (7): 597–606. doi:10.1016/j.dci.2005.09.002. PMID 16386304.
- Yilmaz, N.; Yamaji-Hasegawa, A.; Hullin-Matsuda, F.; Kobayashi, T. (2018). "Molecular mechanisms of action of sphingomyelin-specific pore-forming toxin, lysenin". Seminars in Cell & Developmental Biology. 73: 188–198. doi:10.1016/j.semcdb.2017.07.036. PMID 28751253.
- Sekizawa, Y.; Hagiwara, K.; Nakajima, T.; Kobayashi, H. (1996). "A Novel Protein, Lysenin, that Causes Contraction of the Isolate Rat Aorta: Its Purification from the Coelomic Fluid of the Earthworm, Eisenia foetida". Biomedical Research. 17 (3): 197–203. doi:10.2220/biomedres.17.197.
- ^ De Colibus, L.; Sonnen, A. F.-P.; Morris, K. J.; Siebert, C. A.; Abrusci, P.; Plitzko, J.; Hodnik, V.; Leippe, M.; Volpi, E.; Anderluh, G.; Gilbert, R. J. C. (2012). "Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins And Its Mode of Sphingomyelin Recognition". Structure. 20 (9): 1498–1507. doi:10.1016/j.str.2012.06.011. PMC 3526787. PMID 22819216.
- Bokori-Brown, M.; Martin, T. G.; Naylor, C. E.; Basak, A. K.; Titball, R. W.; Savva, C. G. (2016). "Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein". Nature Communications. 7 (1): 11293. Bibcode:2016NatCo...711293B. doi:10.1038/ncomms11293. PMC 4823867. PMID 27048994.
- ^ Ishitsuka, R.; Kobayashi, T. (2007). "Cholesterol and Lipid/Protein Ratio Control the Oligomerization of a Sphingomyelin-Specific Toxin, Lysenin". Biochemistry. 46 (6): 1495–1502. doi:10.1021/bi061290k. PMID 17243772. S2CID 22016219.
- Simons, K.; Gerl, M. J. (2010). "Revitalizing membrane rafts: new tools and insights". Nature Reviews Molecular Cell Biology. 11 (10): 688–699. doi:10.1038/nrm2977. PMID 20861879. S2CID 1866391.
- Ros, U.; García-Sáez, A. J. (2015). "More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes". The Journal of Membrane Biology. 248 (3): 545–561. doi:10.1007/s00232-015-9820-y. PMID 26087906. S2CID 16305100.
- Yilmaz, N.; Kobayashi, T. (2015). "Visualization of Lipid Membrane Reorganization Induced by a Pore-Forming Toxin Using High-Speed Atomic Force Microscopy". ACS Nano. 9 (8): 7960–7967. doi:10.1021/acsnano.5b01041. PMID 26222645.
- Bokori-Brown, M.; Martin, T. G.; Naylor, C. E.; Basak, A. K.; Titball, R. W.; Savva, C. G. (2016). "Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein". Nature Communications. 7 (1): 11293. Bibcode:2016NatCo...711293B. doi:10.1038/ncomms11293. PMC 4823867. PMID 27048994.
- Quinn, P. J. (2013). "Structure of Sphingomyelin Bilayers and Complexes with Cholesterol Forming Membrane Rafts". Langmuir. 29 (30): 9447–9456. doi:10.1021/la4018129. PMID 23863113.
- Guigas, G.; Weiss, M. (2016). "Effects of protein crowding on membrane systems". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1858 (10): 2441–2450. doi:10.1016/j.bbamem.2015.12.021. PMID 26724385.
- De Colibus, L.; Sonnen, A. F.-P.; Morris, K. J.; Siebert, C. A.; Abrusci, P.; Plitzko, J.; Hodnik, V.; Leippe, M.; Volpi, E.; Anderluh, G.; Gilbert, R. J. C. (2012). "Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins And Its Mode of Sphingomyelin Recognition". Structure. 20 (9): 1498–1507. doi:10.1016/j.str.2012.06.011. PMC 3526787. PMID 22819216.
- Munguira, I.; Casuso, I.; Takahashi, H.; Rico, F.; Miyagi, A.; Chami, M.; Scheuring, S. (2016). "Glasslike Membrane Protein Diffusion in a Crowded Membrane" (PDF). ACS Nano. 10 (2): 2584–2590. doi:10.1021/acsnano.5b07595. PMID 26859708. S2CID 206699095.
- Ishitsuka, R.; Kobayashi, T. (2007). "Cholesterol and Lipid/Protein Ratio Control the Oligomerization of a Sphingomyelin-Specific Toxin, Lysenin". Biochemistry. 46 (6): 1495–1502. doi:10.1021/bi061290k. PMID 17243772. S2CID 22016219.
- Lafont, F.; Van Der Goot, F. G. (2005). "Bacterial invasion via lipid rafts". Cellular Microbiology. 7 (5): 613–620. doi:10.1111/j.1462-5822.2005.00515.x. PMID 15839890. S2CID 26547616.
- ^ Yilmaz, N.; Yamada, T.; Greimel, P.; Uchihashi, T.; Ando, T.; Kobayashi, T. (2013). "Real-Time Visualization of Assembling of a Sphingomyelin-Specific Toxin on Planar Lipid Membranes". Biophysical Journal. 105 (6): 1397–1405. Bibcode:2013BpJ...105.1397Y. doi:10.1016/j.bpj.2013.07.052. PMC 3785888. PMID 24047991.
- Mulvihill, E.; van Pee, K.; Mari, S. A.; Müller, D. J.; Yildiz, Ö. (2015). "Directly Observing the Lipid-Dependent Self-Assembly and Pore-Forming Mechanism of the Cytolytic Toxin Listeriolysin O". Nano Letters. 15 (10): 6965–6973. Bibcode:2015NanoL..15.6965M. doi:10.1021/acs.nanolett.5b02963. PMID 26302195.
- Iacovache, Ioan; De Carlo, Sacha; Cirauqui, Nuria; Dal Peraro, Matteo; van der Goot, F. Gisou; Zuber, Benoît (2016). "Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process". Nature Communications. 7: 12062. Bibcode:2016NatCo...712062I. doi:10.1038/ncomms12062. PMC 4947156. PMID 27405240.
- ^ Bokori-Brown, M.; Martin, T. G.; Naylor, C. E.; Basak, A. K.; Titball, R. W.; Savva, C. G. (2016). "Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein". Nature Communications. 7 (1): 11293. Bibcode:2016NatCo...711293B. doi:10.1038/ncomms11293. PMC 4823867. PMID 27048994.
- Munguira, I. L. B.; Takahashi, H.; Casuso, I.; Scheuring, S. (2017). "Lysenin Toxin Membrane Insertion Is pH-Dependent but Independent of Neighboring Lysenins". Biophysical Journal. 113 (9): 2029–2036. Bibcode:2017BpJ...113.2029M. doi:10.1016/j.bpj.2017.08.056. PMC 5685674. PMID 29117526.
- Munguira, I.L.B. (2019). "Lysenin toxin insertion mechanism is Calcium-dependent". bioRxiv. doi:10.1101/771725.
- Krueger, E.; Bryant, S.; Shrestha, N.; Clark, T.; Hanna, C.; Pink, D.; Fologea, D. (2015). "Intramembrane congestion effects on lysenin channel voltage-induced gating". European Biophysics Journal. 45 (2): 187–194. doi:10.1007/s00249-015-1104-z. PMC 4803513. PMID 26695013.
- Green, D. R. (2000). "Apoptosis and Sphingomyelin Hydrolysis". The Journal of Cell Biology. 150 (1): F5 – F8. doi:10.1083/jcb.150.1.F5. PMC 2185551. PMID 10893276.
- Ros, U.; García-Sáez, A. J. (2015). "More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes". The Journal of Membrane Biology. 248 (3): 545–561. doi:10.1007/s00232-015-9820-y. PMID 26087906. S2CID 16305100.
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. (2003). "Regulation of cell death: the calcium–apoptosis link". Nature Reviews Molecular Cell Biology. 4 (7): 552–565. doi:10.1038/nrm1150. PMID 12838338. S2CID 19079491.
- Yu, S. P. (2003). "Regulation and critical role of potassium homeostasis in apoptosis". Progress in Neurobiology. 70 (4): 363–386. doi:10.1016/s0301-0082(03)00090-x. PMID 12963093. S2CID 13893235.
- Ballarin, L.; Cammarata, M. (2016). Lessons in immunity: from single-cell organisms to mammals. Academic Press. ISBN 9780128032527.
- Kobayashi, H.; Sekizawa, Y.; Aizu, M.; Umeda, M. (2000). "Lethal and non-lethal responses of spermatozoa from a wide variety of vertebrates and invertebrates to lysenin, a protein from the coelomic fluid of the earthworm Eisenia foetida". Journal of Experimental Zoology. 286 (5): 538–549. Bibcode:2000JEZ...286..538K. doi:10.1002/(sici)1097-010x(20000401)286:5<538::aid-jez12>3.0.co;2-w. PMID 10684578.
- Sukumwang, N.; Umezawa, K. (2013). "Earthworm-Derived Pore-Forming Toxin Lysenin and Screening of Its Inhibitors". Toxins. 5 (8): 1392–1401. doi:10.3390/toxins5081392. PMC 3760042. PMID 23965430.
- Kobayashi, H.; Ohta, N.; Umeda, M. (2004). "Biology of lysenin, a protein in the coelomic fluid of the earthworm Eisenia foetida". International Review of Cytology. 236: 45–99. doi:10.1016/S0074-7696(04)36002-X. ISBN 9780123646408. PMID 15261736.
- Swiderska, B.; Kedracka-Krok, S.; Panz, T.; Morgan, A. J.; Falniowski, A.; Grzmil, P.; Plytycz, B. (2017). "Lysenin family proteins in earthworm coelomocytes – Comparative approach". Developmental & Comparative Immunology. 67: 404–412. doi:10.1016/j.dci.2016.08.011. PMID 27567602. S2CID 19895826.
- Berman, E. R. (1991). Biochemistry of the Eye. Springer. doi:10.1007/978-1-4757-9441-0. ISBN 978-1-4757-9441-0. S2CID 41192657.
- Edwards, C. A.; Bohlen, P. J. (1996). Biology and Ecology of Earthworms. Springer Science & Business Media. ISBN 978-0-412-56160-3.
- Bryant, S.; Clark, T.; Thomas, C.; Ware, K.; Bogard, A.; Calzacorta, C.; Prather, D.; Fologea, D. (2018). "Insights into the Voltage Regulation Mechanism of the Pore-Forming Toxin Lysenin". Toxins. 10 (8): 334. doi:10.3390/toxins10080334. PMC 6115918. PMID 30126104.
- Shrestha, N.; Bryant, S. L.; Thomas, C.; Richtsmeier, D.; Pu, X.; Tinker, J.; Fologea, D. (2017). "Stochastic sensing of Angiotensin II with lysenin channels". Scientific Reports. 7 (1): 2448. Bibcode:2017NatSR...7.2448S. doi:10.1038/s41598-017-02438-0. PMC 5446423. PMID 28550293.
- Deamer, D.; Akeson, M.; Branton, D. (2016). "Three decades of nanopore sequencing". Nature Biotechnology. 34 (5): 518–524. doi:10.1038/nbt.3423. PMC 6733523. PMID 27153285.
- Ishitsuka, R.; Kobayashi, T. (2004). "Lysenin: A new tool for investigating membrane lipid organization". Anatomical Science International. 79 (4): 184–190. doi:10.1111/j.1447-073x.2004.00086.x. PMID 15633456. S2CID 1558393.