| Revision as of 14:13, 10 July 2023 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,367 editsm Smokefoot moved page Dimer (chemistry) to Dimerization (chemistry) over redirect: better defined← Previous edit | Latest revision as of 11:51, 19 October 2024 edit undoGünniX (talk | contribs)Extended confirmed users311,149 editsm unbalanced bracketsTag: AWB | ||
| (25 intermediate revisions by 13 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description| |
{{short description|Chemical process of joining two molecular entities by bonds of any kind}} | ||
| {{Redirect|Dimer (chemistry)|other uses|Dimer (disambiguation)}} | |||
| {{refimprove|date=April 2009}} | {{refimprove|date=April 2009}} | ||
| A '''dimer''' ({{IPAc-en|ˈ|d|aɪ|m|ər}}; '']'', "two" + ''-mer'', "parts") is an ] consisting of two ]s joined by bonds that can be either strong or weak, ] or ].<ref>{{cite web |title=Dimer |url=https://www.chem.ucla.edu/~harding/IGOC/D/dimer.html |website=Illustrated Glossary of Organic Chemistry |publisher=UCLA |access-date=12 May 2022}}</ref> Dimers also have significant implications in polymer chemistry; inorganic chemistry, and biochemistry. | |||
| The term ''homodimer'' is used when the two |
In ], '''dimerization''' is the process of joining two identical or similar ] by ]. The resulting bonds can be either strong or weak. Many symmetrical ] are described as '''dimers''', even when the ] is unknown or highly unstable.<ref>{{cite web |title=Dimerization |url=https://goldbook.iupac.org/terms/view/D01744}}</ref> | ||
| The term ''homodimer'' is used when the two subunits are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called ]. When two oppositely-charged ]s associate into dimers, they are referred to as ''Bjerrum pairs'',<ref>{{Cite journal|last1=Adar|first1=Ram M.|last2=Markovich|first2=Tomer|last3=Andelman|first3=David|date=2017-05-17|title=Bjerrum pairs in ionic solutions: A Poisson-Boltzmann approach|journal=The Journal of Chemical Physics|volume=146|issue=19|pages=194904|doi=10.1063/1.4982885|pmid=28527430|issn=0021-9606|arxiv=1702.04853|bibcode=2017JChPh.146s4904A|s2cid=12227786}}</ref> after Danish chemist ]. | |||
| == Noncovalent dimers == | == Noncovalent dimers == | ||
| ]s are often found in the vapour phase.]] | ]s are often found in the vapour phase.]] | ||
| ] ]s form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, ] forms a dimer in the gas phase, where the monomer units are held together by ]s.<ref>{{Cite journal |last1=Karle |first1=J. |last2=Brockway |first2=L. O. |date=1944 |title=An Electron Diffraction Investigation of the Monomers and Dimers of Formic, Acetic and Trifluoroacetic Acids and the Dimer of Deuterium Acetate 1 |url=https://pubs.acs.org/doi/abs/10.1021/ja01232a022 |journal=Journal of the American Chemical Society |language=en |volume=66 |issue=4 |pages=574–584 |doi=10.1021/ja01232a022 |issn=0002-7863}}</ref> |
] ]s form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, ] forms a dimer in the gas phase, where the monomer units are held together by ]s.<ref>{{Cite journal |last1=Karle |first1=J. |last2=Brockway |first2=L. O. |date=1944 |title=An Electron Diffraction Investigation of the Monomers and Dimers of Formic, Acetic and Trifluoroacetic Acids and the Dimer of Deuterium Acetate 1 |url=https://pubs.acs.org/doi/abs/10.1021/ja01232a022 |journal=Journal of the American Chemical Society |language=en |volume=66 |issue=4 |pages=574–584 |doi=10.1021/ja01232a022 |issn=0002-7863}}</ref> Many OH-containing molecules form dimers, e.g. the ]. | ||
| ] and ] are ] structures with a short lifetime. For example, ] do not form stable dimers, but they do form the ] Ar<sub>2</sub>*, Kr<sub>2</sub>* and Xe<sub>2</sub>* under high pressure and electrical stimulation.<ref>{{Cite journal |last=Birks |first=J B |date=1975-08-01 |title=Excimers |url=https://iopscience.iop.org/article/10.1088/0034-4885/38/8/001 |journal=Reports on Progress in Physics |volume=38 |issue=8 |pages=903–974 |doi=10.1088/0034-4885/38/8/001 |s2cid=240065177 |issn=0034-4885}}</ref> | ] and ]es are ] structures with a short lifetime. For example, ] do not form stable dimers, but they do form the ] Ar<sub>2</sub>*, Kr<sub>2</sub>* and Xe<sub>2</sub>* under high pressure and electrical stimulation.<ref>{{Cite journal |last=Birks |first=J B |date=1975-08-01 |title=Excimers |url=https://iopscience.iop.org/article/10.1088/0034-4885/38/8/001 |journal=Reports on Progress in Physics |volume=38 |issue=8 |pages=903–974 |doi=10.1088/0034-4885/38/8/001 |s2cid=240065177 |issn=0034-4885}}</ref> | ||
| == Covalent dimers == | == Covalent dimers == | ||
| ], although this might not be readily apparent on initial inspection.]] | ] gives dicyclopentadiene, although this might not be readily apparent on initial inspection. This dimerization is reversible]] | ||
| ], one of two ] dimers. As evidenced by this molecule's bonds, covalent dimers are usually not similar in structure to their ]s.]] | |||
| ] dimers are often formed by the reaction of two identical compounds e.g.: {{chem2|2A -> A\sA}}. In this example, ] "A" is said to dimerize to give the dimer "{{chem2|A\sA}}". |
] dimers are often formed by the reaction of two identical compounds e.g.: {{chem2|2A -> A\sA}}. In this example, ] "A" is said to dimerize to give the dimer "{{chem2|A\sA}}". | ||
| :<chem>2 C(NR2)2 -> (R2N)2C=C(NR2)2</chem> | |||
| ]s are highly reactive and readily form bonds. | |||
| ] is an asymmetrical dimer of two ] molecules that have reacted in a ] to give the product. Upon heating, it "cracks" (undergoes a retro-Diels-Alder reaction) to give identical monomers: | ] is an asymmetrical dimer of two ] molecules that have reacted in a ] to give the product. Upon heating, it "cracks" (undergoes a retro-Diels-Alder reaction) to give identical monomers: | ||
| :<chem>C10H12 -> 2 C5H6</chem> | :<chem>C10H12 -> 2 C5H6</chem> | ||
| Many nonmetallic elements occur as dimers: ], ], ], and the ]s |
Many nonmetallic elements occur as dimers: ], ], ], and the ]s ], ], ] and ]. Some metals form a proportion of dimers in their vapour phase: ] ({{chem2|Li2}}), ] ({{chem2|Na2}}), ] ({{chem2|K2}}), ] ({{chem2|Rb2}}) and ] ({{chem2|Cs2}}). Such elemental dimers are ] ]s. | ||
| Many small organic molecules, most notably ], easily form dimers. The dimer of formaldehyde ({{chem2|CH2O}}) is ] ({{chem2|C2H4O2}}). | |||
| ] ({{chem2|BH3}}) occurs as the dimer ] ({{chem2|B2H6}}), due to the high ] of the ] center. | |||
| ==Polymer chemistry== | ==Polymer chemistry== | ||
| In the context of ]s, "dimer" also refers to the ] 2, regardless of the stoichiometry or ]s. |
In the context of ]s, "dimer" also refers to the ] 2, regardless of the stoichiometry or ]s. | ||
| One case where this is applicable is with ]s. For example, ] is a dimer of ], even though the formation reaction produces ]: | One case where this is applicable is with ]s. For example, ] is a dimer of ], even though the formation reaction produces ]: | ||
| Line 35: | Line 30: | ||
| Here, the resulting dimer has a stoichiometry different from the initial pair of monomers. | Here, the resulting dimer has a stoichiometry different from the initial pair of monomers. | ||
| Disaccharides need not be composed of the same ] to be considered dimers. An example is ], a dimer of ] and glucose, which follows the same reaction equation as presented above. |
Disaccharides need not be composed of the same ]s to be considered dimers. An example is ], a dimer of ] and glucose, which follows the same reaction equation as presented above. | ||
| Amino acids can also form dimers, which are called ]s. An example is ], consisting of two ] molecules joined by a ]. Other examples include ] and ]. | Amino acids can also form dimers, which are called ]s. An example is ], consisting of two ] molecules joined by a ]. Other examples include ] and ]. | ||
| == Inorganic dimers == | == Inorganic and organometallic dimers == | ||
| Many molecules and ions are described as dimers, even when the monomer is elusive. | |||
| === |
=== Boranes === | ||
| ⚫ | ] | ||
| ] (B<sub>2</sub>H<sub>6</sub>) is an dimer of ], which is elusive and rarely observed. Almost all compounds of the type R2BH exist as dimers.<ref>{{Cite book |last=Shriver |first=Duward |title=Inorganic Chemistry |publisher=W.H. Freeman and Company |year=2014 |isbn=9781429299060 |edition=6th |pages=306–307 |language=English}}</ref> | |||
| === |
===Organoaluminium compounds=== | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] |
] can exist as either monomers or dimers, depending on the ] of the groups attached. For example, ] exists as a dimer, but trimesitylaluminium adopts a monomeric structure.<ref name=":0">{{Cite book |last=Shriver |first=Duward |title=Inorganic Chemistry |publisher=W.H. Freeman and Company |year=2014 |isbn=9781429299060 |edition=6th |pages=377–378 |language=English}}</ref> | ||
| ==== Aluminium ==== | |||
| ===Organochromium compounds=== | |||
| ⚫ | ] | ||
| Cyclopentadienylchromium tricarbonyl dimer exists in measureable equilibrium quantities with the monometallic radical {{chem2|(C5H5)Cr(CO)3}}.<ref>{{cite journal | doi = 10.1021/ja00810a019 | issue = 5| pages = 749–754| title = Unusual structural and magnetic resonance properties of dicyclopentadienylhexacarbonyldichromium| journal = Journal of the American Chemical Society| volume = 96| year = 1974| last1 = Adams| first1 = Richard D.| last2 = Collins| first2 = Douglas E.| last3 = Cotton| first3 = F. Albert}}</ref> | |||
| ] complexes can exist as either monomers or dimers based on the ] of the groups attached. For example, ] or ethylaluminium exists as a dimer, but when a bulkier group is added, the complex exists as a monomer, such as trimesitylaluminium.<ref name=":0">{{Cite book |last=Shriver |first=Duward |title=Inorganic Chemistry |publisher=W.H. Freeman and Company |year=2014 |isbn=9781429299060 |edition=6th |pages=377–378 |language=English}}</ref> | |||
| == Biochemical dimers == | == Biochemical dimers == | ||
| === Pyrimidine dimers === | === Pyrimidine dimers === | ||
| ] (also known as thymine dimers) are formed by a ] from pyrimidine ] when exposed to ultraviolet light.<ref name=":0"/> This cross-linking causes ], which can be ], causing ].<ref name=":0" /> When ] are present, they can block ], decreasing DNA functionality until it is repaired.<ref name=":0" /> | ] (also known as thymine dimers) are formed by a ] from pyrimidine ]s when exposed to ultraviolet light.<ref name=":0"/> This cross-linking causes ], which can be ], causing ]s.<ref name=":0" /> When ]s are present, they can block ]s, decreasing DNA functionality until it is repaired.<ref name=":0" /> | ||
| === Protein dimers === | === Protein dimers === | ||
| ] | ] | ||
| ] arise from the interaction between two ] which can interact further to form larger and more complex ].<ref name=":1">{{Cite journal |last1=Marianayagam |first1=Neelan J. |last2=Sunde |first2=Margaret |last3=Matthews |first3=Jacqueline M. |date=2004 |title=The power of two: protein dimerization in biology |url=http://dx.doi.org/10.1016/j.tibs.2004.09.006 |journal=Trends in Biochemical Sciences |volume=29 |issue=11 |pages=618–625 |doi=10.1016/j.tibs.2004.09.006 |pmid=15501681 |issn=0968-0004}}</ref> For example, ] is formed by the dimerization of ] and ] and this dimer can then ] further to make ].<ref>{{Cite journal |last=Cooper |first=Geoffrey M. |date=2000 |title=Microtubules |url=https://www.ncbi.nlm.nih.gov/books/NBK9932/ |journal=The Cell: A Molecular Approach. 2nd Edition |language=en}}</ref> For symmetric proteins, the larger protein complex can be broken down into smaller identical ], which then dimerize to decrease the genetic code required to make the functional protein.<ref name=":1" /> | ]s arise from the interaction between two ]s which can interact further to form larger and more complex ]s.<ref name=":1">{{Cite journal |last1=Marianayagam |first1=Neelan J. |last2=Sunde |first2=Margaret |last3=Matthews |first3=Jacqueline M. |date=2004 |title=The power of two: protein dimerization in biology |url=http://dx.doi.org/10.1016/j.tibs.2004.09.006 |journal=Trends in Biochemical Sciences |volume=29 |issue=11 |pages=618–625 |doi=10.1016/j.tibs.2004.09.006 |pmid=15501681 |issn=0968-0004}}</ref> For example, ] is formed by the dimerization of ] and ] and this dimer can then ] further to make ]s.<ref>{{Cite journal |last=Cooper |first=Geoffrey M. |date=2000 |title=Microtubules |url=https://www.ncbi.nlm.nih.gov/books/NBK9932/ |journal=The Cell: A Molecular Approach. 2nd Edition |language=en}}</ref> For symmetric proteins, the larger protein complex can be broken down into smaller identical ]s, which then dimerize to decrease the genetic code required to make the functional protein.<ref name=":1" /> | ||
| === G protein-coupled receptors === | === G protein-coupled receptors === | ||
| As the largest and most diverse family of ] within the human genome, ] (GPCR) have been studied extensively, with recent studies supporting their ability to form dimers.<ref>{{Citation |last1=Faron-Górecka |first1=Agata |title=Chapter 10 - Understanding GPCR dimerization |date=2019-01-01 |url=https://www.sciencedirect.com/science/article/pii/S0091679X18301080 |journal=Methods in Cell Biology |volume=149 |pages=155–178 |editor-last=Shukla |editor-first=Arun K. |series=G Protein-Coupled Receptors, Part B |publisher=Academic Press |language=en |doi=10.1016/bs.mcb.2018.08.005 |access-date=2022-10-27 |last2=Szlachta |first2=Marta |last3=Kolasa |first3=Magdalena |last4=Solich |first4=Joanna |last5=Górecki |first5=Andrzej |last6=Kuśmider |first6=Maciej |last7=Żurawek |first7=Dariusz |last8=Dziedzicka-Wasylewska |first8=Marta|pmid=30616817 |isbn=9780128151075 |s2cid=58577416 }}</ref> GPCR dimers include both homodimers and heterodimers formed from related members of the GPCR family.<ref>{{Cite journal |last1=Rios |first1=C. D. |last2=Jordan |first2=B. A. |last3=Gomes |first3=I. |last4=Devi |first4=L. A. |date=2001-11-01 |title=G-protein-coupled receptor dimerization: modulation of receptor function |url=https://www.sciencedirect.com/science/article/pii/S0163725801001607 |journal=Pharmacology & Therapeutics |language=en |volume=92 |issue=2 |pages=71–87 |doi=10.1016/S0163-7258(01)00160-7 |pmid=11916530 |issn=0163-7258}}</ref> While not all, some GPCRs require dimerization to function, such as ]-receptor, emphasizing the importance of dimers in biological systems.<ref>{{Cite journal |last=Lohse |first=Martin J |date=2010-02-01 |title=Dimerization in GPCR mobility and signaling |url=https://www.sciencedirect.com/science/article/pii/S1471489209001672 |journal=Current Opinion in Pharmacology |series=GPCR |language=en |volume=10 |issue=1 |pages=53–58 |doi=10.1016/j.coph.2009.10.007 |pmid=19910252 |issn=1471-4892}}</ref>] | As the largest and most diverse family of ] within the human genome, ]s (GPCR) have been studied extensively, with recent studies supporting their ability to form dimers.<ref>{{Citation |last1=Faron-Górecka |first1=Agata |title=Chapter 10 - Understanding GPCR dimerization |date=2019-01-01 |url=https://www.sciencedirect.com/science/article/pii/S0091679X18301080 |journal=Methods in Cell Biology |volume=149 |pages=155–178 |editor-last=Shukla |editor-first=Arun K. |series=G Protein-Coupled Receptors, Part B |publisher=Academic Press |language=en |doi=10.1016/bs.mcb.2018.08.005 |access-date=2022-10-27 |last2=Szlachta |first2=Marta |last3=Kolasa |first3=Magdalena |last4=Solich |first4=Joanna |last5=Górecki |first5=Andrzej |last6=Kuśmider |first6=Maciej |last7=Żurawek |first7=Dariusz |last8=Dziedzicka-Wasylewska |first8=Marta|pmid=30616817 |isbn=9780128151075 |s2cid=58577416 }}</ref> GPCR dimers include both homodimers and heterodimers formed from related members of the GPCR family.<ref>{{Cite journal |last1=Rios |first1=C. D. |last2=Jordan |first2=B. A. |last3=Gomes |first3=I. |last4=Devi |first4=L. A. |date=2001-11-01 |title=G-protein-coupled receptor dimerization: modulation of receptor function |url=https://www.sciencedirect.com/science/article/pii/S0163725801001607 |journal=Pharmacology & Therapeutics |language=en |volume=92 |issue=2 |pages=71–87 |doi=10.1016/S0163-7258(01)00160-7 |pmid=11916530 |issn=0163-7258}}</ref> While not all, some GPCRs require dimerization to function, such as ]-receptor, emphasizing the importance of dimers in biological systems.<ref>{{Cite journal |last=Lohse |first=Martin J |date=2010-02-01 |title=Dimerization in GPCR mobility and signaling |url=https://www.sciencedirect.com/science/article/pii/S1471489209001672 |journal=Current Opinion in Pharmacology |series=GPCR |language=en |volume=10 |issue=1 |pages=53–58 |doi=10.1016/j.coph.2009.10.007 |pmid=19910252 |issn=1471-4892}}</ref>] | ||
| === Receptor tyrosine kinase === | === Receptor tyrosine kinase === | ||
| Much like for G protein-coupled receptors, dimerization is essential for ] (RTK) to perform their function in ], affecting many different cellular processes.<ref name=":2">{{Cite journal |last=Hubbard |first=Stevan R |date=1999-04-01 |title=Structural analysis of receptor tyrosine kinases |journal=Progress in Biophysics and Molecular Biology |language=en |volume=71 |issue=3 |pages=343–358 |doi=10.1016/S0079-6107(98)00047-9 |pmid=10354703 |issn=0079-6107|doi-access=free }}</ref> RTKs typically exist as monomers, but undergo a ] upon ] binding, allowing them to dimerize with nearby RTKs.<ref>{{Cite journal |last1=Lemmon |first1=Mark A. |last2=Schlessinger |first2=Joseph |date=2010-06-25 |title=Cell Signaling by Receptor Tyrosine Kinases |journal=Cell |language=English |volume=141 |issue=7 |pages=1117–1134 |doi=10.1016/j.cell.2010.06.011 |issn=0092-8674 |pmc=2914105 |pmid=20602996}}</ref><ref>{{Cite journal |last1=Lemmon |first1=Mark A. |last2=Schlessinger |first2=Joseph |last3=Ferguson |first3=Kathryn M. |date=2014-04-01 |title=The EGFR Family: Not So Prototypical Receptor Tyrosine Kinases |journal=Cold Spring Harbor Perspectives in Biology |language=en |volume=6 |issue=4 |pages=a020768 |doi=10.1101/cshperspect.a020768 |issn=1943-0264 |pmid=24691965|pmc=3970421 |doi-access=free }}</ref> The dimerization activates the ] ] ] that are responsible for further ].<ref name=":2" /> | Much like for G protein-coupled receptors, dimerization is essential for ]s (RTK) to perform their function in ], affecting many different cellular processes.<ref name=":2">{{Cite journal |last=Hubbard |first=Stevan R |date=1999-04-01 |title=Structural analysis of receptor tyrosine kinases |journal=Progress in Biophysics and Molecular Biology |language=en |volume=71 |issue=3 |pages=343–358 |doi=10.1016/S0079-6107(98)00047-9 |pmid=10354703 |issn=0079-6107|doi-access=free }}</ref> RTKs typically exist as monomers, but undergo a ] upon ] binding, allowing them to dimerize with nearby RTKs.<ref>{{Cite journal |last1=Lemmon |first1=Mark A. |last2=Schlessinger |first2=Joseph |date=2010-06-25 |title=Cell Signaling by Receptor Tyrosine Kinases |journal=Cell |language=English |volume=141 |issue=7 |pages=1117–1134 |doi=10.1016/j.cell.2010.06.011 |issn=0092-8674 |pmc=2914105 |pmid=20602996}}</ref><ref>{{Cite journal |last1=Lemmon |first1=Mark A. |last2=Schlessinger |first2=Joseph |last3=Ferguson |first3=Kathryn M. |date=2014-04-01 |title=The EGFR Family: Not So Prototypical Receptor Tyrosine Kinases |journal=Cold Spring Harbor Perspectives in Biology |language=en |volume=6 |issue=4 |pages=a020768 |doi=10.1101/cshperspect.a020768 |issn=1943-0264 |pmid=24691965|pmc=3970421 |doi-access=free }}</ref> The dimerization activates the ]ic ] ] that are responsible for further ].<ref name=":2" /> | ||
| == See also == | == See also == | ||
| Line 76: | Line 73: | ||
| == References == | == References == | ||
| * {{cite |
* {{cite web | url=http://goldbook.iupac.org/D01744.html | title=IUPAC "Gold Book" definition | doi=10.1351/goldbook.D01744 | s2cid=242984652 | access-date=2024-07-11| doi-access=free }} | ||
| <references/> | <references/> | ||
| ] | ] | ||
Latest revision as of 11:51, 19 October 2024
Chemical process of joining two molecular entities by bonds of any kind "Dimer (chemistry)" redirects here. For other uses, see Dimer (disambiguation).| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Dimerization" – news · newspapers · books · scholar · JSTOR (April 2009) (Learn how and when to remove this message) |
In chemistry, dimerization is the process of joining two identical or similar molecular entities by bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dimers, even when the monomer is unknown or highly unstable.
The term homodimer is used when the two subunits are identical (e.g. A–A) and heterodimer when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely-charged ions associate into dimers, they are referred to as Bjerrum pairs, after Danish chemist Niels Bjerrum.
Noncovalent dimers

Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Many OH-containing molecules form dimers, e.g. the water dimer.
Excimers and exciplexes are excited structures with a short lifetime. For example, noble gases do not form stable dimers, but they do form the excimers Ar2*, Kr2* and Xe2* under high pressure and electrical stimulation.
Covalent dimers
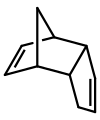
Molecular dimers are often formed by the reaction of two identical compounds e.g.: 2A → A−A. In this example, monomer "A" is said to dimerize to give the dimer "A−A".
Dicyclopentadiene is an asymmetrical dimer of two cyclopentadiene molecules that have reacted in a Diels-Alder reaction to give the product. Upon heating, it "cracks" (undergoes a retro-Diels-Alder reaction) to give identical monomers:
Many nonmetallic elements occur as dimers: hydrogen, nitrogen, oxygen, and the halogens fluorine, chlorine, bromine and iodine. Some metals form a proportion of dimers in their vapour phase: dilithium (Li2), disodium (Na2), dipotassium (K2), dirubidium (Rb2) and dicaesium (Cs2). Such elemental dimers are homonuclear diatomic molecules.
Polymer chemistry
In the context of polymers, "dimer" also refers to the degree of polymerization 2, regardless of the stoichiometry or condensation reactions.
One case where this is applicable is with disaccharides. For example, cellobiose is a dimer of glucose, even though the formation reaction produces water:
Here, the resulting dimer has a stoichiometry different from the initial pair of monomers.
Disaccharides need not be composed of the same monosaccharides to be considered dimers. An example is sucrose, a dimer of fructose and glucose, which follows the same reaction equation as presented above.
Amino acids can also form dimers, which are called dipeptides. An example is glycylglycine, consisting of two glycine molecules joined by a peptide bond. Other examples include aspartame and carnosine.
Inorganic and organometallic dimers
Many molecules and ions are described as dimers, even when the monomer is elusive.
Boranes

Diborane (B2H6) is an dimer of borane, which is elusive and rarely observed. Almost all compounds of the type R2BH exist as dimers.
Organoaluminium compounds

Trialkylaluminium compounds can exist as either monomers or dimers, depending on the steric bulk of the groups attached. For example, trimethylaluminium exists as a dimer, but trimesitylaluminium adopts a monomeric structure.
Organochromium compounds
Cyclopentadienylchromium tricarbonyl dimer exists in measureable equilibrium quantities with the monometallic radical (C5H5)Cr(CO)3.
Biochemical dimers
Pyrimidine dimers
Pyrimidine dimers (also known as thymine dimers) are formed by a photochemical reaction from pyrimidine DNA bases when exposed to ultraviolet light. This cross-linking causes DNA mutations, which can be carcinogenic, causing skin cancers. When pyrimidine dimers are present, they can block polymerases, decreasing DNA functionality until it is repaired.
Protein dimers

Protein dimers arise from the interaction between two proteins which can interact further to form larger and more complex oligomers. For example, tubulin is formed by the dimerization of α-tubulin and β-tubulin and this dimer can then polymerize further to make microtubules. For symmetric proteins, the larger protein complex can be broken down into smaller identical protein subunits, which then dimerize to decrease the genetic code required to make the functional protein.
G protein-coupled receptors
As the largest and most diverse family of receptors within the human genome, G protein-coupled receptors (GPCR) have been studied extensively, with recent studies supporting their ability to form dimers. GPCR dimers include both homodimers and heterodimers formed from related members of the GPCR family. While not all, some GPCRs require dimerization to function, such as GABAB-receptor, emphasizing the importance of dimers in biological systems.

Receptor tyrosine kinase
Much like for G protein-coupled receptors, dimerization is essential for receptor tyrosine kinases (RTK) to perform their function in signal transduction, affecting many different cellular processes. RTKs typically exist as monomers, but undergo a conformational change upon ligand binding, allowing them to dimerize with nearby RTKs. The dimerization activates the cytoplasmic kinase domains that are responsible for further signal transduction.
See also
References
- "IUPAC "Gold Book" definition". doi:10.1351/goldbook.D01744. S2CID 242984652. Retrieved 2024-07-11.
- "Dimerization".
- Adar, Ram M.; Markovich, Tomer; Andelman, David (2017-05-17). "Bjerrum pairs in ionic solutions: A Poisson-Boltzmann approach". The Journal of Chemical Physics. 146 (19): 194904. arXiv:1702.04853. Bibcode:2017JChPh.146s4904A. doi:10.1063/1.4982885. ISSN 0021-9606. PMID 28527430. S2CID 12227786.
- Karle, J.; Brockway, L. O. (1944). "An Electron Diffraction Investigation of the Monomers and Dimers of Formic, Acetic and Trifluoroacetic Acids and the Dimer of Deuterium Acetate 1". Journal of the American Chemical Society. 66 (4): 574–584. doi:10.1021/ja01232a022. ISSN 0002-7863.
- Birks, J B (1975-08-01). "Excimers". Reports on Progress in Physics. 38 (8): 903–974. doi:10.1088/0034-4885/38/8/001. ISSN 0034-4885. S2CID 240065177.
- Shriver, Duward (2014). Inorganic Chemistry (6th ed.). W.H. Freeman and Company. pp. 306–307. ISBN 9781429299060.
- ^ Shriver, Duward (2014). Inorganic Chemistry (6th ed.). W.H. Freeman and Company. pp. 377–378. ISBN 9781429299060.
- Adams, Richard D.; Collins, Douglas E.; Cotton, F. Albert (1974). "Unusual structural and magnetic resonance properties of dicyclopentadienylhexacarbonyldichromium". Journal of the American Chemical Society. 96 (5): 749–754. doi:10.1021/ja00810a019.
- ^ Marianayagam, Neelan J.; Sunde, Margaret; Matthews, Jacqueline M. (2004). "The power of two: protein dimerization in biology". Trends in Biochemical Sciences. 29 (11): 618–625. doi:10.1016/j.tibs.2004.09.006. ISSN 0968-0004. PMID 15501681.
- Cooper, Geoffrey M. (2000). "Microtubules". The Cell: A Molecular Approach. 2nd Edition.
- Faron-Górecka, Agata; Szlachta, Marta; Kolasa, Magdalena; Solich, Joanna; Górecki, Andrzej; Kuśmider, Maciej; Żurawek, Dariusz; Dziedzicka-Wasylewska, Marta (2019-01-01), Shukla, Arun K. (ed.), "Chapter 10 - Understanding GPCR dimerization", Methods in Cell Biology, G Protein-Coupled Receptors, Part B, 149, Academic Press: 155–178, doi:10.1016/bs.mcb.2018.08.005, ISBN 9780128151075, PMID 30616817, S2CID 58577416, retrieved 2022-10-27
- Rios, C. D.; Jordan, B. A.; Gomes, I.; Devi, L. A. (2001-11-01). "G-protein-coupled receptor dimerization: modulation of receptor function". Pharmacology & Therapeutics. 92 (2): 71–87. doi:10.1016/S0163-7258(01)00160-7. ISSN 0163-7258. PMID 11916530.
- Lohse, Martin J (2010-02-01). "Dimerization in GPCR mobility and signaling". Current Opinion in Pharmacology. GPCR. 10 (1): 53–58. doi:10.1016/j.coph.2009.10.007. ISSN 1471-4892. PMID 19910252.
- ^ Hubbard, Stevan R (1999-04-01). "Structural analysis of receptor tyrosine kinases". Progress in Biophysics and Molecular Biology. 71 (3): 343–358. doi:10.1016/S0079-6107(98)00047-9. ISSN 0079-6107. PMID 10354703.
- Lemmon, Mark A.; Schlessinger, Joseph (2010-06-25). "Cell Signaling by Receptor Tyrosine Kinases". Cell. 141 (7): 1117–1134. doi:10.1016/j.cell.2010.06.011. ISSN 0092-8674. PMC 2914105. PMID 20602996.
- Lemmon, Mark A.; Schlessinger, Joseph; Ferguson, Kathryn M. (2014-04-01). "The EGFR Family: Not So Prototypical Receptor Tyrosine Kinases". Cold Spring Harbor Perspectives in Biology. 6 (4): a020768. doi:10.1101/cshperspect.a020768. ISSN 1943-0264. PMC 3970421. PMID 24691965.

