| Revision as of 17:01, 27 November 2023 editPCsci1 (talk | contribs)26 edits →Gene therapy: Updated Gene Therapy section.Tags: Reverted references removed← Previous edit | Latest revision as of 17:50, 24 October 2024 edit undoCitation bot (talk | contribs)Bots5,405,754 edits Altered pmc. Add: pmc, doi-access, pmid, authors 1-1. Removed URL that duplicated identifier. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Jay8g | Category:CS1 maint: PMC format | #UCB_Category 1/3 | ||
| (17 intermediate revisions by 11 users not shown) | |||
| Line 1: | Line 1: | ||
| {{About|the bleeding disorder with factor VIII deficiency|the disorder with factor IX deficiency|haemophilia B}} | {{About|the bleeding disorder with factor VIII deficiency|the disorder with factor IX deficiency|haemophilia B}} | ||
| {{Use British English|date=September 2023}} | {{Use British English|date=September 2023}} | ||
| {{Infobox medical condition |
{{Infobox medical condition | ||
| | name = Haemophilia A | | name = Haemophilia A | ||
| | synonyms = Hemophilia A | | synonyms = Hemophilia A | ||
| Line 30: | Line 30: | ||
| ==Signs and symptoms== | ==Signs and symptoms== | ||
| ] | ] | ||
| Haemophilia |
Haemophilia A's phenotype has a quite wide range of symptoms encompassing both internal and external bleeding episodes. Individuals with more severe haemophilia tend to experience more intense and frequent bleeding, whereas those with mild haemophilia typically exhibit milder symptoms unless subjected to surgical procedures or significant trauma. Those with moderate haemophilia may display variable symptoms, falling within the spectrum between severe and mild forms. | ||
| One common early indicator of haemophilia is prolonged bleeding from ] or ]s. These signs often prompt blood tests that confirm the presence of haemophilia.<ref>{{Cite book|url=https://books.google.com/books?id=hcfvCQAAQBAJ|title=Neonatology at a Glance|last1=Lissauer|first1=Tom|last2=Fanaroff|first2=Avroy A.|last3=Miall|first3=Lawrence|last4=Fanaroff|first4=Jonathan|date=2015-06-10|publisher=John Wiley & Sons|page=135|isbn=9781118767429|language=en}}</ref> In individuals, especially those with moderate or mild haemophilia, any form of trauma can trigger the first significant bleed. Haemophilia substantially elevates the risk of protracted bleeding from ordinary injuries, and in severe cases, bleeding can occur spontaneously without an apparent cause. Bleeding episodes can manifest anywhere in the body. Superficial bleeding resulting from abrasions or shallow lacerations may persist, with scabs easily breaking due to the deficiency of ], potentially leading to re-bleeding.<ref name="gen" /> While superficial bleeding poses challenges, more critical sites of bleeding include:<ref name="nih2" /> | One common early indicator of haemophilia is prolonged bleeding from ] or ]s. These signs often prompt blood tests that confirm the presence of haemophilia.<ref>{{Cite book|url=https://books.google.com/books?id=hcfvCQAAQBAJ|title=Neonatology at a Glance|last1=Lissauer|first1=Tom|last2=Fanaroff|first2=Avroy A.|last3=Miall|first3=Lawrence|last4=Fanaroff|first4=Jonathan|date=2015-06-10|publisher=John Wiley & Sons|page=135|isbn=9781118767429|language=en}}</ref> In individuals, especially those with moderate or mild haemophilia, any form of trauma can trigger the first significant bleed. Haemophilia substantially elevates the risk of protracted bleeding from ordinary injuries, and in severe cases, bleeding can occur spontaneously without an apparent cause. Bleeding episodes can manifest anywhere in the body. Superficial bleeding resulting from abrasions or shallow lacerations may persist, with scabs easily breaking due to the deficiency of ], potentially leading to re-bleeding.<ref name="gen" /> While superficial bleeding poses challenges, more critical sites of bleeding include:<ref name="nih2" /> | ||
| Line 67: | Line 67: | ||
| ] | ] | ||
| ] for self-treatment.]] | ] for self-treatment.]] | ||
| Most individuals with severe haemophilia require regular supplementation with ] ] concentrate ] or ], a ] (VWF) independent, recombinant DNA-derived Factor VIII (FVIII) concentrate shown to prevent bleeding in children and adults.<ref>{{Cite journal |last1=von Drygalski |first1=Annette |last2=Chowdary |first2=Pratima |last3=Kulkarni |first3=Roshni |last4=Susen |first4=Sophie |last5=Konkle |first5=Barbara A. |last6=Oldenburg |first6=Johannes |last7=Matino |first7=Davide |last8=Klamroth |first8=Robert |last9=Weyand |first9=Angela C. |last10=Jimenez-Yuste |first10=Victor |last11=Nogami |first11=Keiji |last12=Poloskey |first12=Stacey |last13=Winding |first13=Bent |last14=Willemze |first14=Annemieke |last15=Knobe |first15=Karin |date=2023-01-26 |title=Efanesoctocog Alfa Prophylaxis for Patients with Severe Hemophilia A |url=http://www.nejm.org/doi/10.1056/NEJMoa2209226 |journal=New England Journal of Medicine |language=en |volume=388 |issue=4 |pages=310–318 |doi=10.1056/NEJMoa2209226 |pmid=36720133 |issn=0028-4793}}</ref><ref>{{Cite journal |last1=Malec |first1=Lynn |last2=Peyvandi |first2=Flora |last3=Chan |first3=Anthony K.C. |last4=Königs |first4=Christoph |last5=Zulfikar |first5=Bulent |last6=Yuan |first6=Huixing |last7=Simpson |first7=Mindy |last8=Álvarez Román |first8=Maria Teresa |last9=Carcao |first9=Manuel |last10=Staber |first10=Janice M. |last11=Dunn |first11=Amy L. |last12=Chou |first12=Sheng-Chieh |last13=d'Oiron |first13=Roseline |last14=Albisetti |first14=Manuela |last15=Demissie |first15=Marek |date=2024-07-18 |title=Efanesoctocog Alfa Prophylaxis for Children with Severe Hemophilia A |url=http://www.nejm.org/doi/10.1056/NEJMoa2312611 |journal=New England Journal of Medicine |language=en |volume=391 |issue=3 |pages=235–246 |doi=10.1056/NEJMoa2312611 |pmid=39018533 |issn=0028-4793}}</ref> Treatment dosing and frequency of plasma concentrate Factor VIII may be variable and individually determined;<ref name="nih2">{{Cite web|url=http://www.nhlbi.nih.gov/health/health-topics/topics/hemophilia/treatment|title=How Is Hemophilia Treated? - NHLBI, NIH|website=www.nhlbi.nih.gov|access-date=2016-07-08}}</ref> dosing of efanesoctocog alfa shown effective is an IV injection of once-weekly 50 IU per kilogram of body weight. | |||
| ⚫ | |||
| ⚫ | In children, an easily accessible intravenous port<ref>{{Cite book|url=https://books.google.com/books?id=JeCD7cUVeTEC|title=Hemophilia and Hemostasis: A Case-Based Approach to Management|last1=Ma|first1=Alice D.|last2=Roberts|first2=Harold R.|last3=Escobar|first3=Miguel A.|date=2012-10-03|publisher=John Wiley & Sons|isbn=9781118439302|language=en}}Google books no page</ref> may have to be inserted to minimise frequent traumatic intravenous ]. These devices have made ] in haemophilia much easier for families because the problems of finding a vein for ] several times a week are eliminated. However, there are risks involved with their use, the most worrisome being that of infection, studies differ but some show an infection rate that is high.<ref>{{Cite journal|last1=Santagostino|first1=Elena|last2=Mancuso|first2=Maria Elisa|date=2008-09-01|title=Barriers to primary prophylaxis in haemophilic children: the issue of the venous access|journal=Blood Transfusion|volume=6|issue=Suppl 2|pages=s12–s16|doi=10.2450/2008.0031-08|issn=1723-2007|pmc=2652218|pmid=19105504}}</ref> These infections can usually be treated with ] antibiotics but sometimes the device must be removed,<ref>{{cite web|title=Guidelines for the Prevention of Intravascular Catheter-Related Infections|url=https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5110a1.htm|website=www.cdc.gov|access-date=8 July 2016}}</ref> also, there are other studies that show a risk of clots forming at the tip of the catheter, rendering it useless. Some individuals with severe haemophilia, and most with moderate and mild haemophilia, treat only as needed without a regular prophylactic schedule.<ref>{{Cite journal|last=Ljung|first=Rolf|date=2007-09-01|title=The risk associated with indwelling catheters in children with haemophilia|journal=British Journal of Haematology|language=en|volume=138|issue=5|pages=580–586|doi=10.1111/j.1365-2141.2007.06703.x|issn=1365-2141|pmid=17686052|s2cid=25748135|doi-access=free}}</ref> Mild haemophiliacs often manage their condition with ], a drug which releases stored factor VIII from blood vessel walls.<ref>{{Cite journal|last1=Franchini|first1=Massimo|last2=Lippi|first2=Giuseppe|date=2011-10-01|title=The use of desmopressin in acquired haemophilia A: a systematic review|journal=Blood Transfusion|volume=9|issue=4|pages=377–382|doi=10.2450/2011.0113-10|issn=1723-2007|pmc=3200405|pmid=21839010}}</ref> | ||
| === Dental considerations === | === Dental considerations === | ||
| If numbing is necessary for dental procedures, the nerve block (typically to the inferior alveolar nerve) should only be given after raising clotting factor levels by appropriate replacement therapy, as there is a risk of bleeding into the muscles along with potential airway compromise due to a haematoma in the retromolar or pterygoid space. The intraligamental technique or interosseous technique should be considered instead of the mandibular block. Articaine has been used as a buccal infiltration to anaesthetize the lower molar teeth. A lingual infiltration also requires appropriate factor replacement since the injection is into an area with a rich plexus of blood vessels and the needle is not adjacent to bone.<ref>''Andrew Brewer, Maria Elvira Correa (May 2006). "Guildelines for Dental Treatment of Patients with Inherited Bleeding Disorders" (PDF). Treatment of Hemophilia. '''40''': 9 – via World Federation of Hemophilia (WFH).''</ref> | |||
| ===Gene therapy=== | ===Gene therapy=== | ||
| In December 2017, it was reported that doctors had used a new form of ] to treat haemophilia A.<ref>{{cite web|url=https://www.bartshealth.nhs.uk/news/groundbreaking-gene-therapy-trial-set-to-cure-haemophilia-a-2529|title=Groundbreaking gene therapy trial set to cure haemophilia A|date=14 December 2017|work=Barts Health NHS Trust|access-date=14 December 2017}}</ref><ref>{{cite web|url=https://www.bbc.com/news/health-42337396|title=Haemophilia A trial results 'mind-blowing'|date=14 December 2017|work=BBC|access-date=14 December 2017}}</ref><ref>{{cite journal|title=AAV5–Factor VIII Gene Transfer in Severe Hemophilia A|date=16 December 2017|journal=New England Journal of Medicine|volume=377|issue=26|pages=2519–2530|doi=10.1056/NEJMoa1708483|pmid=29224506|last1 = Rangarajan|first1 = Savita|last2=Walsh|first2=Liron|last3=Lester|first3=Will|last4=Perry|first4=David|last5=Madan|first5=Bella|last6=Laffan|first6=Michael|last7=Yu|first7=Hua|last8=Vettermann|first8=Christian|last9=Pierce|first9=Glenn F.|last10=Wong|first10=Wing Y.|last11=Pasi|first11=K. John|hdl=10044/1/57163|url=http://qmro.qmul.ac.uk/xmlui/handle/123456789/31880|doi-access=free|hdl-access=free}}</ref> Current treatment efforts utilize adeno-associated virus (AAV) vectors, however recent studies have found lentiviral vectors (LV) as being a more effective alternative. <ref>{{Cite journal |last1=Marchesini |first1=Emanuela |last2=Morfini |first2=Massimo |last3=Valentino |first3=Leonard |date=2021-06-15 |title=Recent Advances in the Treatment of Hemophilia: A Review |journal=Biologics: Targets and Therapy |language=English |volume=15 |pages=221–235 |doi=10.2147/BTT.S252580|doi-access=free |pmid=34163136 |pmc=8214539 }}</ref><ref>{{Cite journal |last1=Milani |first1=Michela |last2=Canepari |first2=Cesare |last3=Assanelli |first3=Simone |last4=Merlin |first4=Simone |last5=Borroni |first5=Ester |last6=Starinieri |first6=Francesco |last7=Biffi |first7=Mauro |last8=Russo |first8=Fabio |last9=Fabiano |first9=Anna |last10=Zambroni |first10=Desirèe |last11=Annoni |first11=Andrea |last12=Naldini |first12=Luigi |last13=Follenzi |first13=Antonia |last14=Cantore |first14=Alessio |date=2024-04-29 |title=GP64-pseudotyped lentiviral vectors target liver endothelial cells and correct hemophilia A mice |journal=EMBO Molecular Medicine |language=en |volume=16 |issue=6 |pages=1427–1450 |doi=10.1038/s44321-024-00072-8 |issn=1757-4684 |pmc=11178766 |pmid=38684862}}</ref> | |||
| Hemophilia is an excellent candidate for gene therapy: it is caused by a single gene, the gene has been cloned, tests to measure factor levels are readily available and, most importantly, the level of factor VIII does not need to be tightly regulated and there are beneficial effects over a wide range of factor VIII activity levels: from a few percent to over 150%. This latter point is important because an individual's response to gene therapy cannot be accurately predicted. <ref>Advances in gene therapy for hemophilia: basis, current status and future perspectives https://link.springer.com/article/10.1007/s12185-018-2513-4</ref> | |||
| BioMarin Pharmaceutical Inc.’s ROCTAVIAN™ (valoctocogene roxaparvovec-rvox), is the first gene therapy to be marketed for adults with severe hemophilia A. BioMarin was granted conditional marketing authorization for ROCTAVIAN by the European Commission on August 24, 2022 and approved by the US Food and Drug Administration (FDA) on June 29, 2023. <ref>FDA Approves First Gene Therapy for Adults with Severe Hemophilia A https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-adults-severe-hemophilia </ref> | |||
| ROCTAVIAN uses a vector (a virus modified to deliver a gene therapy product), derived from an adeno-associated virus serotype 5 (AAV5), which has been stripped of most of its DNA so as to not cause disease. The vector delivers a working copy of the FVIII gene to liver cells, enabling the liver to produce FVIII on its own. ROCTAVIAN is a one-time gene therapy that involves gene-addition: it adds a working copy of the FVIII gene to cells and it is not incorporated into the cells’ genome nor does it modify an individual’s DNA. | |||
| === Monoclonal antibodies === | === Monoclonal antibodies === | ||
| ] ] has been approved by the FDA in 2017 for therapy of hemophilia A.<ref>{{DailyMed|2483adba-fab6-4d1b-96c5-c195577ed071|HEMLIBRA- emicizumab injection, solution}}</ref> | ] ] has been approved by the FDA in 2017 for therapy of hemophilia A.<ref>{{DailyMed|2483adba-fab6-4d1b-96c5-c195577ed071|HEMLIBRA- emicizumab injection, solution}}</ref> | ||
| In July 2024, a recent study published in the ] demonstrated that ], a bioengineered human ] recombinant protein, prophylaxis for children with severe hemophilia A could have therapeutic benefit leading to effective bleeding prevention.<ref>{{Cite journal |last1=Malec |first1=Lynn |last2=Peyvandi |first2=Flora |last3=Chan |first3=Anthony K.C. |last4=Königs |first4=Christoph |last5=Zulfikar |first5=Bulent |last6=Yuan |first6=Huixing |last7=Simpson |first7=Mindy |last8=Álvarez Román |first8=Maria Teresa |last9=Carcao |first9=Manuel |last10=Staber |first10=Janice M. |last11=Dunn |first11=Amy L. |last12=Chou |first12=Sheng-Chieh |last13=d’Oiron |first13=Roseline |last14=Albisetti |first14=Manuela |last15=Demissie |first15=Marek |date=2024-07-18 |title=Efanesoctocog Alfa Prophylaxis for Children with Severe Hemophilia A |url=http://www.nejm.org/doi/10.1056/NEJMoa2312611 |journal=New England Journal of Medicine |language=en |volume=391 |issue=3 |pages=235–246 |doi=10.1056/NEJMoa2312611 |pmid=39018533 |issn=0028-4793}}</ref><ref>{{Cite journal |last=Chowdary |first=Pratima |date=2024-07-18 |editor-last=Phimister |editor-first=Elizabeth G. |title=Bioengineered Factor VIII — More Innovation for Hemophilia A |url=http://www.nejm.org/doi/10.1056/NEJMe2313795 |journal=New England Journal of Medicine |language=en |volume=391 |issue=3 |pages=277–282 |doi=10.1056/NEJMe2313795 |pmid=39018538 |issn=0028-4793}}</ref> | |||
| == Prognosis == | == Prognosis == | ||
| Two Dutch studies have followed haemophilia patients for a number of years.<ref>{{Cite journal|last=Triemstra|first=Mattanja|date=1995-12-01|title=Mortality in Patients with Hemophilia: Changes in a Dutch Population from 1986 to 1992 and 1973 to 1986|journal=Annals of Internal Medicine|volume=123|issue=11|pages=823–7|doi=10.7326/0003-4819-123-11-199512010-00002|pmid=7486463|s2cid=22008880|issn=0003-4819 |
Two Dutch studies have followed haemophilia patients for a number of years.<ref>{{Cite journal|last=Triemstra|first=Mattanja|date=1995-12-01|title=Mortality in Patients with Hemophilia: Changes in a Dutch Population from 1986 to 1992 and 1973 to 1986|journal=Annals of Internal Medicine|volume=123|issue=11|pages=823–7|doi=10.7326/0003-4819-123-11-199512010-00002|pmid=7486463|s2cid=22008880|issn=0003-4819}}</ref><ref name=":0">{{Cite journal|last1=Plug|first1=I.|last2=Van Der Bom|first2=J. G.|last3=Peters|first3=M.|last4=Mauser-Bunschoten|first4=E. P.|last5=De Goede-Bolder|first5=A.|last6=Heijnen|first6=L.|last7=Smit|first7=C.|last8=Willemse|first8=J.|last9=Rosendaal|first9=F. R.|date=2006-03-01|title=Mortality and causes of death in patients with hemophilia, 1992–2001: a prospective cohort study1|journal=Journal of Thrombosis and Haemostasis|language=en|volume=4|issue=3|pages=510–516|doi=10.1111/j.1538-7836.2006.01808.x|pmid=16460432|issn=1538-7836|hdl=1887/5021|s2cid=13651790|hdl-access=free}}</ref> Both studies found that ] were common in haemophiliacs due to the frequent ]s which put them at risk of acquiring ], such as ], ] and ]. In the latest study which followed patients from 1992 to 2001, the male life expectancy was 59 years. If cases with known viral infections were excluded, the life expectancy was 72, close to that of the general population. 26% of the cases died from AIDS and 22% from hepatitis C.<ref name=":0" /> However, these statistics for prognosis are unreliable as there has been marked improvement of ] and efficacy of ] since these studies were done.{{citation needed|date=August 2018}} | ||
| ==Epidemiology== | ==Epidemiology== | ||
| Line 104: | Line 104: | ||
| * {{cite book|last1=Roberts|first1=Harold R.|title=Haemophilia and Haemostasis: A Case-based Approach to Management|publisher=John Wiley & Sons|isbn=9780470766439|url=https://books.google.com/books?id=Qz2XlnVC1f8C&q=Haemophilia+A++diagnosis&pg=PA37|access-date=8 July 2016|language=en|date=2008-04-15}} | * {{cite book|last1=Roberts|first1=Harold R.|title=Haemophilia and Haemostasis: A Case-based Approach to Management|publisher=John Wiley & Sons|isbn=9780470766439|url=https://books.google.com/books?id=Qz2XlnVC1f8C&q=Haemophilia+A++diagnosis&pg=PA37|access-date=8 July 2016|language=en|date=2008-04-15}} | ||
| * {{cite journal|last1=Collins|first1=Peter|last2=Baudo|first2=Francesco|last3=Huth-Kühne|first3=Angela|last4=Ingerslev|first4=Jørgen|last5=Kessler|first5=Craig M|last6=Castellano|first6=Maria E Mingot|last7=Shima|first7=Midori|last8=St-Louis|first8=Jean|last9=Lévesque|first9=Hervé|title=Consensus recommendations for the diagnosis and treatment of acquired hemophilia A|journal=BMC Research Notes|date=7 June 2010|volume=3|pages=161|doi=10.1186/1756-0500-3-161|issn=1756-0500|pmc=2896368|pmid=20529258 |doi-access=free }} | * {{cite journal|last1=Collins|first1=Peter|last2=Baudo|first2=Francesco|last3=Huth-Kühne|first3=Angela|last4=Ingerslev|first4=Jørgen|last5=Kessler|first5=Craig M|last6=Castellano|first6=Maria E Mingot|last7=Shima|first7=Midori|last8=St-Louis|first8=Jean|last9=Lévesque|first9=Hervé|title=Consensus recommendations for the diagnosis and treatment of acquired hemophilia A|journal=BMC Research Notes|date=7 June 2010|volume=3|pages=161|doi=10.1186/1756-0500-3-161|issn=1756-0500|pmc=2896368|pmid=20529258 |doi-access=free }} | ||
| * {{cite journal|last1=Coppola|first1=Antonio|last2=Windyga|first2=Jerzy|last3=Tufano|first3=Antonella|last4=Yeung|first4=Cindy|last5=Di Minno|first5=Matteo Nicola Dario|title=Treatment for preventing bleeding in people with haemophilia or other congenital bleeding disorders undergoing surgery|journal=Cochrane Database of Systematic Reviews|issue=2|pages=CD009961|date=9 February 2015|doi=10.1002/14651858.cd009961.pub2|pmid=25922858|language=en|doi-access=}} | * {{cite journal|last1=Coppola|first1=Antonio|last2=Windyga|first2=Jerzy|last3=Tufano|first3=Antonella|last4=Yeung|first4=Cindy|last5=Di Minno|first5=Matteo Nicola Dario|title=Treatment for preventing bleeding in people with haemophilia or other congenital bleeding disorders undergoing surgery|journal=Cochrane Database of Systematic Reviews|issue=2|pages=CD009961|date=9 February 2015|doi=10.1002/14651858.cd009961.pub2|pmid=25922858|language=en|doi-access=|pmc=11245682}} | ||
| * {{cite journal|last1=Kellogg|first1=TF|title=Steroid balance and tissue cholesterol accumulation in germfree and conventional rats fed diets containing saturated and polyunsaturated fats.|journal=Journal of Lipid Research|date=November 1974|volume=15|issue=6|pages=574–9|pmid=4430880|issn=0253-0716}} | * {{cite journal|last1=Kellogg|first1=TF|title=Steroid balance and tissue cholesterol accumulation in germfree and conventional rats fed diets containing saturated and polyunsaturated fats.|journal=Journal of Lipid Research|date=November 1974|volume=15|issue=6|pages=574–9|pmid=4430880|issn=0253-0716}} | ||
| * {{cite journal|last1=Armstrong|first1=Elina|last2=Hillarp|first2=Andreas|title=Assay discrepancy in mild haemophilia A|journal=European Journal of Haematology. Supplementum|volume=76|pages=48–50|doi=10.1111/ejh.12374|issn=0902-4506|pmid=24957107|year=2014|s2cid=205100329|doi-access=}} | * {{cite journal|last1=Armstrong|first1=Elina|last2=Hillarp|first2=Andreas|title=Assay discrepancy in mild haemophilia A|journal=European Journal of Haematology. Supplementum|volume=76|pages=48–50|doi=10.1111/ejh.12374|issn=0902-4506|pmid=24957107|year=2014|s2cid=205100329|doi-access=}} | ||
| Line 111: | Line 111: | ||
| {{Medical resources | {{Medical resources | ||
| | DiseasesDB = 5555 | | DiseasesDB = 5555 | ||
| | ICD11 = {{ICD11|3B10}} | |||
| | ICD10 = {{ICD10|D|66||d|65}} | | ICD10 = {{ICD10|D|66||d|65}} | ||
| | ICD9 = {{ICD9|286.0}} | | ICD9 = {{ICD9|286.0}} | ||
Latest revision as of 17:50, 24 October 2024
This article is about the bleeding disorder with factor VIII deficiency. For the disorder with factor IX deficiency, see haemophilia B.Medical condition
| Haemophilia A | |
|---|---|
| Other names | Hemophilia A |
 | |
| Protein structure of coagulation factor VIII, of which its deficiency is the cause of haemophilia A. | |
| Specialty | Haematology |
| Symptoms | Prolonged bleeding from common injuries |
| Causes | Factor VIII deficiency |
| Diagnostic method | Bleeding time, coagulation screen, genetic testing |
| Prevention | Hepatitis B vaccine should be considered |
| Treatment | Factor VIII, factor VIII inhibitors, emicizumab |
Haemophilia A (or hemophilia A) is a blood clotting disorder caused by a genetic deficiency in clotting factor VIII, thereby resulting in significant susceptibility to bleeding, both internally and externally. This condition occurs almost exclusively in males born to carrier mothers due to X-linked recessive inheritance. Nevertheless, rare isolated cases do emerge from de novo (spontaneous) mutations.
The medical management of individuals with hemophilia A frequently entails the administration of factor VIII medication through slow intravenous injection. This intervention aims to address and preempt additional bleeding episodes in affected individuals.
Signs and symptoms
Haemophilia A's phenotype has a quite wide range of symptoms encompassing both internal and external bleeding episodes. Individuals with more severe haemophilia tend to experience more intense and frequent bleeding, whereas those with mild haemophilia typically exhibit milder symptoms unless subjected to surgical procedures or significant trauma. Those with moderate haemophilia may display variable symptoms, falling within the spectrum between severe and mild forms.
One common early indicator of haemophilia is prolonged bleeding from venepuncture or heelpricks. These signs often prompt blood tests that confirm the presence of haemophilia. In individuals, especially those with moderate or mild haemophilia, any form of trauma can trigger the first significant bleed. Haemophilia substantially elevates the risk of protracted bleeding from ordinary injuries, and in severe cases, bleeding can occur spontaneously without an apparent cause. Bleeding episodes can manifest anywhere in the body. Superficial bleeding resulting from abrasions or shallow lacerations may persist, with scabs easily breaking due to the deficiency of fibrin, potentially leading to re-bleeding. While superficial bleeding poses challenges, more critical sites of bleeding include:
Complications
One therapeutic conundrum is the development of inhibitor antibodies against factor VIII due to frequent infusions. These develop as the body recognises the infused factor VIII as foreign, as the body does not produce its own copy. In these individuals, activated factor VII, a protein in the extrinsic pathway of the coagulation cascade, can be infused as a treatment for haemorrhage in individuals with haemophilia and antibodies against replacement factor VIII.
Oral Manifestations
The oral manifestations are characterized by frequent bleeding of multiple sites, frequently seen as gingival and postextraction haemorrhages. The symptoms depend on the severity of haemophilia. In the case of severe haemophilia, patients may complain of multiple oral bleeding episodes throughout their life. Haemophilia patients are considered to be a special group of patients as routinely done procedures may be fatal in them. It was seen that almost 14% of all haemophilia patients and 30% of cases with a mild type of haemophilia have been diagnosed early following an episode of severe oral bleeding, of which the most common sites were the labial frenum and the tongue.
Genetics
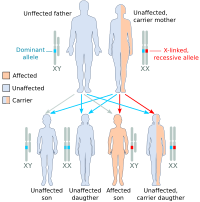
Haemophilia A is inherited as an X-linked recessive trait. It occurs in males and in homozygous females (which is only possible in the daughters of a haemophilic male and a carrier or haemophiliac female). However, mild haemophilia A is known to occur in heterozygous females due to X-inactivation, so it is recommended that levels of factor VIII and IX be measured in all known or potential carriers prior to surgery and in the event of clinically significant bleeding.
About 5-10% of people with haemophilia A are affected because they make a dysfunctional version of the factor VIII protein, while the remainder are affected because they produce factor VIII in insufficient amounts (quantitative deficiency). Of those who have severe deficiency (defined as <1% activity of factor VIII), 45-50% have the same mutation, an inversion within the factor VIII gene that results in total elimination of protein production.
Since both forms of haemophilia can be caused by a variety of different mutations, initial diagnosis and classification is done by measurement of protein activity rather than by genetic tests, though genetic tests are recommended for testing of family members once a known case of haemophilia is identified. Approximately 30% of patients have no family history; their disease is presumably caused by new mutations.
Diagnosis
The diagnosis of haemophilia A may be suspected as coagulation testing reveals an increased partial thromboplastin time (PTT) in the context of a normal prothrombin time (PT) and bleeding time. PTT tests are the first blood test done when haemophilia is indicated. However, the diagnosis is made in the presence of very low levels of factor VIII. A family history is frequently present, although not essential. Recently, genetic testing has been made available to determine an individual's risk of attaining or passing on haemophilia. Diagnosis of haemophilia A also includes a severity level, which can range from mild to severe based on the amount of active and functioning factor VIII detected in the blood. Factor VIII levels do not typically change throughout an individual's lifetime. Severe haemophilia A is the most common severity, occurring in the majority of affected people. Individuals with mild haemophilia often experience few or no bleeding episodes except in the case of serious trauma (i.e. tooth extraction, or surgery).
Severity
There are numerous different mutations which can cause haemophilia A, due to differences in changes to the factor VIII gene (and the resulting protein). Individuals with haemophilia often have some level of active clotting factor. Individuals with less than 1% active factor are classified as having severe haemophilia, those with 1–5% active factor have moderate haemophilia, and those with mild haemophilia have between 5–40% of normal levels of active clotting factor.
Differential diagnosis
Two of the most common differential diagnoses are haemophilia B which is a deficiency in Factor IX and von Willebrand Disease which is a deficiency in von Willebrand factor (needed for the proper functioning of Factor VIII); haemophilia C is also considered.
Treatment


Most individuals with severe haemophilia require regular supplementation with intravenous plasma concentrate factor VIII or efanesoctocog alfa, a von Willebrand factor (VWF) independent, recombinant DNA-derived Factor VIII (FVIII) concentrate shown to prevent bleeding in children and adults. Treatment dosing and frequency of plasma concentrate Factor VIII may be variable and individually determined; dosing of efanesoctocog alfa shown effective is an IV injection of once-weekly 50 IU per kilogram of body weight.
In children, an easily accessible intravenous port may have to be inserted to minimise frequent traumatic intravenous cannulation. These devices have made prophylaxis in haemophilia much easier for families because the problems of finding a vein for infusion several times a week are eliminated. However, there are risks involved with their use, the most worrisome being that of infection, studies differ but some show an infection rate that is high. These infections can usually be treated with intravenous antibiotics but sometimes the device must be removed, also, there are other studies that show a risk of clots forming at the tip of the catheter, rendering it useless. Some individuals with severe haemophilia, and most with moderate and mild haemophilia, treat only as needed without a regular prophylactic schedule. Mild haemophiliacs often manage their condition with desmopressin, a drug which releases stored factor VIII from blood vessel walls.
Dental considerations
If numbing is necessary for dental procedures, the nerve block (typically to the inferior alveolar nerve) should only be given after raising clotting factor levels by appropriate replacement therapy, as there is a risk of bleeding into the muscles along with potential airway compromise due to a haematoma in the retromolar or pterygoid space. The intraligamental technique or interosseous technique should be considered instead of the mandibular block. Articaine has been used as a buccal infiltration to anaesthetize the lower molar teeth. A lingual infiltration also requires appropriate factor replacement since the injection is into an area with a rich plexus of blood vessels and the needle is not adjacent to bone.
Gene therapy
In December 2017, it was reported that doctors had used a new form of gene therapy to treat haemophilia A. Current treatment efforts utilize adeno-associated virus (AAV) vectors, however recent studies have found lentiviral vectors (LV) as being a more effective alternative.
Monoclonal antibodies
Monoclonal antibody emicizumab has been approved by the FDA in 2017 for therapy of hemophilia A.
In July 2024, a recent study published in the New England Journal of Medicine demonstrated that efanesoctocog alfa, a bioengineered human factor VIII recombinant protein, prophylaxis for children with severe hemophilia A could have therapeutic benefit leading to effective bleeding prevention.
Prognosis
Two Dutch studies have followed haemophilia patients for a number of years. Both studies found that viral infections were common in haemophiliacs due to the frequent blood transfusions which put them at risk of acquiring blood borne infections, such as HIV, hepatitis B and hepatitis C. In the latest study which followed patients from 1992 to 2001, the male life expectancy was 59 years. If cases with known viral infections were excluded, the life expectancy was 72, close to that of the general population. 26% of the cases died from AIDS and 22% from hepatitis C. However, these statistics for prognosis are unreliable as there has been marked improvement of infection control and efficacy of anti-retroviral drugs since these studies were done.
Epidemiology
Haemophilia A occurs in approximately 1 in 5,000 males, while the incidence of haemophilia B is 1 in 30,000 in the male population, of these, 85% have haemophilia A and 15% have haemophilia B.
See also
References
- ^ Konkle, Barbara A.; Josephson, Neil C.; Nakaya Fletcher, Shelley (1993-01-01). Pagon, Roberta A.; Adam, Margaret P.; Ardinger, Holly H.; Wallace, Stephanie E.; Amemiya, Anne; Bean, Lora J.H.; Bird, Thomas D.; Fong, Chin-To; Mefford, Heather C. (eds.). Hemophilia A. Seattle (WA): University of Washington, Seattle. PMID 20301578.update 2014
- ^ "Hemophilia A: MedlinePlus Medical Encyclopedia". www.nlm.nih.gov. Retrieved 24 June 2016.
- ^ "Haemophilia A (Factor VIII Deficiency) information | Patient". Patient. Retrieved 24 June 2016.
- World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 259–60. hdl:10665/44053. ISBN 9789241547659.
- Lissauer, Tom; Fanaroff, Avroy A.; Miall, Lawrence; Fanaroff, Jonathan (2015-06-10). Neonatology at a Glance. John Wiley & Sons. p. 135. ISBN 9781118767429.
- ^ "How Is Hemophilia Treated? - NHLBI, NIH". www.nhlbi.nih.gov. Retrieved 2016-07-08.
- Ma, Alice D.; Carrizosa, Daniel (2006-01-01). "Acquired Factor VIII Inhibitors: Pathophysiology and Treatment". ASH Education Program Book. 2006 (1): 432–437. doi:10.1182/asheducation-2006.1.432. ISSN 1520-4391. PMID 17124095.
- Sonu Acharya, Karishma Rathore, Upasana Mahapatra, Sashikant Sethi, Nikita Sahu (2018). "An unusual oral manifestation of hemophilia in a child". Journal of International Oral Health. Retrieved 2019-12-20.
- Nair, Preethi S.; Shetty, S.; Ghosh, Kanjaksha (2012-01-01). "A homozygous female hemophilia A". Indian Journal of Human Genetics. 18 (1): 134–136. doi:10.4103/0971-6866.96685. ISSN 0971-6866. PMC 3385172. PMID 22754241.
- ^ Kliegman, Robert (2011). Nelson textbook of pediatrics (19th ed.). Philadelphia: Saunders. pp. 1700–1. ISBN 978-1-4377-0755-7.
- Bowen, D J (2002). "Haemophilia A and haemophilia B: molecular insights". Molecular Pathology. 55 (1): 1–18. doi:10.1136/mp.55.1.1. PMC 1187139. PMID 11836440.
- "OMIM Entry - # 306700 - HEMOPHILIA A; HEMA". omim.org. Retrieved 2016-07-08.
- Hemophilia A at eMedicine
- "Von Willebrand's Disease. About Von Willebrand's Disease | Patient". Patient. Retrieved 2016-07-08.
- von Drygalski, Annette; Chowdary, Pratima; Kulkarni, Roshni; Susen, Sophie; Konkle, Barbara A.; Oldenburg, Johannes; Matino, Davide; Klamroth, Robert; Weyand, Angela C.; Jimenez-Yuste, Victor; Nogami, Keiji; Poloskey, Stacey; Winding, Bent; Willemze, Annemieke; Knobe, Karin (2023-01-26). "Efanesoctocog Alfa Prophylaxis for Patients with Severe Hemophilia A". New England Journal of Medicine. 388 (4): 310–318. doi:10.1056/NEJMoa2209226. ISSN 0028-4793. PMID 36720133.
- Malec, Lynn; Peyvandi, Flora; Chan, Anthony K.C.; Königs, Christoph; Zulfikar, Bulent; Yuan, Huixing; Simpson, Mindy; Álvarez Román, Maria Teresa; Carcao, Manuel; Staber, Janice M.; Dunn, Amy L.; Chou, Sheng-Chieh; d'Oiron, Roseline; Albisetti, Manuela; Demissie, Marek (2024-07-18). "Efanesoctocog Alfa Prophylaxis for Children with Severe Hemophilia A". New England Journal of Medicine. 391 (3): 235–246. doi:10.1056/NEJMoa2312611. ISSN 0028-4793. PMID 39018533.
- Ma, Alice D.; Roberts, Harold R.; Escobar, Miguel A. (2012-10-03). Hemophilia and Hemostasis: A Case-Based Approach to Management. John Wiley & Sons. ISBN 9781118439302.Google books no page
- Santagostino, Elena; Mancuso, Maria Elisa (2008-09-01). "Barriers to primary prophylaxis in haemophilic children: the issue of the venous access". Blood Transfusion. 6 (Suppl 2): s12–s16. doi:10.2450/2008.0031-08. ISSN 1723-2007. PMC 2652218. PMID 19105504.
- "Guidelines for the Prevention of Intravascular Catheter-Related Infections". www.cdc.gov. Retrieved 8 July 2016.
- Ljung, Rolf (2007-09-01). "The risk associated with indwelling catheters in children with haemophilia". British Journal of Haematology. 138 (5): 580–586. doi:10.1111/j.1365-2141.2007.06703.x. ISSN 1365-2141. PMID 17686052. S2CID 25748135.
- Franchini, Massimo; Lippi, Giuseppe (2011-10-01). "The use of desmopressin in acquired haemophilia A: a systematic review". Blood Transfusion. 9 (4): 377–382. doi:10.2450/2011.0113-10. ISSN 1723-2007. PMC 3200405. PMID 21839010.
- Andrew Brewer, Maria Elvira Correa (May 2006). "Guildelines for Dental Treatment of Patients with Inherited Bleeding Disorders" (PDF). Treatment of Hemophilia. 40: 9 – via World Federation of Hemophilia (WFH).
- "Groundbreaking gene therapy trial set to cure haemophilia A". Barts Health NHS Trust. 14 December 2017. Retrieved 14 December 2017.
- "Haemophilia A trial results 'mind-blowing'". BBC. 14 December 2017. Retrieved 14 December 2017.
- Rangarajan, Savita; Walsh, Liron; Lester, Will; Perry, David; Madan, Bella; Laffan, Michael; Yu, Hua; Vettermann, Christian; Pierce, Glenn F.; Wong, Wing Y.; Pasi, K. John (16 December 2017). "AAV5–Factor VIII Gene Transfer in Severe Hemophilia A". New England Journal of Medicine. 377 (26): 2519–2530. doi:10.1056/NEJMoa1708483. hdl:10044/1/57163. PMID 29224506.
- Marchesini, Emanuela; Morfini, Massimo; Valentino, Leonard (2021-06-15). "Recent Advances in the Treatment of Hemophilia: A Review". Biologics: Targets and Therapy. 15: 221–235. doi:10.2147/BTT.S252580. PMC 8214539. PMID 34163136.
- Milani, Michela; Canepari, Cesare; Assanelli, Simone; Merlin, Simone; Borroni, Ester; Starinieri, Francesco; Biffi, Mauro; Russo, Fabio; Fabiano, Anna; Zambroni, Desirèe; Annoni, Andrea; Naldini, Luigi; Follenzi, Antonia; Cantore, Alessio (2024-04-29). "GP64-pseudotyped lentiviral vectors target liver endothelial cells and correct hemophilia A mice". EMBO Molecular Medicine. 16 (6): 1427–1450. doi:10.1038/s44321-024-00072-8. ISSN 1757-4684. PMC 11178766. PMID 38684862.
- HEMLIBRA- emicizumab injection, solution drug label/data at DailyMed from U.S. National Library of Medicine, National Institutes of Health.
- Malec, Lynn; Peyvandi, Flora; Chan, Anthony K.C.; Königs, Christoph; Zulfikar, Bulent; Yuan, Huixing; Simpson, Mindy; Álvarez Román, Maria Teresa; Carcao, Manuel; Staber, Janice M.; Dunn, Amy L.; Chou, Sheng-Chieh; d’Oiron, Roseline; Albisetti, Manuela; Demissie, Marek (2024-07-18). "Efanesoctocog Alfa Prophylaxis for Children with Severe Hemophilia A". New England Journal of Medicine. 391 (3): 235–246. doi:10.1056/NEJMoa2312611. ISSN 0028-4793. PMID 39018533.
- Chowdary, Pratima (2024-07-18). Phimister, Elizabeth G. (ed.). "Bioengineered Factor VIII — More Innovation for Hemophilia A". New England Journal of Medicine. 391 (3): 277–282. doi:10.1056/NEJMe2313795. ISSN 0028-4793. PMID 39018538.
- Triemstra, Mattanja (1995-12-01). "Mortality in Patients with Hemophilia: Changes in a Dutch Population from 1986 to 1992 and 1973 to 1986". Annals of Internal Medicine. 123 (11): 823–7. doi:10.7326/0003-4819-123-11-199512010-00002. ISSN 0003-4819. PMID 7486463. S2CID 22008880.
- ^ Plug, I.; Van Der Bom, J. G.; Peters, M.; Mauser-Bunschoten, E. P.; De Goede-Bolder, A.; Heijnen, L.; Smit, C.; Willemse, J.; Rosendaal, F. R. (2006-03-01). "Mortality and causes of death in patients with hemophilia, 1992–2001: a prospective cohort study1". Journal of Thrombosis and Haemostasis. 4 (3): 510–516. doi:10.1111/j.1538-7836.2006.01808.x. hdl:1887/5021. ISSN 1538-7836. PMID 16460432. S2CID 13651790.
Further reading
- Casana, P.; Cabrera, N.; Cid, A. R.; Haya, S.; Beneyto, M.; Espinos, C.; Cortina, V.; Dasi, M. A.; Aznar, J. A. (2008-07-01). "Severe and moderate hemophilia A: identification of 38 new genetic alterations". Haematologica. 93 (7): 1091–1094. doi:10.3324/haematol.12344. ISSN 0390-6078. PMID 18403393.
- Roberts, Harold R. (2008-04-15). Haemophilia and Haemostasis: A Case-based Approach to Management. John Wiley & Sons. ISBN 9780470766439. Retrieved 8 July 2016.
- Collins, Peter; Baudo, Francesco; Huth-Kühne, Angela; Ingerslev, Jørgen; Kessler, Craig M; Castellano, Maria E Mingot; Shima, Midori; St-Louis, Jean; Lévesque, Hervé (7 June 2010). "Consensus recommendations for the diagnosis and treatment of acquired hemophilia A". BMC Research Notes. 3: 161. doi:10.1186/1756-0500-3-161. ISSN 1756-0500. PMC 2896368. PMID 20529258.
- Coppola, Antonio; Windyga, Jerzy; Tufano, Antonella; Yeung, Cindy; Di Minno, Matteo Nicola Dario (9 February 2015). "Treatment for preventing bleeding in people with haemophilia or other congenital bleeding disorders undergoing surgery". Cochrane Database of Systematic Reviews (2): CD009961. doi:10.1002/14651858.cd009961.pub2. PMC 11245682. PMID 25922858.
- Kellogg, TF (November 1974). "Steroid balance and tissue cholesterol accumulation in germfree and conventional rats fed diets containing saturated and polyunsaturated fats". Journal of Lipid Research. 15 (6): 574–9. ISSN 0253-0716. PMID 4430880.
- Armstrong, Elina; Hillarp, Andreas (2014). "Assay discrepancy in mild haemophilia A". European Journal of Haematology. Supplementum. 76: 48–50. doi:10.1111/ejh.12374. ISSN 0902-4506. PMID 24957107. S2CID 205100329.
External links
| Classification | D |
|---|---|
| External resources |
| Disorders of bleeding and clotting | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clotting |
| ||||||||||||
| Bleeding |
| ||||||||||||
| X-linked disorders | |||||
|---|---|---|---|---|---|
|