| Revision as of 21:47, 25 June 2021 editTautomers (talk | contribs)Extended confirmed users948 edits →Ring expansion and ring contraction reactions: added to section briefly and removed note.← Previous edit | Latest revision as of 14:18, 13 November 2024 edit undoPreimage (talk | contribs)Extended confirmed users931 edits →Ring-closing reactions: Remove "other reactions, such as an amino group reacting with a hydroxy group, as in the biosynthesis of solanine", added in 08:30, 1 October 2022 (Solanine#Biosynthesis ring-closing product is an imine, not a secondary amine) + fix typo | ||
| (42 intermediate revisions by 17 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Molecule with a ring of bonded atoms}} | {{Short description|Molecule with a ring of bonded atoms}} | ||
| A '''cyclic compound''' (''ring compound'') is a term for a ] in the field of ] in which one or more series of atoms in the compound is connected to form a ]. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are ]s), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (] compounds). Depending on the ring size, the ] of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be ] or non-aromatic, in the latter case, they may vary from being fully ] to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the ]s of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., <17 total atoms) numbers in the many billions. | |||
| A '''cyclic compound''' (or '''ring compound''') is a term for a ] in the field of ] in which one or more series of atoms in the compound is connected to form a ]. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are ]), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (]s with rings containing both carbon and non-carbon). Depending on the ring size, the ] of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be ] or non-aromatic; in the latter case, they may vary from being fully ] to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the ]s of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the many billions. | |||
| <gallery caption="Cyclic compound examples: All-carbon (carbocyclic) and more complex ] cyclic compounds." widths="250 px" align=center> | |||
| Image: Ingenol.svg| ], a complex, ] ], related to but simpler than the ] that follows, which displays a complex ring structure including 3-, 5-, and 7-membered non-], carbocyclic rings. | |||
| <gallery caption="Cyclic compound examples: All-carbon (carbocyclic) and more complex ] cyclic compounds" widths="250 px" align="center"> | |||
| Image:First_four_cycloalkanes.png | ]s, the simplest ]s, including ], ], ], and ]. Note, elsewhere an ] shorthand is used where hydrogen atoms are inferred as present to fill the carbon's valence of 4 (rather than their being shown explicitly). | |||
| Image: |
Image: Ingenol.svg| ], a complex, ] ], related to but simpler than the ] that follows, which displays a complex ring structure including 3-, 5-, and 7-membered non-aromatic, carbocyclic rings. | ||
| Image:First four cycloalkanes - en.svg | ]s, the simplest carbocycles, including ], ], ], and ]. Note, elsewhere an ] shorthand is used where hydrogen atoms are inferred as present to fill the carbon's valence of 4 (rather than their being shown explicitly). | |||
| Image:Taxol.svg | ], another complex, plant-derived ], also a natural product, displaying a complex multi-ring structure including 4-, 6-, and 8-membered rings (carbocyclic and heterocyclic, ] and non-aromatic). | |||
| </gallery> | </gallery> | ||
| Adding to their complexity and number, closing of atoms into rings may lock particular atoms with distinct ] (by ]s) such that ] and ] of the compound results, including some manifestations that are unique to rings (e.g., ]). As well, depending on ring size, the three-dimensional shapes of particular cyclic structures—typically rings of 5-atoms and larger—can vary and interconvert such that ] is displayed. Indeed, the development of this important chemical concept arose, historically, in reference to cyclic compounds. Finally, cyclic compounds, because of the unique shapes, reactivities, properties, and ] that they engender, are the largest majority of all molecules involved in the biochemistry, structure, and function of ]s, and in the man-made molecules (e.g., drugs, herbicides, etc.). | |||
| Adding to their complexity and number, closing of atoms into rings may lock particular atoms with distinct ] (by ]s) such that ] and ] of the compound results, including some manifestations that are unique to rings (e.g., ]). As well, depending on ring size, the three-dimensional shapes of particular cyclic structures – typically rings of five atoms and larger – can vary and interconvert such that ] is displayed. Indeed, the development of this important chemical concept arose historically in reference to cyclic compounds. Finally, cyclic compounds, because of the unique shapes, reactivities, properties, and ] that they engender, are the majority of all molecules involved in the biochemistry, structure, and function of living ]s, and in man-made molecules such as drugs, pesticides, etc. | |||
| ==Structural introduction== | |||
| A ''cyclic compound or ring compound'' is a ] at least some of whose atoms are connected to form a ring.<ref>{{JerryMarch}}.{{page needed|date=June 2015}}</ref>{{rp|unknown}}{{page needed|date=June 2015}} Rings vary in size from 3 to many tens or even hundreds of atoms. Examples of ring compounds readily include cases where: | |||
| ==Structure and classification== | |||
| A cyclic compound or ring compound is a ] in which at least some its atoms are connected to form a ring.<ref>{{JerryMarch}}{{page needed|date=June 2015}}</ref> Rings vary in size from three to many tens or even hundreds of atoms. Examples of ring compounds readily include cases where: | |||
| * all the atoms are carbon (i.e., are ]s), | * all the atoms are carbon (i.e., are ]s), | ||
| * none of the atoms are carbon (inorganic cyclic compounds),<ref>{{cite journal |doi=10.1007/BF01141802 |title=Classification of inorganic cyclic compounds |journal=Journal of Structural Chemistry |volume=2 |issue=3 |pages=350–8 |year=1961 |last1=Halduc |first1=I. |s2cid=93804259 }}</ref> or where | * none of the atoms are carbon (inorganic cyclic compounds),<ref>{{cite journal |doi=10.1007/BF01141802 |title=Classification of inorganic cyclic compounds |journal=Journal of Structural Chemistry |volume=2 |issue=3 |pages=350–8 |year=1961 |last1=Halduc |first1=I. |s2cid=93804259 }}</ref> or where | ||
| * both carbon and non-carbon atoms are present (] compounds). | * both carbon and non-carbon atoms are present (] compounds with rings containing both carbon and non-carbon). | ||
| Common atoms can (as a result of their ]s) form varying numbers of bonds, and many common atoms readily form rings. In addition, depending on the ring size, the ] of the individual links between ring atoms, and their arrangements within the rings, cyclic compounds may be ] or non-aromatic; in the case of non-aromatic cyclic compounds, they may vary from being fully ] to having varying numbers of multiple bonds. As a consequence of the constitutional variability that is ] possible in cyclic structures, the number of possible cyclic structures, even of small size (e.g., <17 atoms) numbers in the many billions.<ref name = ReymondACR15>{{cite journal |doi=10.1021/ar500432k |pmid=25687211 |title=The Chemical Space Project |journal=Accounts of Chemical Research |volume=48 |issue=3 |pages=722–30 |year=2015 |last1=Reymond |first1=Jean-Louis |doi-access=free }}</ref> | Common atoms can (as a result of their ]s) form varying numbers of bonds, and many common atoms readily form rings. In addition, depending on the ring size, the ] of the individual links between ring atoms, and their arrangements within the rings, cyclic compounds may be ] or non-aromatic; in the case of non-aromatic cyclic compounds, they may vary from being fully ] to having varying numbers of multiple bonds. As a consequence of the constitutional variability that is ] possible in cyclic structures, the number of possible cyclic structures, even of small size (e.g., <17 atoms) numbers in the many billions.<ref name = ReymondACR15>{{cite journal |doi=10.1021/ar500432k |pmid=25687211 |title=The Chemical Space Project |journal=Accounts of Chemical Research |volume=48 |issue=3 |pages=722–30 |year=2015 |last1=Reymond |first1=Jean-Louis |doi-access=free }}</ref> | ||
| Moreover, the closing of atoms into rings may lock particular ]–] atoms into place, resulting in ] and ] being associated with the compound, including some manifestations that are unique to rings (e.g., ]);<ref name=Reusch10>William Reusch |
Moreover, the closing of atoms into rings may lock particular ]–] atoms into place, resulting in ] and ] being associated with the compound, including some manifestations that are unique to rings (e.g., ]);<ref name=Reusch10>{{cite book | author = William Reusch | date = 2010 | title = "Stereoisomers Part I" in ''Virtual Textbook of Organic Chemistry'' | publisher = Michigan State University | url = http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/sterisom.htm#start | access-date = 7 April 2015 | archive-date = 10 March 2015 | archive-url = https://web.archive.org/web/20150310162343/http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/sterisom.htm#start | url-status = live }}</ref> As well, depending on ring size, the three-dimensional shapes of particular cyclic structures — typically rings of five atoms and larger — can vary and interconvert such that ] is displayed.<ref name=Reusch10/> | ||
| == |
=== Carbocycles === | ||
| The vast majority of cyclic compounds are ], and of these, a significant and conceptually important portion are composed of rings made only of carbon atoms (i.e., they are carbocycles).{{citation needed|date=April 2015}} | |||
| ] nomenclature has extensive rules to cover the naming of cyclic structures, both as core structures, and as substituents appended to ] structures.{{vague|date=April 2015}}{{citation needed|date=April 2015}} The term ] is used when a ring-containing compound has a ring of 12 or more atoms.<ref name=StillTet81>{{cite journal |doi=10.1016/S0040-4020(01)93273-9 |title=Chemical consequences of conformation in macrocyclic compounds |journal=Tetrahedron |volume=37 |issue=23 |pages=3981–96 |year=1981 |last1=Still |first1=W.Clark |last2=Galynker |first2=Igor }}</ref><ref name=DunitzPersp68>J. D. Dunitz. Perspectives in Structural Chemistry (Edited by J. D. Dunitz and J. A. Ibers), Vol. 2, pp. l-70; Wiley, New York (1968)</ref> The term ] is used when more than one ring appears in a single molecule.{{citation needed|date=April 2015}} ] is formally a polycyclic compound, but is more specifically named as a bicyclic compound. Several examples of macrocyclic and polycyclic structures are given in the final gallery below. | |||
| ===Inorganic cyclic compounds=== | |||
| {{anchor|annular atom}} | |||
| Inorganic atoms form cyclic compounds as well. Examples include ] and ] (e.g. ] {{chem2|S7NH}}, ] {{chem2|(NSCl)3}}, ] {{chem2|S4N4}}), ] (e.g., ] {{chem2|(SiH2)5}}), ] and nitrogen (e.g., ] {{chem2|(NPCl2)3}}), phosphorus and ] (e.g., ]s {{chem2|(PO3−)3}} and other cyclic ] derivatives), ] and oxygen (e.g., ] {{chem2|Na3(BO2)3}}, ]), boron and nitrogen (e.g. ] {{chem2|(BN)3H6}}).{{citation needed|date=April 2015}} When carbon in benzene is "replaced" by other elements, e.g., as in ], ], ], ], and ], aromaticity is retained, and so ] are also known and well-characterized.{{citation needed|date=April 2015}} | |||
| The atoms that are part of the ring structure are called annular atoms.<ref>{{cite book|last1=Morris|first1=Christopher G.|last2=Press|first2=Academic|title=Academic Press Dictionary of Science and Technology|publisher=Gulf Professional Publishing|isbn=9780122004001|page=120|url=https://books.google.com/books?id=nauWlPTBcjIC&pg=PA120|language=en|year=1992}}</ref> | |||
| === Heterocyclic compounds === | |||
| ==Carbocycles== | |||
| A heterocyclic compound is a cyclic compound that has atoms of at least two different ] as members of its ring(s).<ref name=iupac>] </ref> Cyclic compounds that have both carbon and non-carbon atoms present are ] carbon compounds, and the name refers to inorganic cyclic compounds as well (e.g., ]s, which contain only ] and ] in the rings, and ]s, which contain only ] and ] in the rings).<ref name=iupac></ref> Hantzsch–Widman nomenclature is recommended by the IUPAC for naming heterocycles, but many common names remain in regular use.{{citation needed|date=April 2015}} | |||
| The vast majority of cyclic compounds are ], and of these, a significant and conceptually important portion are composed of rings made only of carbon atoms (i.e., they are carbocycles).{{citation needed|date=April 2015}} | |||
| === Macrocycles === | |||
| ==Inorganic cyclic compounds== | |||
| ] | |||
| Inorganic atoms form cyclic compounds as well. Examples include ], ] (e.g., in ]), ] (e.g., in ]s and ] variants), and ] (e.g., in triboric acid).{{citation needed|date=April 2015}} When carbon in benzene is "replaced" by other elements, e.g., as in ], ], ], ], and ], aromaticity is retained, and so ] are known and well-characterized.{{citation needed|date=April 2015}} | |||
| The term ] is used for compounds having a rings of 8 or more atoms.<ref name="StillTet81">{{cite journal |last1=Still |first1=W.Clark |last2=Galynker |first2=Igor |year=1981 |title=Chemical consequences of conformation in macrocyclic compounds |journal=Tetrahedron |volume=37 |issue=23 |pages=3981–96 |doi=10.1016/S0040-4020(01)93273-9}}</ref><ref name="DunitzPersp68">{{cite book |author=J. D. Dunitz |title=Perspectives in Structural Chemistry |date=1968 |publisher=Wiley |editor=J. D. Dunitz and J. A. Ibers |volume=2 |location=New York |pages=1–70}}</ref> Macrocycles may be fully carbocyclic (rings containing only carbon atoms, e.g. ]), heterocyclic containing both carbon and non-carbon atoms (e.g. ]s and ]s containing rings of 8 or more atoms), or non-carbon (containing only non-carbon atoms in the rings, e.g. ]). Heterocycles with carbon in the rings may have limited non-carbon atoms in their rings (e.g., in lactones and lactams whose rings are rich in carbon but have limited number of non-carbon atoms), or be rich in non-carbon atoms and displaying significant symmetry (e.g., in the case of chelating macrocycles). Macrocycles can access a number of stable ]s, with preference to reside in conformations that minimize ] nonbonded interactions within the ring (e.g., with the chair and chair-boat being more stable than the boat-boat conformation for ], because of the interactions depicted by the arcs shown).{{citation needed|date=April 2015}} Medium rings (8-11 atoms) are the most strained, with between 9-13 (kcal/mol) strain energy, and analysis of factors important in the conformations of larger macrocycles can be modeled using medium ring conformations.<ref>Eliel, E.L., Wilen, S.H. and Mander, L.S. ('''1994''') ''Stereochemistry of Organic Compounds,'' John Wiley and Sons, Inc., New York.{{page needed|date=April 2015}}</ref> Conformational analysis of odd-membered rings suggests they tend to reside in less symmetrical forms with smaller energy differences between stable conformations.<ref>{{cite journal |last1=Anet |first1=F.A.L. |last2=St. Jacques |first2=M. |last3=Henrichs |first3=P.M. |last4=Cheng |first4=A.K. |last5=Krane |first5=J. |last6=Wong |first6=L. |year=1974 |title=Conformational analysis of medium-ring ketones |journal=Tetrahedron |volume=30 |issue=12 |pages=1629–37 |doi=10.1016/S0040-4020(01)90685-4}}</ref> | |||
| ], 18-crown-6; B, the simple tetra-aza ], ]; C, an example ], the unsubstituted ]e; D, a mixed ]/], the ]; E, the related ]/imine Jäger macrocycle, and F, the tetracarboxylate-derivative ] macrocycle.]] | |||
| ==Heterocyclic compounds== | |||
| Cyclic compounds that have both carbon and non-carbon atoms present are termed (] compounds);{{citation needed|date=April 2015}} alternatively the name can refer to inorganic cyclic compounds, such as siloxanes and borazines, that have more than one type of atom in their rings.{{citation needed|date=April 2015}} Hantzsch–Widman nomenclature is recommended by the IUPAC for naming heterocycles, but many common names remain in regular use.{{citation needed|date=April 2015}} | |||
| === Nomenclature === | |||
| ==Aromaticity== | |||
| ] nomenclature has extensive rules to cover the naming of cyclic structures, both as core structures, and as substituents appended to ] structures.{{citation needed|date=April 2015}} The term ] is used when a ring-containing compound has a ring of 12 or more atoms.<ref name="StillTet81" /><ref name="DunitzPersp68" /> The term ] is used when more than one ring appears in a single molecule. ] is formally a polycyclic compound, but is more specifically named as a bicyclic compound. Several examples of macrocyclic and polycyclic structures are given in the final gallery below. | |||
| Cyclic compounds may or may not exhibit ]; ] is an example of an aromatic cyclic compound, while ] is non-aromatic. In organic chemistry, the term aromaticity is used to describe a cyclic (ring-shaped), planar (flat) molecule that exhibits unusual stability as compared to other geometric or connective arrangements of the same set of atoms. As a result of their stability, it is very difficult to cause aromatic molecules to break apart and to react with other substances. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have especial stability (low reactivity). | |||
| {{anchor|annular atom}}The atoms that are part of the ring structure are called annular atoms.<ref>{{cite book |last1=Morris |first1=Christopher G. |url=https://books.google.com/books?id=nauWlPTBcjIC&pg=PA120 |title=Academic Press Dictionary of Science and Technology |last2=Press |first2=Academic |publisher=Gulf Professional Publishing |year=1992 |isbn=9780122004001 |page=120 |language=en |access-date=2020-09-14 |archive-url=https://web.archive.org/web/20210413203802/https://books.google.com/books?id=nauWlPTBcjIC&pg=PA120 |archive-date=2021-04-13 |url-status=live}}</ref> | |||
| Since one of the most commonly encountered aromatic systems of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA. A functional group or other substituent that is aromatic is called an aryl group. | |||
| == Isomerism == | |||
| The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (aromas), unlike pure saturated hydrocarbons. Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds (how they smell), although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene. | |||
| === Stereochemistry === | |||
| In terms of the electronic nature of the molecule, aromaticity describes a conjugated system often made of alternating single and double bonds in a ring. This configuration allows for the electrons in the molecule’s pi system to be delocalized around the ring, increasing the molecule's stability. The molecule cannot be represented by one structure, but rather a resonance hybrid of different structures, such as with the two resonance structures of benzene. These molecules cannot be found in either one of these representations, with the longer single bonds in one location and the shorter double bond in another (See Theory below). Rather, the molecule exhibits bond lengths in between those of single and double bonds. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.{{citation needed|date=April 2015}} | |||
| The closing of atoms into rings may lock particular atoms with distinct ] by functional groups such that the result is ] and ] of the compound, including some manifestations that are unique to rings (e.g., ]).<ref name="Reusch10" /> | |||
| === Conformational isomerism === | |||
| ==Simple, mono-cyclic examples== | |||
| {{Image frame|width=200|content=] | |||
| The following are examples of simple and aromatic carbocycles, inorganic cyclic compounds, and heterocycles: | |||
| |caption=Two conformers of ], the ''chair'' at left, and the ''boat'' at right. Axial and equatorial hydrogen atoms are denoted with an ''a'' and ''e'', respectively.}} | |||
| <gallery caption="Simple mono-cyclic compounds: Carbocyclic, inorganic, and heterocyclic (aromatic and non-aromatic) examples."> | |||
| Image:Cycloheptane.png | ], a simple 7-membered carbocyclic compound, ] hydrogens shown (non-aromatic). | |||
| Image:Benz4.png | ], a 6-membered carbocyclic compound. ] hydrogens shown, and 6 electrons shown as ] through drawing of circle (aromatic). | |||
| Image:Cyclooctasulfur_structural_formula_3D.svg | ''Cyclo''-], an 8-membered inorganic cyclic compound (non-aromatic). | |||
| Image:Pentazole.png | ], a 5-membered inorganic cyclic compound (aromatic). | |||
| Image:Azetidine structure.svg| ], a 4-membered ] (aza) hetero-cyclic compound, ] hydrogen atoms implied, not shown (non-aromatic). | |||
| Image:Pyridine.svg|], a 6 membered heterocyclic compound, ] hydrogen atoms implied, not shown, and ] π-electrons shown as discrete bonds (aromatic). | |||
| </gallery> | |||
| Depending on ring size, the three-dimensional shapes of particular cyclic structures—typically rings of 5-atoms and larger—can vary and interconvert such that ] is displayed.<ref name="Reusch10" /> Indeed, the development of this important chemical concept arose, historically, in reference to cyclic compounds. For instance, ]s—six membered ]s with no double bonds, to which various substituents might be attached, see image—display an equilibrium between two conformations, the ''chair'' and the ''boat,'' as shown in the image. | |||
| ==Stereochemistry== | |||
| The closing of atoms into rings may lock particular atoms with distinct ] by functional groups such that the result is ] and ] of the compound, including some manifestations that are unique to rings (e.g., ]).<ref name="Reusch10"/> | |||
| The chair conformation is the favored configuration, because in this conformation, the ], ], and ] that are otherwise possible are minimized.<ref name="Reusch10" /> Which of the ''possible'' chair conformations predominate in cyclohexanes bearing one or more substituents depends on the substituents, and where they are located on the ring; generally, "bulky" substituents—those groups with large ''],'' or groups that are otherwise repulsive in their ]{{citation needed|date=June 2015}}—prefer to occupy an equatorial location.<ref name="Reusch10" /> An example of interactions within a molecule that would lead to ], leading to a shift in equilibrium from boat to chair, is the interaction between the two ]s in ''cis''-1,4-dimethylcyclohexane. In this molecule, the two methyl groups are in opposing positions of the ring (1,4-), and their ''cis'' stereochemistry projects both of these groups toward the same side of the ring. Hence, if forced into the higher energy boat form, these methyl groups are in steric contact, repel one another, and drive the equilibrium toward the chair conformation.<ref name="Reusch10" /> | |||
| ==Conformational isomerism== | |||
| {{multiple image | |||
| | width = 300 | |||
| | footer = ''General description.'' The structures are shown in line angle representation, though in the image at left, the lines projecting from the cyclohexane are not terminal methyl groups; rather, they indicate ''possible'' positions that might be occupied by ] (]) attached to the ring. In the image at left, those groups projecting upward and downward are termed ''axial substituents'' ('''a'''), and those groups projecting around the conceptual ] are termed ''equatorial substituents'' ('''e'''). Note, in general, the axial substituents are closer in space to one another (allowing for repulsive interactions); moreover, in the boat form, axial substituents in directly opposing positions (12 o'clock and 6 o'clock, termed "1,4-") are very close in space, and therefore give rise to even greater repulsion. These and other types of ] are used to explain the observation that ''the chair conformation of cyclohexanes is the favored conformation''.<ref name=Reusch10/> | |||
| | image1 = Chair-Boat-Conformation_general.svg | |||
| | alt1 = To be supplied | |||
| | caption1 = '''Chair and boat conformers in ]s.''' Two conformers of cyclohexane, the ''chair'' at left, and the ''boat'' at right (in German, respectively, ''Sessel'' and ''Wanne'', the latter meaning "bath"). | |||
| | image2 = Cis14dimethyl cyclohexane2 HD.jpg | |||
| | alt2 = To be supplied | |||
| | caption2 = '''''cis''-1,4-Dimethylcyclohexane''', in chair form, minimising steric interactions between the ]s in the directly opposing 1,4-positions of the cyclohexane ring. | |||
| }} | |||
| ==Aromaticity== | |||
| Depending on ring size, the three-dimensional shapes of particular cyclic structures—typically rings of 5-atoms and larger—can vary and interconvert such that ] is displayed.<ref name=Reusch10/> Indeed, the development of this important chemical concept arose, historically, in reference to cyclic compounds. For instance, ]s—six membered ]s with no double bonds, to which various substituents might be attached, see image—display an equilibrium between two conformations, the ''chair'' and the ''boat,'' as shown in the image. | |||
| {{Copying within Misplaced Pages|Aromaticity}} | |||
| Cyclic compounds may or may not exhibit ]; ] is an example of an aromatic cyclic compound, while ] is non-aromatic. In organic chemistry, the term aromaticity is used to describe a cyclic (ring-shaped), planar (flat) molecule that exhibits unusual stability as compared to other geometric or connective arrangements of the same set of atoms. As a result of their stability, it is very difficult to cause aromatic molecules to break apart and to react with other substances. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have especial stability (low reactivity). | |||
| Since one of the most commonly encountered aromatic systems of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA. A functional group or other substituent that is aromatic is called an aryl group. | |||
| The chair conformation is the favored configuration, because in this conformation, the ], ], and ] that are otherwise possible are minimized.<ref name=Reusch10/> Which of the ''possible'' chair conformations predominate in cyclohexanes bearing one or more substituents depends on the substiuents, and where they are located on the ring; generally, "bulky" substituents—those groups with large ''],'' or groups that are otherwise repulsive in their ]{{citation needed|date=June 2015}}—prefer to occupy an equatorial location.<ref name=Reusch10/> An example of interactions within a molecule that would lead to ], leading to a shift in equilibrium from boat to chair, is the interaction between the two ]s in ''cis''-1,4-dimethylcyclohexane. In this molecule, the two methyl groups are in opposing positions of the ring (1,4-), and their ''cis'' stereochemistry projects both of these groups toward the same side of the ring. Hence, if forced into the higher energy boat form, these methyl groups are in steric contact, repel one another, and drive the equilibrium toward the chair conformation.{{citation needed|date=June 2015}} | |||
| The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (aromas), unlike pure saturated hydrocarbons. Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds (how they smell), although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene. | |||
| ==Macrocycles== | |||
| ] | |||
| The term ] is used for compounds having a rings of 8 or more atoms.<ref name=StillTet81/><ref name=DunitzPersp68/> Macrocycles may be fully carbocyclic, heterocyclic but having limited heteroatoms (e.g., in ]s and ]s), or be rich in heteroatoms and displaying significant symmetry (e.g., in the case of chelating macrocycles). Macrocycles can access a number of stable ]s, with preference to reside in conformations that minimize ] nonbonded interactions within the ring (e.g., with the chair and chair-boat being more stable than the boat-boat conformation for cyclooctane, because of the interactions depicted by the arcs shown).{{citation needed|date=April 2015}} Medium rings (8-11 atoms) are the most strained, with between 9-13 (kcal/mol) strain energy, and analysis of factors important in the conformations of larger macrocycles can be modeled using medium ring conformations.<ref>Eliel, E.L., Wilen, S.H. and Mander, L.S. ('''1994''') ''Stereochemistry of Organic Compounds,'' John Wiley and Sons, Inc., New York.{{page needed|date=April 2015}}</ref>{{page needed|date=April 2015}} Conformational analysis of odd-membered rings suggests they tend to reside in less symmetrical forms with smaller energy differences between stable conformations.<ref>{{cite journal |doi=10.1016/S0040-4020(01)90685-4 |title=Conformational analysis of medium-ring ketones |journal=Tetrahedron |volume=30 |issue=12 |pages=1629–37 |year=1974 |last1=Anet |first1=F.A.L. |last2=St. Jacques |first2=M. |last3=Henrichs |first3=P.M. |last4=Cheng |first4=A.K. |last5=Krane |first5=J. |last6=Wong |first6=L. }}{{primary source inline|date=April 2015}}</ref>{{primary source inline|date=April 2015}} | |||
| In terms of the electronic nature of the molecule, aromaticity describes a conjugated system often made of alternating single and double bonds in a ring. This configuration allows for the electrons in the molecule's pi system to be delocalized around the ring, increasing the molecule's stability. The molecule cannot be represented by one structure, but rather a resonance hybrid of different structures, such as with the two resonance structures of benzene. These molecules cannot be found in either one of these representations, with the longer single bonds in one location and the shorter double bond in another (See Theory below). Rather, the molecule exhibits bond lengths in between those of single and double bonds. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.{{citation needed|date=April 2015}} | |||
| ], 18-crown-6; B, the simple tetra-aza ], ]; C, an example ], the unsubstituted ]e; D, a mixed ]/], the ]; E, the related ]/imine Jäger macrocycle, and F, the tetracarboxylate-derivative ] macrocycle.]] | |||
| == |
==Principal uses== | ||
| Because of the unique shapes, reactivities, properties, and ] that they engender, cyclic compounds are the largest majority of all molecules involved in the biochemistry, structure, and function of ]s, and in the man-made molecules (e.g., drugs, herbicides, etc.) through which man attempts to exert control over nature and biological systems. | Because of the unique shapes, reactivities, properties, and ] that they engender, cyclic compounds are the largest majority of all molecules involved in the biochemistry, structure, and function of ]s, and in the man-made molecules (e.g., drugs, herbicides, etc.) through which man attempts to exert control over nature and biological systems. | ||
| == Synthetic reactions == | |||
| ==Complex and polycyclic examples== | |||
| The following are examples of cyclic compounds exhibiting more complex ring systems and stereochemical features: | |||
| <gallery caption="Complex cyclic compounds: Macrocyclic and polycyclic examples" widths="250 px"> | |||
| Image:Naphtalene-diagram.png | ], technically a polycyclic, more specifically a bicyclic compound, with circles showing ] of π-electrons (aromatic). | |||
| Image:Cis-trans isomerism of decahydronaphthalene.svg | ] (decahydronaphthalene), the fully saturated derivative of ], showing the two ] possible for "fusing" the two rings together, and how this impacts the shapes available to this bicyclic compound (non-aromatic). | |||
| Image:Longifolene.PNG|], a ] ], and an example of a tricyclic molecule (non-aromatic). | |||
| Image:TaxolNumberingScheme.svg | ], a polycyclic ] with a tricyclic core: with a heterocyclic, 4-membered D ring, fused to further 6- and 8-membered carbocyclic (A/C and B) rings (non-aromatic), and with three further pendant ]-rings on its "tail", and attached to C-2 (abbrev. Ph, C<sub>6</sub>H<sub>5</sub>; aromatics). | |||
| Image:Paclitaxel_JMolBiol_2001_1045.jpg | A representative three-dimensional shape adopted by ], as a result of its unique cyclic structure.<ref>{{cite journal |doi=10.1006/jmbi.2001.5077 |pmid=11700061 |title=Refined structure of αβ-tubulin at 3.5 Å resolution |journal=Journal of Molecular Biology |volume=313 |issue=5 |pages=1045–57 |year=2001 |last1=Löwe |first1=J |last2=Li |first2=H |last3=Downing |first3=K.H |last4=Nogales |first4=E |url=https://zenodo.org/record/1229896 }}</ref> | |||
| Image:Cholesterol.svg|], another terpene natural product, in particular, a ], a class of tetracyclic molecules (non-aromatic). | |||
| Image:Benzo-a-pyrene.svg|pyrene]], a pentacyclic compound both natural and man-made, and ] π-electrons shown as discrete bonds (aromatic). | |||
| Image:Pagodane.svg|], a complex, highly symmetric, man-made polycyclic compound (non-aromatic). | |||
| Image:Brevetoxin A.svg|], a ] with ten rings, all fused, and all ], and a toxic component associated with the organisms responsible for ]s. The R group at right refers to one of several possible four-carbon side chains (see main ] article; non-aromatic). | |||
| </gallery> | |||
| ==Synthetic reactions altering rings== | |||
| ===Important general reactions for forming rings=== | ===Important general reactions for forming rings=== | ||
| ] | ] | ||
| There are a variety of specialized reactions whose use is solely the formation of rings, and these will be discussed below. In addition to those, there are a wide variety of ''general'' organic reactions that historically have been crucial in the development, first, of understanding the concepts of ring chemistry, and second, of reliable procedures for preparing ring structures in high ], and with defined orientation of ring substituents (i.e., defined ]). These general reactions include: | There are a variety of specialized reactions whose use is solely the formation of rings, and these will be discussed below. In addition to those, there are a wide variety of ''general'' organic reactions that historically have been crucial in the development, first, of understanding the concepts of ring chemistry, and second, of reliable procedures for preparing ring structures in high ], and with defined orientation of ring substituents (i.e., defined ]). These general reactions include: | ||
| * ]; | * ]; | ||
| Line 104: | Line 77: | ||
| === Ring-closing reactions=== | === Ring-closing reactions=== | ||
| In organic chemistry, a variety of synthetic |
In organic chemistry, a variety of synthetic procedures are particularly useful in closing carbocyclic and other rings; these are termed ''ring-closing reactions''. Examples include: | ||
| * ]; | * ]; | ||
| * the ]; | * the ] of an ]; | ||
| * the ] and other ] reactions; | * the ], between a conjugated ] and a substituted ], and other ] reactions; | ||
| * the ]; | * the ], originally being the cyclization of a ]; | ||
| * various ]s; | * various ]s; | ||
| * ] reactions, which also can be used to accomplish a specific type of ]; | * ] reactions, which also can be used to accomplish a specific type of ]; | ||
| * the ] |
* the ], in which two ] groups combine to form a ] group with loss of {{chem2|CO2}} and {{chem2|H2O}}; | ||
| * the ] |
* the ] converting a beta ] to an ] | ||
| ===Ring-opening reactions === | ===Ring-opening reactions === | ||
| Line 121: | Line 94: | ||
| {{Main|Ring expansion and ring contraction}} | {{Main|Ring expansion and ring contraction}} | ||
| Ring expansion reactions are common in ], and are frequently encountered in ]. Ring expansions and contractions can involve the insertion of a functional group such as the case with ] of cyclic ketones, rearrangements of cyclic carbocycles as seen in ] ], or collapse or rearrangement of ] as several examples. | Ring expansion and contraction reactions are common in ], and are frequently encountered in ]s. Ring expansions and contractions can involve the insertion of a functional group such as the case with ] of cyclic ketones, rearrangements of cyclic carbocycles as seen in ] ]s, or collapse or rearrangement of ]s as several examples. | ||
| == Examples == | |||
| === Simple, mono-cyclic examples === | |||
| The following are examples of simple and aromatic carbocycles, inorganic cyclic compounds, and heterocycles: | |||
| <gallery caption="Simple mono-cyclic compounds: Carbocyclic, inorganic, and heterocyclic (aromatic and non-aromatic) examples."> | |||
| Image:Cycloheptane.png | ], a simple 7-membered carbocyclic compound, ] hydrogens shown (non-aromatic). | |||
| Image:Benzene-6H-delocalized.svg | ], a 6-membered carbocyclic organic compound. ] hydrogens shown, and 6 electrons shown as ] through drawing of circle (aromatic). | |||
| Image:Cyclooctasulfur_structural_formula_3D.svg | ''Cyclo''-], an 8-membered inorganic cyclic compound (non-aromatic). | |||
| Image:1,3-Selenium hexasulfide.png|], an 8-membered inorganic heterocyclic compound (non-aromatic). | |||
| Image:Pentasilolane.svg|], a 5-membered inorganic cyclic compound (non-aromatic). | |||
| Image:Hexamethylcyclotrisiloxan.svg|], a 6-membered organic heterocyclic compound (non-aromatic). | |||
| Image:Hexachlorotriphosphazene-2D-dimensions.png|], a 6-membered inorganic heterocyclic compound (aromatic). | |||
| Image:Borazine-dimensions-2D.svg|], a 6-membered inorganic heterocyclic compound (may be aromatic). | |||
| Image:Pentazole.svg | ], a 5-membered inorganic cyclic compound (aromatic). | |||
| Image:Azetidine structure.svg| ], a 4-membered ] (aza) heterocyclic compound, ] hydrogen atoms implied, not shown (non-aromatic). | |||
| Image:Caprolactam-2D-skeletal.png|], a 7-membered heterocyclic organic compound (non-aromatic). | |||
| Image:Pyridine.svg|], a 6 membered heterocyclic compound, ] hydrogen atoms implied, not shown, and ] π-electrons shown as discrete bonds (aromatic). | |||
| </gallery> | |||
| === Complex and polycyclic examples === | |||
| The following are examples of cyclic compounds exhibiting more complex ring systems and stereochemical features: | |||
| <gallery caption="Complex cyclic compounds: Macrocyclic and polycyclic examples" widths="250 px"> | |||
| Image:Naphtalene topo.svg | ], technically a polycyclic, more specifically a bicyclic compound, with circles showing ] of π-electrons (aromatic). | |||
| Image:Cis-trans isomerism of decahydronaphthalene.svg | ] (decahydronaphthalene), the fully saturated derivative of ], showing the two ] possible for "fusing" the two rings together, and how this impacts the shapes available to this bicyclic compound (non-aromatic). | |||
| Image:Longifolene plus acsv.svg|], a ] ], and an example of a tricyclic molecule (non-aromatic). | |||
| Image:TaxolNumberingScheme.svg | ], a polycyclic ] with a tricyclic core: with a heterocyclic, 4-membered D ring, fused to further 6- and 8-membered carbocyclic (A/C and B) rings (non-aromatic), and with three further pendant ]-rings on its "tail", and attached to C-2 (abbrev. Ph, C<sub>6</sub>H<sub>5</sub>; aromatics). | |||
| Image:Paclitaxel_JMolBiol_2001_1045.jpg | A representative three-dimensional shape adopted by ], as a result of its unique cyclic structure.<ref>{{cite journal |doi=10.1006/jmbi.2001.5077 |pmid=11700061 |title=Refined structure of αβ-tubulin at 3.5 Å resolution |journal=Journal of Molecular Biology |volume=313 |issue=5 |pages=1045–57 |year=2001 |last1=Löwe |first1=J |last2=Li |first2=H |last3=Downing |first3=K.H |last4=Nogales |first4=E |url=https://zenodo.org/record/1229896 |access-date=2020-09-14 |archive-date=2021-01-22 |archive-url=https://web.archive.org/web/20210122161041/https://zenodo.org/record/1229896 |url-status=live }}</ref> | |||
| Image:Cholesterol.svg|], another terpene natural product, in particular, a ], a class of tetracyclic molecules (non-aromatic). | |||
| Image:Benzo-a-pyrene.svg|pyrene]], a pentacyclic compound both natural and man-made, and ] π-electrons shown as discrete bonds (aromatic). | |||
| Image:Pagodane.svg|], a complex, highly symmetric, man-made polycyclic compound (non-aromatic). | |||
| Image:Brevetoxin A.svg|], a ] with ten rings, all fused, and all ], and a toxic component associated with the organisms responsible for ]s. The R group at right refers to one of several possible four-carbon side chains (see main ] article; non-aromatic). | |||
| </gallery> | |||
| ==See also== | ==See also== | ||
| *] | *] | ||
| *] | |||
| *] | *] | ||
| *] | |||
| *] | *] | ||
| Line 147: | Line 151: | ||
| {{Authority control}} | {{Authority control}} | ||
| ] | |||
| ] | ] | ||
| ] | |||
Latest revision as of 14:18, 13 November 2024
Molecule with a ring of bonded atomsA cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (heterocyclic compounds with rings containing both carbon and non-carbon). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the many billions.
- Cyclic compound examples: All-carbon (carbocyclic) and more complex natural cyclic compounds
-
 Ingenol, a complex, terpenoid natural product, related to but simpler than the paclitaxel that follows, which displays a complex ring structure including 3-, 5-, and 7-membered non-aromatic, carbocyclic rings.
Ingenol, a complex, terpenoid natural product, related to but simpler than the paclitaxel that follows, which displays a complex ring structure including 3-, 5-, and 7-membered non-aromatic, carbocyclic rings.
-
 Cycloalkanes, the simplest carbocycles, including cyclopropane, cyclobutane, cyclopentane, and cyclohexane. Note, elsewhere an organic chemistry shorthand is used where hydrogen atoms are inferred as present to fill the carbon's valence of 4 (rather than their being shown explicitly).
Cycloalkanes, the simplest carbocycles, including cyclopropane, cyclobutane, cyclopentane, and cyclohexane. Note, elsewhere an organic chemistry shorthand is used where hydrogen atoms are inferred as present to fill the carbon's valence of 4 (rather than their being shown explicitly).
-
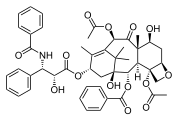 Paclitaxel, another complex, plant-derived terpenoid, also a natural product, displaying a complex multi-ring structure including 4-, 6-, and 8-membered rings (carbocyclic and heterocyclic, aromatic and non-aromatic).
Paclitaxel, another complex, plant-derived terpenoid, also a natural product, displaying a complex multi-ring structure including 4-, 6-, and 8-membered rings (carbocyclic and heterocyclic, aromatic and non-aromatic).
Adding to their complexity and number, closing of atoms into rings may lock particular atoms with distinct substitution (by functional groups) such that stereochemistry and chirality of the compound results, including some manifestations that are unique to rings (e.g., configurational isomers). As well, depending on ring size, the three-dimensional shapes of particular cyclic structures – typically rings of five atoms and larger – can vary and interconvert such that conformational isomerism is displayed. Indeed, the development of this important chemical concept arose historically in reference to cyclic compounds. Finally, cyclic compounds, because of the unique shapes, reactivities, properties, and bioactivities that they engender, are the majority of all molecules involved in the biochemistry, structure, and function of living organisms, and in man-made molecules such as drugs, pesticides, etc.
Structure and classification
A cyclic compound or ring compound is a compound in which at least some its atoms are connected to form a ring. Rings vary in size from three to many tens or even hundreds of atoms. Examples of ring compounds readily include cases where:
- all the atoms are carbon (i.e., are carbocycles),
- none of the atoms are carbon (inorganic cyclic compounds), or where
- both carbon and non-carbon atoms are present (heterocyclic compounds with rings containing both carbon and non-carbon).
Common atoms can (as a result of their valences) form varying numbers of bonds, and many common atoms readily form rings. In addition, depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, cyclic compounds may be aromatic or non-aromatic; in the case of non-aromatic cyclic compounds, they may vary from being fully saturated to having varying numbers of multiple bonds. As a consequence of the constitutional variability that is thermodynamically possible in cyclic structures, the number of possible cyclic structures, even of small size (e.g., <17 atoms) numbers in the many billions.
Moreover, the closing of atoms into rings may lock particular functional group–substituted atoms into place, resulting in stereochemistry and chirality being associated with the compound, including some manifestations that are unique to rings (e.g., configurational isomers); As well, depending on ring size, the three-dimensional shapes of particular cyclic structures — typically rings of five atoms and larger — can vary and interconvert such that conformational isomerism is displayed.
Carbocycles
The vast majority of cyclic compounds are organic, and of these, a significant and conceptually important portion are composed of rings made only of carbon atoms (i.e., they are carbocycles).
Inorganic cyclic compounds
Inorganic atoms form cyclic compounds as well. Examples include sulfur and nitrogen (e.g. heptasulfur imide S7NH, trithiazyl trichloride (NSCl)3, tetrasulfur tetranitride S4N4), silicon (e.g., cyclopentasilane (SiH2)5), phosphorus and nitrogen (e.g., hexachlorophosphazene (NPCl2)3), phosphorus and oxygen (e.g., metaphosphates (PO−3)3 and other cyclic phosphoric acid derivatives), boron and oxygen (e.g., sodium metaborate Na3(BO2)3, borax), boron and nitrogen (e.g. borazine (BN)3H6). When carbon in benzene is "replaced" by other elements, e.g., as in borabenzene, silabenzene, germanabenzene, stannabenzene, and phosphorine, aromaticity is retained, and so aromatic inorganic cyclic compounds are also known and well-characterized.
Heterocyclic compounds
A heterocyclic compound is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Cyclic compounds that have both carbon and non-carbon atoms present are heterocyclic carbon compounds, and the name refers to inorganic cyclic compounds as well (e.g., siloxanes, which contain only silicon and oxygen in the rings, and borazines, which contain only boron and nitrogen in the rings). Hantzsch–Widman nomenclature is recommended by the IUPAC for naming heterocycles, but many common names remain in regular use.
Macrocycles

The term macrocycle is used for compounds having a rings of 8 or more atoms. Macrocycles may be fully carbocyclic (rings containing only carbon atoms, e.g. cyclooctane), heterocyclic containing both carbon and non-carbon atoms (e.g. lactones and lactams containing rings of 8 or more atoms), or non-carbon (containing only non-carbon atoms in the rings, e.g. diselenium hexasulfide). Heterocycles with carbon in the rings may have limited non-carbon atoms in their rings (e.g., in lactones and lactams whose rings are rich in carbon but have limited number of non-carbon atoms), or be rich in non-carbon atoms and displaying significant symmetry (e.g., in the case of chelating macrocycles). Macrocycles can access a number of stable conformations, with preference to reside in conformations that minimize transannular nonbonded interactions within the ring (e.g., with the chair and chair-boat being more stable than the boat-boat conformation for cyclooctane, because of the interactions depicted by the arcs shown). Medium rings (8-11 atoms) are the most strained, with between 9-13 (kcal/mol) strain energy, and analysis of factors important in the conformations of larger macrocycles can be modeled using medium ring conformations. Conformational analysis of odd-membered rings suggests they tend to reside in less symmetrical forms with smaller energy differences between stable conformations.

Nomenclature
IUPAC nomenclature has extensive rules to cover the naming of cyclic structures, both as core structures, and as substituents appended to alicyclic structures. The term macrocycle is used when a ring-containing compound has a ring of 12 or more atoms. The term polycyclic is used when more than one ring appears in a single molecule. Naphthalene is formally a polycyclic compound, but is more specifically named as a bicyclic compound. Several examples of macrocyclic and polycyclic structures are given in the final gallery below.
The atoms that are part of the ring structure are called annular atoms.
Isomerism
Stereochemistry
The closing of atoms into rings may lock particular atoms with distinct substitution by functional groups such that the result is stereochemistry and chirality of the compound, including some manifestations that are unique to rings (e.g., configurational isomers).
Conformational isomerism
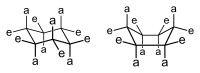 Two conformers of cyclohexane, the chair at left, and the boat at right. Axial and equatorial hydrogen atoms are denoted with an a and e, respectively.
Two conformers of cyclohexane, the chair at left, and the boat at right. Axial and equatorial hydrogen atoms are denoted with an a and e, respectively.
Depending on ring size, the three-dimensional shapes of particular cyclic structures—typically rings of 5-atoms and larger—can vary and interconvert such that conformational isomerism is displayed. Indeed, the development of this important chemical concept arose, historically, in reference to cyclic compounds. For instance, cyclohexanes—six membered carbocycles with no double bonds, to which various substituents might be attached, see image—display an equilibrium between two conformations, the chair and the boat, as shown in the image.
The chair conformation is the favored configuration, because in this conformation, the steric strain, eclipsing strain, and angle strain that are otherwise possible are minimized. Which of the possible chair conformations predominate in cyclohexanes bearing one or more substituents depends on the substituents, and where they are located on the ring; generally, "bulky" substituents—those groups with large volumes, or groups that are otherwise repulsive in their interactions—prefer to occupy an equatorial location. An example of interactions within a molecule that would lead to steric strain, leading to a shift in equilibrium from boat to chair, is the interaction between the two methyl groups in cis-1,4-dimethylcyclohexane. In this molecule, the two methyl groups are in opposing positions of the ring (1,4-), and their cis stereochemistry projects both of these groups toward the same side of the ring. Hence, if forced into the higher energy boat form, these methyl groups are in steric contact, repel one another, and drive the equilibrium toward the chair conformation.
Aromaticity
| This article's edit history is not complete. Some of the article text's edit history exists at Aromaticity due to copying and pasting between articles. This may be a violation of the CC BY-SA and/or GFDL if proper attribution was not made in an edit summary or on the talk page. Please see Misplaced Pages:Merge and Misplaced Pages:How to break up a page for details of when such copying and pasting is acceptable and when it is not, and how to correctly attribute using links in the edit summaries. You can also read the "copying within Misplaced Pages" guideline for an overview of the issues involved. |
Cyclic compounds may or may not exhibit aromaticity; benzene is an example of an aromatic cyclic compound, while cyclohexane is non-aromatic. In organic chemistry, the term aromaticity is used to describe a cyclic (ring-shaped), planar (flat) molecule that exhibits unusual stability as compared to other geometric or connective arrangements of the same set of atoms. As a result of their stability, it is very difficult to cause aromatic molecules to break apart and to react with other substances. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have especial stability (low reactivity).
Since one of the most commonly encountered aromatic systems of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA. A functional group or other substituent that is aromatic is called an aryl group.
The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (aromas), unlike pure saturated hydrocarbons. Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds (how they smell), although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.
In terms of the electronic nature of the molecule, aromaticity describes a conjugated system often made of alternating single and double bonds in a ring. This configuration allows for the electrons in the molecule's pi system to be delocalized around the ring, increasing the molecule's stability. The molecule cannot be represented by one structure, but rather a resonance hybrid of different structures, such as with the two resonance structures of benzene. These molecules cannot be found in either one of these representations, with the longer single bonds in one location and the shorter double bond in another (See Theory below). Rather, the molecule exhibits bond lengths in between those of single and double bonds. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.
Principal uses
Because of the unique shapes, reactivities, properties, and bioactivities that they engender, cyclic compounds are the largest majority of all molecules involved in the biochemistry, structure, and function of living organisms, and in the man-made molecules (e.g., drugs, herbicides, etc.) through which man attempts to exert control over nature and biological systems.
Synthetic reactions
Important general reactions for forming rings
There are a variety of specialized reactions whose use is solely the formation of rings, and these will be discussed below. In addition to those, there are a wide variety of general organic reactions that historically have been crucial in the development, first, of understanding the concepts of ring chemistry, and second, of reliable procedures for preparing ring structures in high yield, and with defined orientation of ring substituents (i.e., defined stereochemistry). These general reactions include:
- Acyloin condensation;
- Anodic oxidations; and
- the Dieckmann condensation as applied to ring formation.
Ring-closing reactions
In organic chemistry, a variety of synthetic procedures are particularly useful in closing carbocyclic and other rings; these are termed ring-closing reactions. Examples include:
- alkyne trimerisation;
- the Bergman cyclization of an enediyne;
- the Diels–Alder, between a conjugated diene and a substituted alkene, and other cycloaddition reactions;
- the Nazarov cyclization reaction, originally being the cyclization of a divinyl ketone;
- various radical cyclizations;
- ring-closing metathesis reactions, which also can be used to accomplish a specific type of polymerization;
- the Ruzicka large ring synthesis, in which two carboxyl groups combine to form a carbonyl group with loss of CO2 and H2O;
- the Wenker synthesis converting a beta amino alcohol to an aziridine
Ring-opening reactions
A variety of further synthetic procedures are particularly useful in opening carbocyclic and other rings, generally which contain a double bound or other functional group "handle" to facilitate chemistry; these are termed ring-opening reactions. Examples include:
- ring opening metathesis, which can also be used to accomplish a specific type of polymerization.
Ring expansion and ring contraction reactions
Main article: Ring expansion and ring contractionRing expansion and contraction reactions are common in organic synthesis, and are frequently encountered in pericyclic reactions. Ring expansions and contractions can involve the insertion of a functional group such as the case with Baeyer–Villiger oxidation of cyclic ketones, rearrangements of cyclic carbocycles as seen in intramolecular Diels-Alder reactions, or collapse or rearrangement of bicyclic compounds as several examples.
Examples
Simple, mono-cyclic examples
The following are examples of simple and aromatic carbocycles, inorganic cyclic compounds, and heterocycles:
- Simple mono-cyclic compounds: Carbocyclic, inorganic, and heterocyclic (aromatic and non-aromatic) examples.
-
 Cycloheptane, a simple 7-membered carbocyclic compound, methylene hydrogens shown (non-aromatic).
Cycloheptane, a simple 7-membered carbocyclic compound, methylene hydrogens shown (non-aromatic).
-
 Benzene, a 6-membered carbocyclic organic compound. methine hydrogens shown, and 6 electrons shown as delocalized through drawing of circle (aromatic).
Benzene, a 6-membered carbocyclic organic compound. methine hydrogens shown, and 6 electrons shown as delocalized through drawing of circle (aromatic).
-
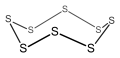 Cyclo-octasulfur, an 8-membered inorganic cyclic compound (non-aromatic).
Cyclo-octasulfur, an 8-membered inorganic cyclic compound (non-aromatic).
-
 Diselenium hexasulfide, an 8-membered inorganic heterocyclic compound (non-aromatic).
Diselenium hexasulfide, an 8-membered inorganic heterocyclic compound (non-aromatic).
-
 Cyclopentasilane, a 5-membered inorganic cyclic compound (non-aromatic).
Cyclopentasilane, a 5-membered inorganic cyclic compound (non-aromatic).
-
 Hexamethylcyclotrisiloxane, a 6-membered organic heterocyclic compound (non-aromatic).
Hexamethylcyclotrisiloxane, a 6-membered organic heterocyclic compound (non-aromatic).
-
 Hexachlorophosphazene, a 6-membered inorganic heterocyclic compound (aromatic).
Hexachlorophosphazene, a 6-membered inorganic heterocyclic compound (aromatic).
-
 Borazine, a 6-membered inorganic heterocyclic compound (may be aromatic).
Borazine, a 6-membered inorganic heterocyclic compound (may be aromatic).
-
 Pentazole, a 5-membered inorganic cyclic compound (aromatic).
Pentazole, a 5-membered inorganic cyclic compound (aromatic).
-
 Azetidine, a 4-membered nitrogen (aza) heterocyclic compound, methylene hydrogen atoms implied, not shown (non-aromatic).
Azetidine, a 4-membered nitrogen (aza) heterocyclic compound, methylene hydrogen atoms implied, not shown (non-aromatic).
-
 Caprolactam, a 7-membered heterocyclic organic compound (non-aromatic).
Caprolactam, a 7-membered heterocyclic organic compound (non-aromatic).
-
 Pyridine, a 6 membered heterocyclic compound, methine hydrogen atoms implied, not shown, and delocalized π-electrons shown as discrete bonds (aromatic).
Pyridine, a 6 membered heterocyclic compound, methine hydrogen atoms implied, not shown, and delocalized π-electrons shown as discrete bonds (aromatic).
Complex and polycyclic examples
The following are examples of cyclic compounds exhibiting more complex ring systems and stereochemical features:
- Complex cyclic compounds: Macrocyclic and polycyclic examples
-
 Naphthalene, technically a polycyclic, more specifically a bicyclic compound, with circles showing delocalization of π-electrons (aromatic).
Naphthalene, technically a polycyclic, more specifically a bicyclic compound, with circles showing delocalization of π-electrons (aromatic).
-
 Decalin (decahydronaphthalene), the fully saturated derivative of naphthalene, showing the two stereochemistries possible for "fusing" the two rings together, and how this impacts the shapes available to this bicyclic compound (non-aromatic).
Decalin (decahydronaphthalene), the fully saturated derivative of naphthalene, showing the two stereochemistries possible for "fusing" the two rings together, and how this impacts the shapes available to this bicyclic compound (non-aromatic).
-
 Longifolene, a terpene natural product, and an example of a tricyclic molecule (non-aromatic).
Longifolene, a terpene natural product, and an example of a tricyclic molecule (non-aromatic).
-
 Paclitaxel, a polycyclic natural product with a tricyclic core: with a heterocyclic, 4-membered D ring, fused to further 6- and 8-membered carbocyclic (A/C and B) rings (non-aromatic), and with three further pendant phenyl-rings on its "tail", and attached to C-2 (abbrev. Ph, C6H5; aromatics).
Paclitaxel, a polycyclic natural product with a tricyclic core: with a heterocyclic, 4-membered D ring, fused to further 6- and 8-membered carbocyclic (A/C and B) rings (non-aromatic), and with three further pendant phenyl-rings on its "tail", and attached to C-2 (abbrev. Ph, C6H5; aromatics).
-
 A representative three-dimensional shape adopted by paclitaxel, as a result of its unique cyclic structure.
A representative three-dimensional shape adopted by paclitaxel, as a result of its unique cyclic structure.
-
 Cholesterol, another terpene natural product, in particular, a steroid, a class of tetracyclic molecules (non-aromatic).
Cholesterol, another terpene natural product, in particular, a steroid, a class of tetracyclic molecules (non-aromatic).
-
 Benzopyrene, a pentacyclic compound both natural and man-made, and delocalized π-electrons shown as discrete bonds (aromatic).
Benzopyrene, a pentacyclic compound both natural and man-made, and delocalized π-electrons shown as discrete bonds (aromatic).
-
 Pagodane, a complex, highly symmetric, man-made polycyclic compound (non-aromatic).
Pagodane, a complex, highly symmetric, man-made polycyclic compound (non-aromatic).
-
 Brevetoxin A, a natural product with ten rings, all fused, and all heterocyclic, and a toxic component associated with the organisms responsible for red tides. The R group at right refers to one of several possible four-carbon side chains (see main Brevetoxin article; non-aromatic).
Brevetoxin A, a natural product with ten rings, all fused, and all heterocyclic, and a toxic component associated with the organisms responsible for red tides. The R group at right refers to one of several possible four-carbon side chains (see main Brevetoxin article; non-aromatic).
See also
References
- March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.). New York: Wiley. ISBN 9780471854722. OCLC 642506595.
- Halduc, I. (1961). "Classification of inorganic cyclic compounds". Journal of Structural Chemistry. 2 (3): 350–8. doi:10.1007/BF01141802. S2CID 93804259.
- Reymond, Jean-Louis (2015). "The Chemical Space Project". Accounts of Chemical Research. 48 (3): 722–30. doi:10.1021/ar500432k. PMID 25687211.
- ^ William Reusch (2010). "Stereoisomers Part I" in Virtual Textbook of Organic Chemistry. Michigan State University. Archived from the original on 10 March 2015. Retrieved 7 April 2015.
- ^ IUPAC Gold Book heterocyclic compounds
- ^ Still, W.Clark; Galynker, Igor (1981). "Chemical consequences of conformation in macrocyclic compounds". Tetrahedron. 37 (23): 3981–96. doi:10.1016/S0040-4020(01)93273-9.
- ^ J. D. Dunitz (1968). J. D. Dunitz and J. A. Ibers (ed.). Perspectives in Structural Chemistry. Vol. 2. New York: Wiley. pp. 1–70.
- Eliel, E.L., Wilen, S.H. and Mander, L.S. (1994) Stereochemistry of Organic Compounds, John Wiley and Sons, Inc., New York.
- Anet, F.A.L.; St. Jacques, M.; Henrichs, P.M.; Cheng, A.K.; Krane, J.; Wong, L. (1974). "Conformational analysis of medium-ring ketones". Tetrahedron. 30 (12): 1629–37. doi:10.1016/S0040-4020(01)90685-4.
- Morris, Christopher G.; Press, Academic (1992). Academic Press Dictionary of Science and Technology. Gulf Professional Publishing. p. 120. ISBN 9780122004001. Archived from the original on 2021-04-13. Retrieved 2020-09-14.
- Löwe, J; Li, H; Downing, K.H; Nogales, E (2001). "Refined structure of αβ-tubulin at 3.5 Å resolution". Journal of Molecular Biology. 313 (5): 1045–57. doi:10.1006/jmbi.2001.5077. PMID 11700061. Archived from the original on 2021-01-22. Retrieved 2020-09-14.
Further reading
- Jürgen-Hinrich Fuhrhop & Gustav Penzlin, 1986, "Organic synthesis: concepts, methods, starting materials," Weinheim, BW, DEU:VCH, ISBN 0895732467, see , accessed 19 June 2015.
- Michael B. Smith & Jerry March, 2007, "March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure," 6th Ed., New York, NY, USA:Wiley & Sons, ISBN 0470084944, see , accessed 19 June 2015.
- Francis A. Carey & Richard J. Sundberg, 2006, "Title Advanced Organic Chemistry: Part A: Structure and Mechanisms," 4th Edn., New York, NY, USA:Springer Science & Business Media, ISBN 0306468565, see , accessed 19 June 2015.
- Michael B. Smith, 2011, "Organic Chemistry: An Acid—Base Approach," Boca Raton, FL, USA:CRC Press, ISBN 1420079212, see , accessed 19 June 2015.
- Jonathan Clayden, Nick Greeves & Stuart Warren, 2012, "Organic Chemistry," Oxford, Oxon, GBR:Oxford University Press, ISBN 0199270295, see , accessed 19 June 2015.
- László Kürti & Barbara Czakó, 2005, "Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms, Amsterdam, NH, NLD:Elsevier Academic Press, 2005ISBN 0124297854, see , accessed 19 June 2015.
External links
- Polycyclic+Compounds at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Macrocyclic+Compounds at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
