| Revision as of 00:11, 2 June 2009 edit93.34.214.143 (talk) →North African admixtureTag: references removed← Previous edit | Latest revision as of 00:36, 8 December 2024 edit undo2001:8f8:1e3d:14bc:249e:dd15:9cc0:ff5c (talk)No edit summaryTags: Visual edit Mobile edit Mobile web edit | ||
| Line 1: | Line 1: | ||
| {{Short description|none}} | |||
| {{Expert-subject|Molecular and Cellular Biology|date=November 2008}} | |||
| {{Disputed|date=July 2007}} | |||
| {{EngvarB|date=November 2016}} | |||
| The genetic history of Europe can be inferred by observing the patterns of genetic diversity across the continent and comparing them with the patterns on the adjacent land masses. These patterns can be found by using classical ]s or by using molecular genetics (], ] and ]). Most data is from modern populations, but there is a small amount of information from ancient DNA. ] have a complicated ] and ] history, including many layers of successive ] between different time periods, from the first appearance of '']'' in the ] to contemporary ]. | |||
| ] and its descendants.]] | |||
| ==Relation of Neanderthals and modern humans== | |||
| Before modern humans arrived in Europe some 45,000 years ago, the continent was inhabited by ]s, who did not die out until about 25,000 years ago. There continues to be much speculation as to whether the two genetic groups interbred. Analysis of the DNA distributions of the two groups shows significant difference, so any such offspring were unlikely to be fertile. | |||
| [[File:European genetic structure (based on SNPs) PC analysis.png|thumb|400px| The European genetic structure today (based on 273,464 SNPs). Three levels of structure as revealed by PC analysis are shown: A) inter-continental; B) intra-continental; and C) inside a single country (Estonia), where median values of the PC1&2 are shown. D) European map illustrating the origin of sample and population size. CEU – Utah residents with ancestry from Northern and Western Europe, CHB – Han Chinese from Beijing, JPT – Japanese from Tokyo, and YRI – Yoruba from Ibadan, Nigeria.<ref name="FleischerNelis2009">{{cite journal | vauthors = Nelis M, Esko T, Mägi R, Zimprich F, Zimprich A, Toncheva D, Karachanak S, Piskácková T, Balascák I, Peltonen L, Jakkula E, Rehnström K, Lathrop M, Heath S, Galan P, Schreiber S, Meitinger T, Pfeufer A, Wichmann HE, Melegh B, Polgár N, Toniolo D, Gasparini P, D'Adamo P, Klovins J, Nikitina-Zake L, Kucinskas V, Kasnauskiene J, Lubinski J, Debniak T, Limborska S, Khrunin A, Estivill X, Rabionet R, Marsal S, Julià A, Antonarakis SE, Deutsch S, Borel C, Attar H, Gagnebin M, Macek M, Krawczak M, Remm M, Metspalu A | display-authors = 6 | title = Genetic structure of Europeans: a view from the North-East | journal = PLOS ONE | volume = 4 | issue = 5 | pages = e5472 | year = 2009 | pmid = 19424496 | pmc = 2675054 | doi = 10.1371/journal.pone.0005472 | doi-access = free | bibcode = 2009PLoSO...4.5472N }}</ref> | |||
| ==Relation of present population to other populations== | |||
| ]] | |||
| ===Classical genetics studies=== | |||
| {| class="wikitable" align="right" width="280px" | |||
| |+''' Percentage genetic distances among major continents based on 120 classical polymorphisms ''' | |||
| ! ||Africa || Oceania || East Asia || Europe | |||
| |- | |||
| |Oceania || 24.7 || || || | |||
| |- | |||
| |East Asia || 20.6 || 10 || || | |||
| |- | |||
| |Europe || 16.6 || 13.5 || 9.7 || | |||
| |- | |||
| |America || 22.6 || 14.6 || 8.9 || 9.5 | |||
| |} | |||
| <!-- Deleted image removed: ] --> | |||
| In the 1990s, a study by ] of the Stanford University School of Medicine, using 120 blood polymorphisms, provided information on genetic relatedness of the various continental populations.<ref name="cavalli2">{{cite journal |author=Cavalli-Sforza LL |title=Genes, peoples, and languages |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=94 |issue=15 |pages=7719–24 |year=1997 |month=July |pmid=9223254 |pmc=33682 |url=http://www.pnas.org/cgi/content/full/94/15/7719}}</ref> ] is a measure of genetic difference between two populations. It is based on the principle that two populations that share similar frequencies of a trait are more closely related than populations that have more divergent frequencies of the trait. In its simplest form, it is the difference in frequencies of a particular trait between two populations. For example, the frequency of ] negative individuals is 50.4% among ], 41.2% in ] and 41.1% in ]. Thus regarding the RH negative trait, the genetic difference between the Basques and ] is 9.2% and the genetic difference between the French and the ] is 0.1%. Averaged over several traits this approach can give a measure of the overall genetic relatedness of various populations.<ref name="CavalliSforza">{{cite book |author=Cavalli-Sforza, L. L. |title=Genes, peoples, and languages |publisher=University of California Press |location=Berkeley |year=2001 |isbn=0-520-22873-1 }}</ref> | |||
| The '''genetic history of Europe''' includes information around the formation, ], and other ]-specific information about ]s ], or living in ]. | |||
| According to the study, all non-African populations are more closely related to each other than to Africans; this supports the hypothesis that all non-Africans descend from a single African population. The genetic distance from Africa to Europe (16.6) is shorter than the genetic distance from Africa to East Asia (20.6), and much shorter than that from Africa to Australia (24.7). The simplest explanation for this short genetic distance, according to Cavalli-Sforza, is that substantial gene exchange has taken place between Africa and its neighbouring continents, after the major "]" emigration which was ancestral to ]. Cavalli-Sforza suggested that this admixture took place 30,000 years ago. The overall contributions from Asia and Africa to the modern population were estimated to be around two-thirds and one-third, respectively. Europe's genetic variation is about a third of that of other continents.<ref name="CavalliSforza"/><ref></ref> The timing and number of important human migrations out of Africa remain a major point of discussion today. | |||
| ] (EEMH) lineages between 40 and 26 ka (]) were still part of a large Western Eurasian "meta-population", related to Central and Western Asian populations.<ref name=Seguin-Orlando2014>{{cite journal | vauthors = Seguin-Orlando A, Korneliussen TS, Sikora M, Malaspinas AS, Manica A, Moltke I, Albrechtsen A, Ko A, Margaryan A, Moiseyev V, Goebel T, Westaway M, Lambert D, Khartanovich V, Wall JD, Nigst PR, Foley RA, Lahr MM, Nielsen R, Orlando L, Willerslev E | display-authors = 6 | title = Paleogenomics. Genomic structure in Europeans dating back at least 36,200 years | journal = Science | volume = 346 | issue = 6213 | pages = 1113–1118 | date = November 2014 | pmid = 25378462 | doi = 10.1126/science.aaa0114 | s2cid = 206632421 | bibcode = 2014Sci...346.1113S | url = https://escholarship.org/uc/item/6mb1d8p0 }}</ref> | |||
| According to Guglielmino et al. (1990), | |||
| Divergence into genetically distinct sub-populations within Western Eurasia is a result of increased ] and ]s during the ] (LGM, ]).<ref name=LGMpressures>{{cite journal | vauthors = Beleza S, Santos AM, McEvoy B, Alves I, Martinho C, Cameron E, Shriver MD, Parra EJ, Rocha J | display-authors = 6 | title = The timing of pigmentation lightening in Europeans | journal = Molecular Biology and Evolution | volume = 30 | issue = 1 | pages = 24–35 | date = January 2013 | pmid = 22923467 | pmc = 3525146 | doi = 10.1093/molbev/mss207 }}</ref> | |||
| By the end of the LGM, after 20 ka, A Western European lineage, dubbed ] (WHG) emerged from the ] ] during the ].<ref name=Jones2015>{{cite journal | vauthors = Jones ER, Gonzalez-Fortes G, Connell S, Siska V, Eriksson A, Martiniano R, McLaughlin RL, Gallego Llorente M, Cassidy LM, Gamba C, Meshveliani T, Bar-Yosef O, Müller W, Belfer-Cohen A, Matskevich Z, Jakeli N, Higham TF, Currat M, Lordkipanidze D, Hofreiter M, Manica A, Pinhasi R, Bradley DG | display-authors = 6 | title = Upper Palaeolithic genomes reveal deep roots of modern Eurasians | journal = Nature Communications | volume = 6 | issue = 1 | pages = 8912 | date = November 2015 | pmid = 26567969 | pmc = 4660371 | doi = 10.1038/ncomms9912 | bibcode = 2015NatCo...6.8912J }}</ref> These mesolithic hunter-gatherer cultures are subsequently replaced in the ] as a result of the arrival of ] (EEF) lineages derived from mesolithic populations of West Asia (] and the ]).<ref>Population replacement in the Neolithic, and again in the Bronze Age, was nearly complete in ], the Mesolithic WHG population accounting for just about 10% of the ancestry of the modern indigenous British population. | |||
| <blockquote>Principal coordinate analysis shows that Lapps/Sami are almost exactly intermediate between people located geographically near the Ural mountains and speaking Uralic languages, and central and northern Europeans. Hungarians and Finns are definitely closer to Europeans. An analysis of genetic admixture between Uralic and European ancestors shows that Lapps/Sami are slightly more than 50% European, Hungarians are 87% European, and Finns are 90% European. There is basic agreement between these conclusions and historical data on Hungary. Less is known about Finns and very little about Lapps/Sami.<ref>{{cite journal |author=Guglielmino CR, Piazza A, Menozzi P, Cavalli-Sforza LL |title=Uralic genes in Europe |journal=Am. J. Phys. Anthropol. |volume=83 |issue=1 |pages=57–68 |year=1990 |month=September |pmid=2221031 |doi=10.1002/ajpa.1330830107 }}</ref></blockquote> | |||
| {{cite journal | vauthors = Olalde I, Brace S, Allentoft ME, Armit I, Kristiansen K, Booth T, Rohland N, Mallick S, Szécsényi-Nagy A, Mittnik A, Altena E, Lipson M, Lazaridis I, Harper TK, Patterson N, Broomandkhoshbacht N, Diekmann Y, Faltyskova Z, Fernandes D, Ferry M, Harney E, de Knijff P, Michel M, Oppenheimer J, Stewardson K, Barclay A, Alt KW, Liesau C, Ríos P, Blasco C, Miguel JV, García RM, Fernández AA, Bánffy E, Bernabò-Brea M, Billoin D, Bonsall C, Bonsall L, Allen T, Büster L, Carver S, Navarro LC, Craig OE, Cook GT, Cunliffe B, Denaire A, Dinwiddy KE, Dodwell N, Ernée M, Evans C, Kuchařík M, Farré JF, Fowler C, Gazenbeek M, Pena RG, Haber-Uriarte M, Haduch E, Hey G, Jowett N, Knowles T, Massy K, Pfrengle S, Lefranc P, Lemercier O, Lefebvre A, Martínez CH, Olmo VG, Ramírez AB, Maurandi JL, Majó T, McKinley JI, McSweeney K, Mende BG, Modi A, Kulcsár G, Kiss V, Czene A, Patay R, Endrődi A, Köhler K, Hajdu T, Szeniczey T, Dani J, Bernert Z, Hoole M, Cheronet O, Keating D, Velemínský P, Dobeš M, Candilio F, Brown F, Fernández RF, Herrero-Corral AM, Tusa S, Carnieri E, Lentini L, Valenti A, Zanini A, Waddington C, Delibes G, Guerra-Doce E, Neil B, Brittain M, Luke M, Mortimer R, Desideri J, Besse M, Brücken G, Furmanek M, Hałuszko A, Mackiewicz M, Rapiński A, Leach S, Soriano I, Lillios KT, Cardoso JL, Pearson MP, Włodarczak P, Price TD, Prieto P, Rey PJ, Risch R, Rojo Guerra MA, Schmitt A, Serralongue J, Silva AM, Smrčka V, Vergnaud L, Zilhão J, Caramelli D, Higham T, Thomas MG, Kennett DJ, Fokkens H, Heyd V, Sheridan A, Sjögren KG, Stockhammer PW, Krause J, Pinhasi R, Haak W, Barnes I, Lalueza-Fox C, Reich D | display-authors = 6 | title = The Beaker phenomenon and the genomic transformation of northwest Europe | journal = Nature | volume = 555 | issue = 7695 | pages = 190–196 | date = March 2018 | pmid = 29466337 | pmc = 5973796 | doi = 10.1038/nature25738 | bibcode = 2018Natur.555..190O }}</ref> | |||
| In the ], there were again substantial population replacements in parts of Europe by the intrusion of ] (WSH) lineages from the ]s, arising from admixture between ] (EHG) and peoples related to Near Easterners. These Bronze Age population replacements are associated with the ] and ] cultures archaeologically and with the ] linguistically.<ref>{{Cite journal |last1=Haak |first1=Wolfgang |last2=Lazaridis |first2=Iosif |last3=Patterson |first3=Nick |last4=Rohland |first4=Nadin |last5=Mallick |first5=Swapan |last6=Llamas |first6=Bastien |last7=Brandt |first7=Guido |last8=Nordenfelt |first8=Susanne |last9=Harney |first9=Eadaoin |last10=Stewardson |first10=Kristin |last11=Fu |first11=Qiaomei |last12=Mittnik |first12=Alissa |last13=Bánffy |first13=Eszter |last14=Economou |first14=Christos |last15=Francken |first15=Michael |date=2015|title=Massive migration from the steppe was a source for Indo-European languages in Europe |journal=Nature |language=en |volume=522 |issue=7555 |pages=207–211 |doi=10.1038/nature14317 |issn=1476-4687 |pmc=5048219 |pmid=25731166|arxiv=1502.02783 |bibcode=2015Natur.522..207H }}</ref><ref name="Lazaridis2014">{{cite journal |display-authors=6 |vauthors=Lazaridis I, Patterson N, Mittnik A, Renaud G, Mallick S, Kirsanow K, Sudmant PH, Schraiber JG, Castellano S, Lipson M, Berger B, Economou C, Bollongino R, Fu Q, Bos KI, Nordenfelt S, Li H, de Filippo C, Prüfer K, Sawyer S, Posth C, Haak W, Hallgren F, Fornander E, Rohland N, Delsate D, Francken M, Guinet JM, Wahl J, Ayodo G, Babiker HA, Bailliet G, Balanovska E, Balanovsky O, Barrantes R, Bedoya G, Ben-Ami H, Bene J, Berrada F, Bravi CM, Brisighelli F, Busby GB, Cali F, Churnosov M, Cole DE, Corach D, Damba L, van Driem G, Dryomov S, Dugoujon JM, Fedorova SA, Gallego Romero I, Gubina M, Hammer M, Henn BM, Hervig T, Hodoglugil U, Jha AR, Karachanak-Yankova S, Khusainova R, Khusnutdinova E, Kittles R, Kivisild T, Klitz W, Kučinskas V, Kushniarevich A, Laredj L, Litvinov S, Loukidis T, Mahley RW, Melegh B, Metspalu E, Molina J, Mountain J, Näkkäläjärvi K, Nesheva D, Nyambo T, Osipova L, Parik J, Platonov F, Posukh O, Romano V, Rothhammer F, Rudan I, Ruizbakiev R, Sahakyan H, Sajantila A, Salas A, Starikovskaya EB, Tarekegn A, Toncheva D, Turdikulova S, Uktveryte I, Utevska O, Vasquez R, Villena M, Voevoda M, Winkler CA, Yepiskoposyan L, Zalloua P, Zemunik T, Cooper A, Capelli C, Thomas MG, Ruiz-Linares A, Tishkoff SA, Singh L, Thangaraj K, Villems R, Comas D, Sukernik R, Metspalu M, Meyer M, Eichler EE, Burger J, Slatkin M, Pääbo S, Kelso J, Reich D, Krause J |date=September 2014 |title=Ancient human genomes suggest three ancestral populations for present-day Europeans |journal=Nature |volume=513 |issue=7518 |pages=409–413 |arxiv=1312.6639 |bibcode=2014Natur.513..409L |doi=10.1038/nature13673 |pmc=4170574 |pmid=25230663}}</ref> | |||
| As a result of the population movements during the Mesolithic to Bronze Age, modern European populations are distinguished by differences in WHG, EEF and ] (ANE) ancestry.<ref>Since Lazaridis et al. (2014), further studies have refined the picture of interbreeding between EEF and WHG. | |||
| === DNA studies === | |||
| In a 2017 analysis of 180 ancient DNA datasets of the Chalcolithic and Neolithic periods from Hungary, Germany and Spain | |||
| Studies of the genetic history of Europe have been done using ] (mtDNA), ] DNA and ]. The first allows to trace female lines, the second allows to trace male lines, while the third provides many possible markers (over 500,0000 have been used) but allows descents only to be determined on a statistical basis because of recombination of male/female DNA. By comparing the polymorphisms found in DNA analyses, haplogroup trees have been defined. These have made use of both ] and ], which produce information about long and short timescale changes respectively. | |||
| evidence was found of a prolonged period of EEF-WHG interbreeding. Admixture took place regionally, from local hunter-gatherer populations, so that populations from the three regions (Germany, Iberia and Hungary) were genetically distinguishable at all stages of the Neolithic period, with a gradually increasing ratio of WHG ancestry of farming populations over time. This suggests that after the initial expansion of early farmers, there were no further long-range migrations substantial enough to homogenize the farming population, and that farming and hunter-gatherer populations existed side by side for many centuries, with ongoing gradual admixture throughout the 5th to 4th millennia ] (rather than a single admixture event on initial contact). | |||
| {{cite journal | vauthors = Lipson M, Szécsényi-Nagy A, Mallick S, Pósa A, Stégmár B, Keerl V, Rohland N, Stewardson K, Ferry M, Michel M, Oppenheimer J, Broomandkhoshbacht N, Harney E, Nordenfelt S, Llamas B, Gusztáv Mende B, Köhler K, Oross K, Bondár M, Marton T, Osztás A, Jakucs J, Paluch T, Horváth F, Csengeri P, Koós J, Sebők K, Anders A, Raczky P, Regenye J, Barna JP, Fábián S, Serlegi G, Toldi Z, Gyöngyvér Nagy E, Dani J, Molnár E, Pálfi G, Márk L, Melegh B, Bánfai Z, Domboróczki L, Fernández-Eraso J, Antonio Mujika-Alustiza J, Alonso Fernández C, Jiménez Echevarría J, Bollongino R, Orschiedt J, Schierhold K, Meller H, Cooper A, Burger J, Bánffy E, Alt KW, Lalueza-Fox C, Haak W, Reich D | display-authors = 6 | title = Parallel palaeogenomic transects reveal complex genetic history of early European farmers | journal = Nature | volume = 551 | issue = 7680 | pages = 368–372 | date = November 2017 | pmid = 29144465 | pmc = 5973800 | doi = 10.1038/nature24476 | bibcode = 2017Natur.551..368L }}</ref><ref name="ann">{{Cite web|url=https://www.science.org/content/article/theres-no-such-thing-pure-european-or-anyone-else|title=There's no such thing as a 'pure' European—or anyone else|date=May 15, 2017|website=Science | AAAS}}</ref><ref name="Natgeo">{{cite magazine|url=https://www.nationalgeographic.com/culture/2019/07/first-europeans-immigrants-genetic-testing-feature/|archive-url=https://web.archive.org/web/20190709130347/https://www.nationalgeographic.com/culture/2019/07/first-europeans-immigrants-genetic-testing-feature/|url-status=dead|archive-date=July 9, 2019|title=Genetic testing reveals that Europe is a melting pot, made of immigrants|magazine=National Geographic| vauthors = Curry A |year=2019}}</ref> | |||
| Admixture rates varied geographically; in the late Neolithic, WHG ancestry in farmers in Hungary was at around 10%, in Germany around 25% and in Iberia as high as 50%.<ref>Lipson et al. (2017), </ref> The contribution of EEF is more significant in Mediterranean Europe, and declines towards northern and northeastern Europe, where WHG ancestry is stronger; the ] are considered to be the closest European group to the population of the EEF. | |||
| Ethnogenesis of the modern ] in the ] is associated with numerous admixture events, primarily those associated with the ] and the ], associated with the ], ], and ] expansions | |||
| Worldwide DNA studies have shown that a group of ''Homo sapiens'' left Africa for the ] some 80,000 years ago. Some of their descendants entered Europe about 30,000 years later. The Y-chromosome and the mtDNA haplogroups found in Europe differ in their frequency distributions from those in Africa, Asia and the Middle East. However some predominantly European haplogroups are found in Asia and vice versa. A similar correspondence is found between North Africa and Europe.<ref> See for example Stephen Oppenheimer, ''Out of Eden - the peopling of the World'', or Brian Sykes, ''The Seven Daughters of Eve''.</ref> | |||
| Research into the genetic history of Europe became possible in the second half of the 20th century, but did not yield results with high resolution before the 1990s. In the 1990s, preliminary results became possible, but they remained mostly limited to studies of ] and ] lineages. ] became more easily accessible in the 2000s, and since the mid-2010s, results of previously unattainable resolution, many of them based on full-genome analysis of ancient DNA, have been published at an accelerated pace.<ref name="Dutchen 2015">{{cite web| vauthors = Dutchen S |title=Farming's in Their DNA|url=http://hms.harvard.edu/news/farmings-their-dna|publisher=]|access-date=25 November 2015|date=November 23, 2015}}</ref><ref name="Fu 2016"/> | |||
| This relationship of European to Asian populations has been shown for example by the spread of the Y-chromosome marker Haplogroup N, in Northern Europe. . Several studies strongly suggest a pattern of migrations from Asia to Northern Europe over the last 4000 years. For example, the studies by Zerjal et al. 1997, Su et al. 1999, and Lell et al. 2002 established a significant presence of this Asian marker in different European peoples, ranging from a 52% in Finns, 47% in Lithuanians, 37% in Estonians and 32% in Latvians to 14% in Russians, 11% in Ukranians, 8% in North Swedes, 6% in Gotlanders, 6% in Norwegians, 4% in Poles, 3% in Germans and 1% in Turks, among others. | |||
| == Prehistory == | |||
| == European population structure == | |||
| {{multiple image | |||
| {{POV-section|date=December 2008}} | |||
| | align = right | |||
| In 2006, an autosomal analysis comparing samples from various European populations concluded that “there is a consistent and reproducible distinction between ‘northern’ and ‘southern’ European population groups”. Most individual participants with southern European ancestry (], ], ], ], and ]) have >85% membership in the ‘southern’ population; and most northern, western, eastern, and central Europeans have >90% in the ‘northern’ population group. ]ish as well as ]ish origin also showed >85% membership in the ‘southern’ population, consistent with a later ] origin of these ethnic groups". Many of the participants in this study were actually American citizens who self identified with different European ethnicities and were not Europeans<ref>{{cite journal |author=Seldin MF, Shigeta R, Villoslada P, ''et al.'' |title=European population substructure: clustering of northern and southern populations |journal=PLoS Genet. |volume=2 |issue=9 |pages=e143 |year=2006 |month=September |pmid=17044734 |pmc=1564423 |doi=10.1371/journal.pgen.0020143 |url=http://genetics.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pgen.0020143}}</ref>. | |||
| | caption_align = center | |||
| | direction = vertical | |||
| | header = | |||
| | total_width = 340 | |||
| | image1 = Neanderthal distribution.jpg | |||
| | caption1 = Distribution of the ], and main sites | |||
| | image2 = | |||
| | caption2 = | |||
| | footer = | |||
| | alt1 = | |||
| }} | |||
| Due to natural selection, the percentage of Neanderthal DNA in ancient Europeans gradually decreased over time. From 45,000 BP to 7,000 BP, the percentage dropped from around 3–6% to 2%.<ref name="Fu 2016">{{cite journal | vauthors = Fu Q, Posth C, Hajdinjak M, Petr M, Mallick S, Fernandes D, Furtwängler A, Haak W, Meyer M, Mittnik A, Nickel B, Peltzer A, Rohland N, Slon V, Talamo S, Lazaridis I, Lipson M, Mathieson I, Schiffels S, Skoglund P, Derevianko AP, Drozdov N, Slavinsky V, Tsybankov A, Cremonesi RG, Mallegni F, Gély B, Vacca E, Morales MR, Straus LG, Neugebauer-Maresch C, Teschler-Nicola M, Constantin S, Moldovan OT, Benazzi S, Peresani M, Coppola D, Lari M, Ricci S, Ronchitelli A, Valentin F, Thevenet C, Wehrberger K, Grigorescu D, Rougier H, Crevecoeur I, Flas D, Semal P, Mannino MA, Cupillard C, Bocherens H, Conard NJ, Harvati K, Moiseyev V, Drucker DG, Svoboda J, Richards MP, Caramelli D, Pinhasi R, Kelso J, Patterson N, Krause J, Pääbo S, Reich D | display-authors = 6 | title = The genetic history of Ice Age Europe | journal = Nature | volume = 534 | issue = 7606 | pages = 200–205 | date = June 2016 | pmid = 27135931 | pmc = 4943878 | doi = 10.1038/nature17993 | bibcode = 2016Natur.534..200F }}</ref> The removal of Neanderthal-derived alleles occurred more frequently around genes than other parts of the genome.<ref name="Fu 2016"/> | |||
| === Palaeolithic === | |||
| Somewhat contradicting these findings, a similar study in 2007 using samples exclusively from Europe found that the most important genetic differentiation in Europe occurs on a line from the north to the south-east (northern Europe to the Balkans), with another east-west axis of differentiation across Europe. Its findings were consistent with earlier results based on mtDNA and Y-chromosonal DNA that support the theory that modern ] (Spanish and Portuguese) hold the most ancient European genetic ancestry, as well as separating Basques and Sami from other European populations. It confirmed that the ] and ] cluster with other Northern and Eastern Europeans such as ] and ], while some Basque and Italian individuals also clustered with Northern Europeans. Despite these stratifications, it noted the unusually high degree of European homogeneity: "there is low apparent diversity in Europe with the entire continent-wide samples only marginally more dispersed than single population samples elsewhere in the world."<ref></ref> | |||
| {{Further|Peopling of Europe|Archaic human admixture with modern humans#Neanderthals|Paleolithic Europe}} | |||
| ]s inhabited much of Europe and western Asia from as far back as 130,000 years ago. They existed in Europe as late as 30,000 years ago. They were eventually replaced by ]s (AMH; sometimes known as ]s), who began to appear in Europe circa 40,000 years ago. Given that the two hominid species likely coexisted in Europe, anthropologists have long wondered whether the two interacted.<ref name="sciencemag1525">Even before the advent of genetic studies, some anthropologists believed they had discovered skeletons representing Neanderthal-modern human 'hybrids'. These results were deemed 'ambiguous'. Archaeological evidence points to an abrupt change from Neanderthal artefacts to those related to AMH during the Upper Palaeolithic.{{cite journal | vauthors = Klein RG | title = Paleoanthropology. Whither the Neanderthals? | journal = Science | volume = 299 | issue = 5612 | pages = 1525–1527 | date = March 2003 | pmid = 12624250 | doi = 10.1126/science.1082025 | s2cid = 161836323 }}</ref> The question was resolved only in 2010, when it was established that Eurasian populations exhibit Neanderthal admixture, estimated at 1.5–2.1% on average.<ref name=pruf13comal>{{cite journal | vauthors = Prüfer K, Racimo F, Patterson N, Jay F, Sankararaman S, Sawyer S, Heinze A, Renaud G, Sudmant PH, de Filippo C, Li H, Mallick S, Dannemann M, Fu Q, Kircher M, Kuhlwilm M, Lachmann M, Meyer M, Ongyerth M, Siebauer M, Theunert C, Tandon A, Moorjani P, Pickrell J, Mullikin JC, Vohr SH, Green RE, Hellmann I, Johnson PL, Blanche H, Cann H, Kitzman JO, Shendure J, Eichler EE, Lein ES, Bakken TE, Golovanova LV, Doronichev VB, Shunkov MV, Derevianko AP, Viola B, Slatkin M, Reich D, Kelso J, Pääbo S | display-authors = 6 | title = The complete genome sequence of a Neanderthal from the Altai Mountains | journal = Nature | volume = 505 | issue = 7481 | pages = 43–49 | date = January 2014 | pmid = 24352235 | pmc = 4031459 | doi = 10.1038/nature12886 | orig-year = Online 2013 | bibcode = 2014Natur.505...43P }}</ref> The question now became whether this admixture had taken place in Europe, or rather in the Levant, prior to AMH migration into Europe. | |||
| There has also been speculation about the inheritance of specific genes from Neanderthals. For example, one ] locus ]21.3 which is split into deep genetic lineages H1 and H2. Since the H2 lineage seems restricted to European populations, several authors had argued for inheritance from Neanderthals beginning in 2005.<ref>{{cite journal | vauthors = Hardy J, Pittman A, Myers A, Gwinn-Hardy K, Fung HC, de Silva R, Hutton M, Duckworth J | display-authors = 6 | title = Evidence suggesting that Homo neanderthalensis contributed the H2 MAPT haplotype to Homo sapiens | journal = Biochemical Society Transactions | volume = 33 | issue = Pt 4 | pages = 582–585 | date = August 2005 | pmid = 16042549 | doi = 10.1042/bst0330582 | quote = "We suggest that the H2 haplotype is derived from Homo neanderthalensis and entered H. sapiens populations during the coexistence of these species in Europe from approx. 45 000 to 18 000 years ago and that the H2 haplotype has been under selection pressure since that time, possibly because of the role of this H1 haplotype in neurodegenerative disease."..."The tau (MAPT ) locus is very unusual. Over a region of approx. 1.8 Mb, there are two haplotype clades in European populations, H1 and H2 . In other populations, only the H1 occurs and shows a normal pattern of recombination" }}</ref><ref>{{cite journal | vauthors = Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S, Curley R, Cumming S, Dunn C, Kalaitzopoulos D, Porter K, Prigmore E, Krepischi-Santos AC, Varela MC, Koiffmann CP, Lees AJ, Rosenberg C, Firth HV, de Silva R, Carter NP | display-authors = 6 | title = Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability | journal = Nature Genetics | volume = 38 | issue = 9 | pages = 1032–1037 | date = September 2006 | pmid = 16906163 | doi = 10.1038/ng1858 | s2cid = 38047848 }}</ref><ref>{{cite journal | vauthors = Zody MC, Jiang Z, Fung HC, Antonacci F, Hillier LW, Cardone MF, Graves TA, Kidd JM, Cheng Z, Abouelleil A, Chen L, Wallis J, Glasscock J, Wilson RK, Reily AD, Duckworth J, Ventura M, Hardy J, Warren WC, Eichler EE | display-authors = 6 | title = Evolutionary toggling of the MAPT 17q21.31 inversion region | journal = Nature Genetics | volume = 40 | issue = 9 | pages = 1076–1083 | date = September 2008 | pmid = 19165922 | pmc = 2684794 | doi = 10.1038/ng.193 }}</ref><ref>] and microcephalin FAQ ] </ref><ref>{{cite journal | vauthors = Almos PZ, Horváth S, Czibula A, Raskó I, Sipos B, Bihari P, Béres J, Juhász A, Janka Z, Kálmán J | display-authors = 6 | title = H1 tau haplotype-related genomic variation at 17q21.3 as an Asian heritage of the European Gypsy population | journal = Heredity | volume = 101 | issue = 5 | pages = 416–419 | date = November 2008 | pmid = 18648385 | doi = 10.1038/hdy.2008.70 | quote = In this study, we examine the frequency of a 900 kb inversion at 17q21.3 in the Gypsy and Caucasian populations of Hungary, which may reflect the Asian origin of Gypsy populations. Of the two haplotypes (H1 and H2), H2 is thought to be exclusively of Caucasian origin, and its occurrence in other racial groups is likely to reflect admixture. In our sample, the H1 haplotype was significantly more frequent in the Gypsy population (89.8 vs 75.5%, P<0.001) and was in Hardy–Weinberg disequilibrium (P=0.017). The 17q21.3 region includes the gene of microtubule-associated protein tau, and this result might imply higher sensitivity to H1 haplotype-related multifactorial tauopathies among Gypsies. | doi-access = free }}</ref> | |||
| In fact, according to another European wide study, the main components in the European genomes appear to derive from ancestors whose features were similar to those of modern Basques and Near Easterners, with average values greater than 35% for both these parental populations, regardless of whether or not molecular information is taken into account. The lowest degree of both Basque and Near Eastern admixture is found in ], whereas the highest values are, respectively, 70% ("]") in Spain and more than 60% ("]ern") in the Balkans.<ref name=DupanloupT03>{{cite journal |author=Dupanloup I, Bertorelle G, Chikhi L, Barbujani G |title=Estimating the impact of prehistoric admixture on the genome of Europeans |journal=Mol. Biol. Evol. |volume=21 |issue=7 |pages=1361–72 |year=2004 |month=July |pmid=15044595 |doi=10.1093/molbev/msh135 |url=http://mbe.oxfordjournals.org/cgi/content/full/21/7/1361 |quote=}}</ref> | |||
| However the preliminary results from the sequencing of the full Neanderthal Genome at that time (2009), failed to uncover evidence of interbreeding between Neanderthals and modern humans.<ref>{{cite journal |url=https://www.nytimes.com/2009/02/13/science/13neanderthal.html |title=Scientists in Germany Draft Neanderthal Genome|date=2009-12-02 | vauthors = Wade N |access-date=2010-05-03|newspaper=The New York Times}}</ref><ref>{{cite journal|url=http://news.bbc.co.uk/2/hi/7886477.stm|title=Neanderthals 'distinct from us'|journal=BBC|date=2009-12-02}}</ref> | |||
| By 2010, findings by ] (Max Planck Institute for Evolutionary Anthropology at Leipzig, Germany), Richard E. Green (University of California, Santa Cruz), and ] (Harvard Medical School), comparing the genetic material from the bones of three Neanderthals with that from five modern humans, did show a relationship between Neanderthals and modern people outside Africa. | |||
| ====Upper Paleolithic==== | |||
| In 2008, two international research teams published analyses of large-scale genotyping of large samples of Europeans, revealing relatively little genetic differentiation between the various European ] sampled. (The samples for these papers used overlapped somewhat, though the papers were not collaborative.) But the number of ] included in the analysis was sufficient to detect the geographic region an individual comes from to within about 840 km (for 90% of individuals), as long as the individual's recent ancestry is from that region (for example if an individual has parents from different regions of Europe, then that individual will be placed on the map exactly ] between the parent's populations of origin, and not near either parental population). Southern Europeans have more genetic diversity, having both less ] and greater ], indicating a larger effective population size and/or population expansion from southern to northern Europe, as expected. Populations did not form clusters as previous studies have found (see Seldin ''et al.'' 2006 and Bauchett ''et al.'' 2007), but showed a correlation between genetic distance and geographic distance. The researchers take this observation to imply that, genetically speaking, Europeans are not distributed into discrete, populations.<ref>{{cite journal |author=Novembre J, Johnson T, Bryc K, ''et al.'' |title=Genes mirror geography within Europe |journal=Nature |volume=456 |issue=7218 |pages=98–101 |year=2008 |month=November |pmid=18758442 |doi=10.1038/nature07331 }}</ref><ref>{{cite journal |author=Lao O, Lu TT, Nothnagel M, ''et al.'' |title=Correlation between genetic and geographic structure in Europe |journal=Curr. Biol. |volume=18 |issue=16 |pages=1241–8 |year=2008 |month=August |pmid=18691889 |doi=10.1016/j.cub.2008.07.049 |url=http://linkinghub.elsevier.com/retrieve/pii/S0960-9822(08)00956-1}}</ref> | |||
| {{main|Upper Paleolithic|Early European modern humans}} | |||
| ] | |||
| It is thought that modern humans began to inhabit Europe during the Upper Paleolithic about 40,000 years ago. Some evidence shows the spread of the ] culture.<ref name = "Milisauskas_2002">{{cite book| vauthors = Milisauskas S |title=European Prehistory: a survey |year=2002 |publisher=]|isbn=978-0-306-46793-6}}</ref>{{rp|59}} | |||
| From a purely patrilineal, ] perspective, it appears that ], ] and ] may be those with the oldest presence in Europe. They have been found in some of the oldest human remains sequenced from the ]. However, other haplogroups are far more common among modern European males, because of later demographic changes. | |||
| A very recent study in May 2009 <ref>, Nelis et al. 2009</ref> that studied 19 populations from Europe using 270,000 SNPs highlighted the genetic diversity or European populations corresponding to the northwest to southeast gradient and distinguished "four several distinct regions" within Europe: | |||
| * ] | |||
| * the ] region (], ] and ]), Eastern ] and ] | |||
| * Central and Western Europe | |||
| * ], "with the southern Italians being more distant" | |||
| In this study, Fst (]) was found to correlate considerably with geographic distances ranging from ≤0.0010 for neighbouring populations to 0.0200-0.0230 for Southern Italy and Finland. For comparisons, pair-wise Fst of non-european samples were as follows: Europeans – Africans (Yoruba) 0.1530; Europeans – Chinese 0.1100; Africans (Yoruba) – Chinese 0.1900 <ref></ref>. | |||
| Currently the oldest sample of ] (M170), which is now relatively common and widespread within Europe, has been found to be Krems WA3 from Lower Austria dating back to about 30–31,000 ybp.<ref>{{Cite journal|last1=Teschler-Nicola|first1=Maria|last2=Fernandes|first2=Daniel|last3=Händel|first3=Marc|last4=Einwögerer|first4=Thomas|last5=Simon|first5=Ulrich|last6=Neugebauer-Maresch|first6=Christine|last7=Tangl|first7=Stefan|last8=Heimel|first8=Patrick|last9=Dobsak|first9=Toni|last10=Retzmann|first10=Anika|last11=Prohaska|first11=Thomas|date=2020-11-06|title=Ancient DNA reveals monozygotic newborn twins from the Upper Palaeolithic|journal=Communications Biology|language=en|volume=3|issue=1|page=650|doi=10.1038/s42003-020-01372-8|pmid=33159107|pmc=7648643|issn=2399-3642|doi-access=free}}</ref> At about this time, an Upper Palaeolithic culture also appeared, known as the ].<ref name="Semino_2000"/> | |||
| == Haplogroups in Europe == | |||
| === Human Y-chromosome DNA haplogroups === | |||
| ] (purple) and ] (red). Two of the three most common ] in ]. Black represents all the other haplogroups.]] | |||
| There are three major ] ]s which largely account for most of Europe's present-day ]<ref name="DNA Heritage">{{cite journal |author=Semino O, Passarino G, Oefner PJ, ''et al.'' |title=The genetic legacy of Paleolithic Homo sapiens sapiens in extant Europeans: a Y chromosome perspective |journal=Science |volume=290 |issue=5494 |pages=1155–9 |year=2000 |month=November |pmid=11073453 |url=http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=11073453}} Note: Haplogroup names are different in this article. For example: Haplogroup I is referred as M170</ref>. | |||
| * ] ] is common on the western ], from the ] (comprising ] and ]) to ], ], ] and ], and ]. | |||
| * ] ] is common across ], the ], ], and up into ], as well as a very high amount in the western ]. | |||
| * ] ] is common in ] and ] (and is also common in ] and the ]). | |||
| Earlier research into Y-DNA had instead focused on ] (M173): the most populous lineage among living European males; R1 was also believed to have emerged ~ 40,000 BP in ].<ref name="Semino_2000" /><ref name="Wells_2001" /> However, it is now estimated that R1 emerged substantially more recently: a 2008 study dated the most recent common ancestor of haplogroup IJ to 38,500 and haplogroup R1 to 18,000 BP. This suggested that haplogroup IJ colonists formed the first wave and haplogroup R1 arrived much later.<ref>{{cite journal | vauthors = Karafet TM, Mendez FL, Meilerman MB, Underhill PA, Zegura SL, Hammer MF | title = New binary polymorphisms reshape and increase resolution of the human Y chromosomal haplogroup tree | journal = Genome Research | volume = 18 | issue = 5 | pages = 830–838 | date = May 2008 | pmid = 18385274 | pmc = 2336805 | doi = 10.1101/gr.7172008 }}</ref> | |||
| Most common of all haplogroups among western Europeans is ].<ref></ref><ref></ref> The following values of Hg ] are: ]: 89.0%; ]: 88.1%; ]: 81.5%; ]: 77.1%; ]: 68.8; Non-Basque Spaniards: 68.0 (]: 79.2; ]: 65.5); ]: 63.0; ] (North): 62.0%; ] (North-central): 62.0; ] (Central): 61.9%; Portuguese (South): 56.0%; ]: 52.2%; ]: 41.7%, ]: 47.9; ] & ]: 35.6%; Italians (]): 32.4; ]: 25.9; ]: 22.8%; Italians (]): 22.1%; ]: 21%; ]: 20.0; ]: 18.0%; ]: 17.6%; ]: 17.0%; ]: 16.4%; ]: 16.3%; ] (mainland): 15.7%; ]: 13.3%; ]: 10.6%; ]: 9.0%. <ref>{{cite journal |author=Pericić M, Lauc LB, Klarić IM, ''et al.'' |title=High-resolution phylogenetic analysis of southeastern Europe traces major episodes of paternal gene flow among Slavic populations |journal=Mol. Biol. Evol. |volume=22 |issue=10 |pages=1964–75 |year=2005 |month=October |pmid=15944443 |doi=10.1093/molbev/msi185 |url=http://mbe.oxfordjournals.org/cgi/content/full/22/10/1964 |quote=}}</ref> | |||
| Thus the genetic data suggests that, at least from the perspective of patrilineal ancestry, separate groups of modern humans took two routes into Europe: from the Middle East via the Balkans and another from Central Asia via the ], to the north of the ]. | |||
| Among European populations, diversity is highest in Eastern Europe, despite lower frequencies. Diversity analysis indicates that all European variants of R1b shared an existence in Central Asia (]) before migrating to ] and then splitting into two major migrations, moving primarily along rivers and coastlines.<ref></ref> | |||
| Martin Richards ''et al.'' found that 15–40% of extant mtDNA lineages trace back to the Palaeolithic migrations (depending on whether one allows for multiple founder events).<ref name = "Richards_2000">{{cite journal | vauthors = Richards M, Macaulay V, Hickey E, Vega E, Sykes B, Guida V, Rengo C, Sellitto D, Cruciani F, Kivisild T, Villems R, Thomas M, Rychkov S, Rychkov O, Rychkov Y, Gölge M, Dimitrov D, Hill E, Bradley D, Romano V, Calì F, Vona G, Demaine A, Papiha S, Triantaphyllidis C, Stefanescu G, Hatina J, Belledi M, Di Rienzo A, Novelletto A, Oppenheim A, Nørby S, Al-Zaheri N, Santachiara-Benerecetti S, Scozari R, Torroni A, Bandelt HJ | display-authors = 6 | title = Tracing European founder lineages in the Near Eastern mtDNA pool | journal = American Journal of Human Genetics | volume = 67 | issue = 5 | pages = 1251–1276 | date = November 2000 | pmid = 11032788 | pmc = 1288566 | doi = 10.1016/S0002-9297(07)62954-1 }}</ref> MtDNA haplogroup U5, dated to be ~ 40–50 kYa, arrived during the first early upper Palaeolithic colonisation. Individually, it accounts for 5–15% of total mtDNA lineages. Middle U.P. movements are marked by the haplogroups HV, I and U4. HV split into Pre-V (around 26,000 years old) and the larger branch H, both of which spread over Europe, possibly via Gravettian contacts.<ref name="Semino_2000"/><ref>{{cite journal | vauthors = Torroni A, Bandelt HJ, Macaulay V, Richards M, Cruciani F, Rengo C, Martinez-Cabrera V, Villems R, Kivisild T, Metspalu E, Parik J, Tolk HV, Tambets K, Forster P, Karger B, Francalacci P, Rudan P, Janicijevic B, Rickards O, Savontaus ML, Huoponen K, Laitinen V, Koivumäki S, Sykes B, Hickey E, Novelletto A, Moral P, Sellitto D, Coppa A, Al-Zaheri N, Santachiara-Benerecetti AS, Semino O, Scozzari R | display-authors = 6 | title = A signal, from human mtDNA, of postglacial recolonization in Europe | journal = American Journal of Human Genetics | volume = 69 | issue = 4 | pages = 844–852 | date = October 2001 | pmid = 11517423 | pmc = 1226069 | doi = 10.1086/323485 }}</ref> | |||
| Each haplogroup ] has ]s.<ref></ref> R1a and R1b are subclades of ]<ref></ref> Two main subgroups of ] are ], which according to the ] "has highest frequency in Scandinavia, Iceland, and northwest Europe" and I-S31, which according to the International Society of Genetic Genealogy, "includes I-P37.2, which is the most common form in the Balkans and Sardinia, and I-S23/I-S30/I-S32/I-S33, which reaches its highest frequency along the northwest coast of continental Europe."<ref></ref> | |||
| ] accounts for about half the gene lines in Europe, with many subgroups. The above mtDNA lineages or their precursors, are most likely to have arrived into Europe via the Middle East. This contrasts with Y ], whereby some 50%-plus of male lineages are characterised by the R1 superfamily, which is of possible central Asian origin.{{citation needed|date=November 2021}} Ornella Semino postulates that these differences "may be due in part to the apparent more recent molecular age of Y chromosomes relative to other loci, suggesting more rapid replacement of previous Y chromosomes. Gender-based differential migratory demographic behaviors will also influence the observed patterns of mtDNA and Y variation"{{citation needed|date=November 2021}}. | |||
| The extent of the population changes resulting from the introduction of ] technology into ] are still debated. There is evidence both for and against a dominating affect from "]" from the ]: G Barbujani and L Chikhi (2006) state, | |||
| ====Last Glacial Maximum==== | |||
| :Genetic studies have failed to settle the controversy so far, because they have been interpreted in different ways... A rather heated debate followed, and is still continuing."<ref>{{cite journal |author=Barbujani G, Chikhi L |title=Population genetics: DNAs from the European Neolithic |journal=Heredity |volume=97 |issue=2 |pages=84–5 |year=2006 |month=August |pmid=16721387 |doi=10.1038/sj.hdy.6800852 |url=http://www.nature.com/hdy/journal/v97/n2/full/6800852a.html}}</ref><ref name=DupanloupT03/><ref>{{cite journal |author=Dupanloup I, Bertorelle G, Chikhi L, Barbujani G |title=Estimating the impact of prehistoric admixture on the genome of Europeans |journal=Mol. Biol. Evol. |volume=21 |issue=7 |pages=1361–72 |year=2004 |month=July |pmid=15044595 |doi=10.1093/molbev/msh135 |url=http://mbe.oxfordjournals.org/cgi/content/full/21/7/1361 |quote=}}</ref> | |||
| {{main|Early European modern humans}} | |||
| {{Further|Last Glacial Maximum|Last Glacial Maximum refugia}} | |||
| [[Image:Europe20000ya.png|thumb|250px|European LGM refuges, 20 kya<br /> | |||
| {{legend|#c54b00|Solutrean and Proto-Solutrean Cultures}} | |||
| {{legend|#ca00b0|Epi-Gravettian Culture}} | |||
| ]] | |||
| The Last Glacial Maximum ("LGM") started c. 30 ka BCE, at the end of ], leading to a depopulation of Northern Europe. According to the classical model, people took refuge in climatic sanctuaries (or refugia) as follows: | |||
| * Northern Iberia and Southwest ], together making up the "Franco-Cantabrian" refugium | |||
| * The Balkans | |||
| * ] and more generally the northern coast of the Black Sea<ref name="Semino_2000"/> | |||
| * Italy.<ref>{{cite journal | vauthors = Pala M, Achilli A, Olivieri A, Hooshiar Kashani B, Perego UA, Sanna D, Metspalu E, Tambets K, Tamm E, Accetturo M, Carossa V, Lancioni H, Panara F, Zimmermann B, Huber G, Al-Zahery N, Brisighelli F, Woodward SR, Francalacci P, Parson W, Salas A, Behar DM, Villems R, Semino O, Bandelt HJ, Torroni A | display-authors = 6 | title = Mitochondrial haplogroup U5b3: a distant echo of the epipaleolithic in Italy and the legacy of the early Sardinians | journal = American Journal of Human Genetics | volume = 84 | issue = 6 | pages = 814–821 | date = June 2009 | pmid = 19500771 | pmc = 2694970 | doi = 10.1016/j.ajhg.2009.05.004 }}</ref> | |||
| This event decreased the overall genetic diversity in Europe, a "result of drift, consistent with an inferred population bottleneck during the Last Glacial Maximum".<ref name="Wells_2001">{{cite journal | vauthors = Wells RS, Yuldasheva N, Ruzibakiev R, Underhill PA, Evseeva I, Blue-Smith J, Jin L, Su B, Pitchappan R, Shanmugalakshmi S, Balakrishnan K, Read M, Pearson NM, Zerjal T, Webster MT, Zholoshvili I, Jamarjashvili E, Gambarov S, Nikbin B, Dostiev A, Aknazarov O, Zalloua P, Tsoy I, Kitaev M, Mirrakhimov M, Chariev A, Bodmer WF | display-authors = 6 | title = The Eurasian heartland: a continental perspective on Y-chromosome diversity | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 98 | issue = 18 | pages = 10244–10249 | date = August 2001 | pmid = 11526236 | pmc = 56946 | doi = 10.1073/pnas.171305098 | bibcode = 2001PNAS...9810244W | doi-access = free }}</ref> As the glaciers receded from about 16,000–13,000 years ago, Europe began to be slowly repopulated by people from refugia, leaving genetic signatures.<ref name="Semino_2000" /> | |||
| Also, around 4,500 years ago, ] began moving westward from west of the ], and seems to follow closely the spread of the ].<ref name="DNA Heritage"/> It is also present at high frequencies in northern Russians, reflecting the absorption of Finno-Ugric tribes.<ref>{{cite journal |author=Balanovsky O, Rootsi S, Pshenichnov A, ''et al.'' |title=Two sources of the Russian patrilineal heritage in their Eurasian context |journal=Am. J. Hum. Genet. |volume=82 |issue=1 |pages=236–50 |year=2008 |month=January |pmid=18179905 |pmc=2253976 |doi=10.1016/j.ajhg.2007.09.019 |url=http://www.ajhg.org/AJHG/fulltext/S0002-9297(07)00025-0}}</ref> | |||
| Some Y haplogroup I clades appear to have diverged from their parental haplogroups sometime during or shortly after the LGM.<ref name=rootsi/> | |||
| === Human mitochondrial DNA haplogroups === | |||
| There have been a number of studies about the ] (]) in Europe. According to the ] Library in Finland: | |||
| Cinnioglu sees evidence for the existence of an Anatolian refuge, which also harboured Hg R1b1b2.<ref>Cinnioglu et al. ''Excavating Y-chromosome haplotype strata in Anatolia''. 2003</ref> Today, R1b dominates the y chromosome landscape of western Europe, including the British Isles, suggesting that there could have been large population composition changes based on migrations after the LGM. | |||
| <blockquote>Classical polymorphic markers (i.e. blood groups, protein electromorphs and HLA antigenes) have suggested that Europe is a genetically homogeneous continent with a few outliers such as the ], ], ] and Basques (Cavalli-Sforza et al. 1993, Piazza 1993). The analysis of mtDNA sequences has also shown a high degree of homogeneity among European populations, and the genetic distances have been found to be much smaller than between populations on other continents, especially Africa (Comas et al. 1997).</blockquote> | |||
| Semino, Passarino and Pericic place the origins of haplogroup '''R1a''' within the Ukrainian ] refuge. Its current distribution in eastern Europe and parts of Scandinavia are in part reflective of a re-peopling of Europe from the southern Russian/Ukrainian steppes after the ].<ref name="Peričic_2005" /><ref name = "Passarino_2001">{{cite journal | vauthors = Passarino G, Semino O, Magri C, Al-Zahery N, Benuzzi G, Quintana-Murci L, Andellnovic S, Bullc-Jakus F, Liu A, Arslan A, Santachiara-Benerecetti AS | display-authors = 6 | title = The 49a,f haplotype 11 is a new marker of the EU19 lineage that traces migrations from northern regions of the Black Sea | journal = Human Immunology | volume = 62 | issue = 9 | pages = 922–932 | date = September 2001 | pmid = 11543894 | doi = 10.1016/S0198-8859(01)00291-9 }}</ref><ref name="Semino_2000" /> | |||
| <blockquote>The mtDNA haplogroups<ref></ref> of Europeans are surveyed by using a combination of data from RFLP analysis of the coding region and sequencing of the hypervariable segment I. About 99% of European mtDNAs fall into one of ten haplogroups: H, I, J, K, M, T, U, V, W or X (Torroni et al. 1996a). Each of these is defined by certain relatively ancient and stable polymorphic sites located in the coding region (Torroni et al. 1996a)... Haplogroup H, which is defined by the absence of a AluI site at bp 7025, is the most prevalent, comprising half of all Europeans (Torroni et al. 1996a, Richards et al. 1998)... Six of the European haplogroups (H, I, J, K, T and W) are essentially confined to European populations (Torroni et al. 1994, 1996a), and probably originated after the ancestral Caucasoids became genetically separated from the ancestors of the modern Africans and Asians.<ref>, Oulu University Library (Finland)</ref></blockquote> | |||
| From an mtDNA perspective, Richards ''et al.'' found that the majority of mtDNA diversity in Europe is accounted for by post-glacial re-expansions during the late upper Palaeolithic/ Mesolithic. "The regional analyses lend some support to the suggestion that much of western and central Europe was repopulated largely from the southwest when the climate improved. The lineages involved include much of the most common haplogroup, H, as well as much of K, T, W, and X." The study could not determine whether there were new migrations of mtDNA lineages from the near east during this period; a significant input was deemed unlikely.<ref name = "Richards_2000" /> | |||
| ==Migrations into Europe== | |||
| The ] of the European peoples can be traced by the examination of ] sites, ], and by the examination of the ] of the people who live in Europe now, or from recovered ]. Much of this research is ongoing, and discoveries are still being continually made, so theories rise and fall. Although it is possible to track the various migrations of people across Europe using founder analysis of DNA, most information on these movements comes from archeology. | |||
| The alternative model of more refugees was discussed by Bilton et al.<ref>{{cite journal | vauthors = Bilton DT, Mirol PM, Mascheretti S, Fredga K, Zima J, Searle JB | title = Mediterranean Europe as an area of endemism for small mammals rather than a source for northwards postglacial colonization | journal = Proceedings. Biological Sciences | volume = 265 | issue = 1402 | pages = 1219–1226 | date = July 1998 | pmid = 9699314 | pmc = 1689182 | doi = 10.1098/rspb.1998.0423 }}</ref> | |||
| Five patterns of genetic relatedness have been identified since the early "principle components" studies of Cavalli-Sforza: | |||
| #A cline of genes with highest frequencies in the Middle East, spreading to lowest levels northwest. | |||
| #A cline of genes with highest frequencies amongst Finnish and Saami in the extreme north east, and spreading to lowest frequencies in the south west. | |||
| #A cline of genes with highest frequencies in the area of the lower Don and Volga rivers in southern Russia, and spreading to lowest frequencies in Iberia, Southern Italy, Greece and the areas inhabited Saami speakers in the extreme north of Scandinavia. | |||
| #A cline of genes with highest frequencies in the Balkans and Southern Italy, spreading to lowest levels in Britain and the Basque country. | |||
| #A cline of genes with highest frequencies in the Basque country, and lower levels beyond the area of Iberia and Southern France. | |||
| From a study of 51 individuals, researchers were able to identify five separate genetic clusters of ancient Eurasians during the LGM: the ] (34,000–26,000 years ago), associated with the ]; the Mal'ta Cluster (24,000–17,000), associated with the ], the ] (19,000–14,000 years ago), associated with the ]; the ] (14,000–7,000 years ago) and the ] (13,000 to 10,000 years ago).<ref name="Fu 2016"/> | |||
| The exact causes of each of these is the subject of continuing discussion concerning different regions of Europe, as well as different periods of time... | |||
| From around 37,000 years ago, all ancient Europeans began to share some ancestry with modern Europeans.<ref name="Fu 2016"/> This founding population is represented by GoyetQ116-1, a 35,000 year old specimen from Belgium.<ref name="Fu 2016"/> This lineage disappears from the record and is not found again until 19,000 BP in Spain at El Mirón, which shows strong affinities to GoyetQ116-1.<ref name="Fu 2016"/> During this interval, the distinct Věstonice Cluster is predominant in Europe, even at ].<ref name="Fu 2016"/> The re-expansion of the El Mirón Cluster coincided with warming temperatures following the retreat of the glaciers during the ].<ref name="Fu 2016"/> From 37,000 to 14,000 years ago, the population of Europe consisted of an isolated population descended from a founding population that didn't interbreed significantly with other populations.<ref name="Dutchen 2016">{{cite web|vauthors = Dutchen S |title=History on Ice|url=http://hms.harvard.edu/news/history-ice|publisher=]|access-date=11 May 2016|date=May 2, 2016}}</ref> | |||
| ===Gene flow from Africa=== | |||
| ] from Africa into Europe according to Semino et al 2004]] | |||
| Gene flow from Africa is primarily represented by ], which entered Europe in the late pleistocene or early neolithic. At least 7.2% of European Males carry the African Haplogroup.<ref name="cruciani2004">{{cite journal|last=Cruciani et al|year=2004 |url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1181964 |title=Phylogeographic Analysis of Haplogroup E3b (E-M215) Y Chromosomes Reveals Multiple Migratory Events Within and Out Of Africa}}</ref> | |||
| ===Mesolithic=== | |||
| The Mediterranean Sea and the Sahara Desert were formidable barriers to gene flow between Sub-Saharan Africa and Europe. But Europe was periodically accessible by Africans due to fluctuations in the size and climate of the Sahara. At the ], Africa and Europe are separated by only 15km of Ocean. At the Suez, Eurasia is connected to Africa. The Nile river valley, which runs from East Africa to the Mediterranean Sea served as a bidirectional corridor in the Sahara desert, that frequently connected people from Sub-Saharan Africa with the peoples of Eurasia. | |||
| {{see|Mesolithic Europe|Western hunter-gatherer|Caucasus hunter-gatherer}} | |||
| <ref></ref> | |||
| ] (post-LGM) populations had diverged significantly due to their relative isolation over several millennia, to the harsh selection pressures during the LGM, and to the ]s caused by the rapid expansion from ] in the beginning Mesolithic.<ref name=LGMpressures/> | |||
| By the end of the LGM, around 19 to 11 ka, the familiar varieties of Eurasian phenotypes had emerged. However, the lineage of Mesolithic hunter-gatherers of Western Europe (WHG) does not survive as a majority contribution in any modern population. They were most likely blue eyed, and retained the dark skin pigmentation of pre-LGM EEMH.<ref name="Mathieson 2015"/> The ] and ] variations for blue eyes are derived from the WHG lineage were also found in the ].<ref name="Mathieson 2015"/>{{contradict-inline|date=August 2019}}<!--if it is found both in WHG and ANE, why the claim that it is derived from WHG?--> | |||
| Around 14,000 years ago, the ] shifted away from GoyetQ116-1 affinity and started to show more affinity with the Near East, a shift which coincided with the warming temperatures of the ] interstadial.<ref name="Fu 2016"/> This genetic shift shows that Near East populations had probably already begun moving into Europe during the end of the Upper Paleolithic, about 6,000 years earlier than previously thought, before the introduction of farming.<ref name="Dutchen 2016"/> A few specimens from the Villabruna Cluster also show genetic affinities for East Asians that are derived from gene flow.<ref name="Fu 2016"/><ref name="Dutchen 2016"/> | |||
| === Migrations before the Last Glacial Maximum=== | |||
| The ] variation for blue eyes first appears around 13,000 to 14,000 years ago in Italy and the Caucasus.<ref name="Fu 2016"/> | |||
| <!-- Deleted image removed: ] ] ('']'') tooth found in ], ], in June 2007]] --> | |||
| The ] pigmentation characteristic of modern Europeans is estimated to have spread across Europe in a "selective sweep" during the | |||
| {{see|Paleolithic Europe}} | |||
| Mesolithic (19 to 11 ka). The associated ] ] and ] alleles emerge around 19 ka, still during the LGM, most likely in the Caucasus.<ref name="LGMpressures"/><ref name="Jones2015"/> | |||
| ===Neolithic=== | |||
| ] | |||
| {{Further|Neolithic Europe|Neolithic Revolution|Early European Farmers|Holocene}} | |||
| ] period in the ]<ref>{{cite journal | vauthors = Sikora M, Carpenter ML, Moreno-Estrada A, Henn BM, Underhill PA, Sánchez-Quinto F, Zara I, Pitzalis M, Sidore C, Busonero F, Maschio A, Angius A, Jones C, Mendoza-Revilla J, Nekhrizov G, Dimitrova D, Theodossiev N, Harkins TT, Keller A, Maixner F, Zink A, Abecasis G, Sanna S, Cucca F, Bustamante CD | display-authors = 6 | title = Population genomic analysis of ancient and modern genomes yields new insights into the genetic ancestry of the Tyrolean Iceman and the genetic structure of Europe | journal = PLOS Genetics | volume = 10 | issue = 5 | pages = e1004353 | date = May 2014 | pmid = 24809476 | pmc = 4014435 | doi = 10.1371/journal.pgen.1004353 | doi-access = free }}</ref>]] | |||
| ] | |||
| A big cline in genetic variation that has long been recognised in Europe seems to show important dispersals from the direction of the Middle East. This has often been linked to the spread of farming technology during the Neolithic, which has been argued to be one of the most important periods in determining modern European genetic diversity. | |||
| The Neolithic started with the introduction of farming, beginning in SE Europe approximately 10,000–3000 BCE, and extending into NW Europe between 4500 and 1700 BCE. During this era, the ] led to drastic economic as well as socio-cultural changes in Europe and this is also thought to have had a big effect on Europe's genetic diversity, especially concerning genetic lineages entering Europe from the Middle East into the Balkans. There were several phases of this period: | |||
| Modern humans (]) began to colonize Europe in the Paleolithic about 40,000 years ago, as evidenced by the spread of the ]. Modern humans may have arrived along two major routes either side of the ]. By about 25,000 years ago, the prior inhabitants (our cousin species '']'') were either killed off or absorbed into the population and ultimately became extinct.<ref>{{cite journal |author=Klein RG |title=Paleoanthropology. Whither the Neanderthals? |journal=Science |volume=299 |issue=5612 |pages=1525–7 |year=2003 |month=March |pmid=12624250 |doi=10.1126/science.1082025 |url=http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=12624250}}</ref> Martin Richards ''et al.'' found that 21% of extant studied the mtDNA lines of Europe came from pre-glacial migrations.<ref name=Richards00>{{cite journal |author=Richards M, Macaulay V, Hickey E, ''et al.'' |title=Tracing European founder lineages in the Near Eastern mtDNA pool |journal=Am. J. Hum. Genet. |volume=67 |issue=5 |pages=1251–76 |year=2000 |month=November |pmid=11032788 |pmc=1288566 |url=http://linkinghub.elsevier.com/retrieve/pii/S0002-9297(07)62954-1}}</ref> The two main haplogroups were U and HV, which arrived around 50,000 and 35,000 years ago respectively. Early on, HV split into Pre-V (around 26,000 years old) and the larger branch H, both of which spread over all Europe. Haplogroup H accounts for about half the gene lines in Europe, with many subgroups. The large Y-chromosome haplogroup R1 moved into Central Europe from the east 30,000 years ago and to the south-west before the LGM. | |||
| * In a late European Mesolithic prelude to the Neolithic, it appears that Near Eastern peoples from areas that already had farming, and who also had sea-faring technology, had a transient presence in Greece (for example at ]).<ref>Perlès C, Monthel G ( 2001) The Early Neolithic in Greece: The First Farming Communities in Europe. Cambridge University Press, Cambridge.</ref><ref>Runnels C (2003) The origins of the Greek Neolithic: a personal view, in Ammerman and Biagi (2003 eds).</ref> | |||
| * There is consensus that ] and the main breeds of animals and plants which are farmed entered Europe from somewhere in the area of the ] and specifically the ] region from the ] to ].<ref name = "Milisauskas_2002" />{{rp|1143, 1150}}<ref>{{cite journal | vauthors = Zeder MA | title = Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 105 | issue = 33 | pages = 11597–11604 | date = August 2008 | pmid = 18697943 | pmc = 2575338 | doi = 10.1073/pnas.0801317105 | name-list-style = vanc | doi-access = free | bibcode = 2008PNAS..10511597Z }}</ref> (Less certainly, this ] is sometimes argued to have in turn been partly triggered by movements of people and technology coming across the Sinai from Africa.) For more see ]. | |||
| * A later stage of the Neolithic, the so-called ], saw an introduction of ] into the Levant, Balkans and Southern Italy (it had been present in the area of modern Sudan for some time before it is found in the ], but it is thought to have developed independently), and this may have also been a period of cultural transfer from the Levant into the Balkans. | |||
| An important issue regarding the genetic impact of neolithic technologies in Europe is the manner by which they were transferred into Europe. Farming was introduced by a significant migration of farmers from the Near East (Cavalli-Sforza's biological ] model) or a "]" or a combination of the two, and ] have tried to clarify whether any genetic signatures of Near Eastern origin correspond to the expansion routes postulated by the archaeological evidence.<ref name = "Milisauskas_2002" />{{rp|146}} | |||
| === Last Glacial Maximum: Refugia=== | |||
| {{See|Last Glacial Maximum}} | |||
| Martin Richards estimated that only 11% of European mtDNA is due to immigration in this period, suggesting that farming was spread primarily due to being adopted by indigenous Mesolithic populations, rather than due to immigration from Near East. Gene flow from SE to NW Europe seems to have continued in the Neolithic, the percentage significantly declining towards the British Isles. ] also suggested that the largest admixture to the European Paleolithic/Mesolithic stock was due to the ] of the 7th to 5th millennia BCE.<ref>{{cite book | vauthors = Piazza A, Cavalli-Sforza LL, Menozzi P |title=The history and geography of human genes |publisher=Princeton University Press |location=Princeton, N.J |year=1994 |isbn=978-0-691-08750-4}}</ref> Three main mtDNA gene groups have been identified as contributing Neolithic entrants into Europe: J, T1 and U3 (in that order of importance). With others, they amount up to around 20% of the ].<ref name = "Richards_2000" /> | |||
| About 25,000 years ago began the last very cold period (the ], LGM), rendering much of Europe uninhabitable. Humans may only have occupied certain regions of Europe at this time. These areas are often called refuges (or refugia) and were located along the northern ] and ] coasts, as well as in the ] and ]. As ] from about 16,000 years ago, the populations that had occupied the refuges are thought to have begun to spread and colonise northern Europe.<ref>{{cite journal |author=Torroni A, Bandelt HJ, Macaulay V, ''et al.'' |title=A signal, from human mtDNA, of postglacial recolonization in Europe |journal=Am. J. Hum. Genet. |volume=69 |issue=4 |pages=844–52 |year=2001 |month=October |pmid=11517423 |pmc=1226069 |doi=10.1086/323485 |url=http://linkinghub.elsevier.com/retrieve/pii/S0002-9297(07)61139-2}}</ref><ref name="DNA Heritage"/> Pre-V's daughter group V (from about 16,300 years ago) gave the first indication of spread from the Galacian refuge, being found mostly in western and northern regions. It is found with highest frequency in ] and ], but also in south-west France and with declining frequency up the Atlantic coast. Subgroups of haplogroup H seem to have originated in the Iberian refuge and spread, for example, into the British Isles. Presumably as a result of genetic drift, only one Y-chromosome subgroup (R1b) emerged from the Iberian refuge and colonised western Europe. Its frequency of occurrence declines from 90% in Basques to 50% in Germans and 0% around the eastern Baltic coast. In the north-west R1b is partly replaced by R1a, coming from an eastern refuge. There is also an exclusively European haplogroup I, deriving from the Balkans but with high frequencies in Scandinavia and northern Germany. Martin Richards estimates that overall around 50% of mtDNA arrived in the Late Upper Paleolithic, while only around 20% of Y-chromosomes did. | |||
| In 2000, Semino's study on Y DNA revealed the presence of haplotypes belonging to the large clade ] (E-M35). These were predominantly found in the southern Balkans, southern Italy and parts of Iberia. Semino connected this pattern, along with J haplogroup subclades, to be the Y-DNA component of Cavalli-Sforza's Neolithic demic-diffusion of farmers from the Near East.<ref name="Semino_2000" />{{rp|Here, the clade E-M35 is referred to as "Eu 4"}} Rosser et al. rather saw it as a (direct) 'North African component' in European genealogy, although they did not propose a timing and mechanism to account for it.<ref name = "Rosser_2000">{{cite journal | vauthors = Rosser ZH, Zerjal T, Hurles ME, Adojaan M, Alavantic D, Amorim A, Amos W, Armenteros M, Arroyo E, Barbujani G, Beckman G, Beckman L, Bertranpetit J, Bosch E, Bradley DG, Brede G, Cooper G, Côrte-Real HB, de Knijff P, Decorte R, Dubrova YE, Evgrafov O, Gilissen A, Glisic S, Gölge M, Hill EW, Jeziorowska A, Kalaydjieva L, Kayser M, Kivisild T, Kravchenko SA, Krumina A, Kucinskas V, Lavinha J, Livshits LA, Malaspina P, Maria S, McElreavey K, Meitinger TA, Mikelsaar AV, Mitchell RJ, Nafa K, Nicholson J, Nørby S, Pandya A, Parik J, Patsalis PC, Pereira L, Peterlin B, Pielberg G, Prata MJ, Previderé C, Roewer L, Rootsi S, Rubinsztein DC, Saillard J, Santos FR, Stefanescu G, Sykes BC, Tolun A, Villems R, Tyler-Smith C, Jobling MA | display-authors = 6 | title = Y-chromosomal diversity in Europe is clinal and influenced primarily by geography, rather than by language | journal = American Journal of Human Genetics | volume = 67 | issue = 6 | pages = 1526–1543 | date = December 2000 | pmid = 11078479 | pmc = 1287948 | doi = 10.1086/316890 | url = http://www.ajhg.org/AJHG/abstract/S0002-9297(07)63221-2 | url-status = dead | archive-url = https://web.archive.org/web/20080506041100/http://www.ajhg.org/AJHG/abstract/S0002-9297(07)63221-2 | archive-date = 2008-05-06 }}</ref><ref name = "Underhill_2007">{{cite journal | vauthors = Underhill PA, Kivisild T | title = Use of y chromosome and mitochondrial DNA population structure in tracing human migrations | journal = Annual Review of Genetics | volume = 41 | pages = 539–564 | year = 2007 | pmid = 18076332 | doi = 10.1146/annurev.genet.41.110306.130407 }}</ref> also described E1b1b as representing a late-] migration from Africa to Europe over the ] in ], evidence for which does not show up in mitochondrial DNA.<ref>Y chromosome data show a signal for a separate late-Pleistocene migration from Africa to Europe via Sinai as evidenced through the distribution of haplogroup E3b lineages, which is not manifested in mtDNA haplogroup distributions.</ref> | |||
| It is thought that there were refugia in the Balkans and the Ukraine that acted as source areas for the repopulation of Europe after the LGM. Associated with these areas are Y-chromosomes I and R1a respectively. People from these refuges reached north-west Europe, while R1a also spread into central Asia and as far as ] and ]. There was a major incursion of Haplogroup N into north-eastern Europe. South-eastern Europe shows the incursion of several haplogroups mainly found in the Middle East. | |||
| Concerning timing the distribution and diversity of V13 however, Battaglia<ref name="Battaglia_2008" /> proposed an earlier movement whereby the E-M78* lineage ancestral to all modern E-V13 men moved rapidly out of a Southern Egyptian homeland and arrived in Europe with only ] technologies. They then suggest that the E-V13 sub-clade of E-M78 only expanded subsequently as native Balkan 'foragers-cum-farmers' adopted Neolithic technologies from the Near East. They propose that the first major dispersal of E-V13 from the Balkans may have been in the direction of the ] with the ] ] culture often referred to as ''Impressa'' or ],<ref name="Peričic_2005" /> rather propose that the main route of E-V13 spread was along the Vardar-Morava-Danube river 'highway' system. | |||
| ===After the LGM=== | |||
| {{see|Mesolithic Europe}} | |||
| In contrast to Battaglia, Cruciani<ref name = "Cruciani_2007">{{cite journal | vauthors = Cruciani F, La Fratta R, Trombetta B, Santolamazza P, Sellitto D, Colomb EB, Dugoujon JM, Crivellaro F, Benincasa T, Pascone R, Moral P, Watson E, Melegh B, Barbujani G, Fuselli S, Vona G, Zagradisnik B, Assum G, Brdicka R, Kozlov AI, Efremov GD, Coppa A, Novelletto A, Scozzari R | display-authors = 6 | title = Tracing past human male movements in northern/eastern Africa and western Eurasia: new clues from Y-chromosomal haplogroups E-M78 and J-M12 | journal = Molecular Biology and Evolution | volume = 24 | issue = 6 | pages = 1300–1311 | date = June 2007 | pmid = 17351267 | doi = 10.1093/molbev/msm049 | url = http://mbe.oxfordjournals.org/cgi/reprint/24/6/1300 | url-status = dead | access-date = 2009-07-22 | doi-access = free | archive-url = https://wayback.archive-it.org/all/20171010093322/https://academic.oup.com/mbe/article/24/6/1300/984002/Tracing-Past-Human-Male-Movements-in-Northern | archive-date = 2017-10-10 }} Also see .</ref> tentatively suggested (i) a different point where the V13 mutation happened on its way from Egypt to the Balkans via the Middle East, and (ii) a later dispersal time. The authors proposed that the V13 mutation first appeared in western Asia, where it is found in low but significant frequencies, whence it entered the Balkans sometime after 11 kYa. It later experienced a rapid dispersal which he dated to c. 5300 years ago in Europe, coinciding with the Balkan Bronze Age. Like Peričic et al. they consider that "the dispersion of the E-V13 and J-M12 haplogroups seems to have mainly followed the river waterways connecting the southern Balkans to north-central Europe". | |||
| ] | |||
| More recently, Lacan<ref name="Lacan_2011">{{cite journal | vauthors = Lacan M, Keyser C, Ricaut FX, Brucato N, Duranthon F, Guilaine J, Crubézy E, Ludes B | title = Ancient DNA reveals male diffusion through the Neolithic Mediterranean route | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 108 | issue = 24 | pages = 9788–91 | date = June 2011 | pmid = 21628562 | pmc = 3116412 | doi = 10.1073/pnas.1100723108 | bibcode = 2011PNAS..108.9788L | doi-access = free }}</ref> announced that a 7000-year-old skeleton in a Neolithic context in a Spanish funeral cave, was an E-V13 man. (The other specimens tested from the same site were in ], which has been found in Neolithic contexts throughout Europe.) Using 7 STR markers, this specimen was identified as being similar to modern individuals tested in ], ], ], ], and ]. The authors therefore proposed that, whether or not the modern distribution of E-V13 of today is a result of more recent events, E-V13 was already in Europe within the Neolithic, carried by early farmers from the Eastern Mediterranean to the Western Mediterranean, much earlier than the Bronze Age. This supports the proposals of Battaglia et al. rather than Cruciani et al. at least concerning earliest European dispersals, but E-V13 may have dispersed more than once. Even more recent than the Bronze Age, it has also been proposed that modern E-V13's modern distribution in Europe is at least partly caused by Roman era movements of people.<ref name="Bird 2007">{{cite journal | vauthors = Bird S | title = Haplogroup E3b1a2 as a Possible Indicator of Settlement in Roman Britain by Soldiers of Balkan Origin | journal = Journal of Genetic Genealogy | year = 2007 | volume = 3 | issue = 2 | url = http://www.jogg.info/32/bird.htm | access-date = 2009-08-25 | archive-date = 2016-04-22 | archive-url = https://web.archive.org/web/20160422005652/http://www.jogg.info/32/bird.htm | url-status = dead }}</ref> (See below.) | |||
| After a less severe cold event around 12000-10000 years ago, there was an increasing use of ] and reliance on the coast and sea. Styles of tool making varied by location, suggesting that the population of Europe was settling down. Northern Europe was first settled in the Mesolithic. Martin Richards showed that about 11% of modern mtDNA arrived from the Middle East during the Mesolithic.<ref name=Richards00/> These types include T, T2 and K which show a significant decline from SE to NW Europe. However ] says that there was further gene flow from Iberia to NW Europe.<ref>The Origins of the British</ref> He has identified four mtDNA subgroups that expanded into western Europe and nine Y-chromosome R1b descendant clusters that expanded in the Mesolithic. In northern Europe there was also mtDNA input from Asia, while the male gene flow was largely from SE Europe and Asia, including descendants of haplogroups R1a and N3, though there was also R1b input. | |||
| The migration of Neolithic farmers into Europe brought along several new adaptations.<ref name="Mathieson 2015"/> The variation for light skin colour was introduced to Europe by the ].<ref name="Mathieson 2015"/> After the arrival of the neolithic farmers, a ] mutation was selected for, a mutation which probably arose to deal with ] deficiency but increases the risk of ], ], and ]. | |||
| === Holocene migrations === | |||
| {{see|Neolithic Europe|Neolithic Revolution|Holocene}} | |||
| ===Bronze Age=== | |||
| ====Entry via the Balkans from the Near East==== | |||
| {{Further|Bronze Age Europe}} | |||
| The ] saw the development of long-distance ], particularly along the Atlantic Coast and in the Danube valley. There was migration from Norway to ] and ] in this period (and to a lesser extent to mainland Scotland and Ireland). There was also migration from Germany to eastern England. Martin Richards estimated that there was about 4% mtDNA immigration to Europe in the Bronze Age. | |||
| The early Holocene saw the continuation of Mesolithic technology in many parts of Europe, and an apparent decrease of population in some parts of southern Europe to very low levels<ref>(Perlès 2001, Ch. 4), Runnels (2003).</ref>. | |||
| ]]] | |||
| Neolithic ] technology entered Europe during the Holocene, first in Greece, and is considered to have made important changes to Europe's genetic make-up. The duration of the Neolithic varied from place to place, starting with the introduction of farming and ending with the introduction of bronze implements. In SE Europe it was approximately 7000-3000 BC while in NW Europe 4500-1700 BC. Besides the introduction of new plants and animals, the Neolithic also saw the beginning of the use of ]. Pottery remains allow the tracing of the movement of ideas and possibly people across Europe. | |||
| Another theory about the origin of the ] centres around a hypothetical ] people, who, according to the ], can be traced to north of the Black and Caspian Seas at about 4500 BCE.<ref name = Reich>{{cite journal | vauthors = Haak W, Lazaridis I, Patterson N, Rohland N, Mallick S, Llamas B, Brandt G, Nordenfelt S, Harney E, Stewardson K, Fu Q, Mittnik A, Bánffy E, Economou C, Francken M, Friederich S, Pena RG, Hallgren F, Khartanovich V, Khokhlov A, Kunst M, Kuznetsov P, Meller H, Mochalov O, Moiseyev V, Nicklisch N, Pichler SL, Risch R, Rojo Guerra MA, Roth C, Szécsényi-Nagy A, Wahl J, Meyer M, Krause J, Brown D, Anthony D, Cooper A, Alt KW, Reich D | display-authors = 6 | title = Massive migration from the steppe was a source for Indo-European languages in Europe | journal = Nature | volume = 522 | issue = 7555 | pages = 207–211 | date = June 2015 | pmid = 25731166 | pmc = 5048219 | doi = 10.1038/nature14317 | arxiv = 1502.02783 | bibcode = 2015Natur.522..207H }}</ref> They ] and possibly invented the wooden disk ], and are considered to have spread their culture and genes across Europe.<ref name = Callaway>{{cite journal | vauthors = Callaway E |title=European languages linked to migration from the east |journal=Nature |date=12 February 2015 |doi=10.1038/nature.2015.16919 |s2cid=184180681 |doi-access=free }}</ref> The Y ] is a proposed marker of these "Kurgan" genes, as is the Y ], although these haplogroups as a whole may be much older than the language family.<ref>{{cite journal | vauthors = Underhill PA, Myres NM, Rootsi S, Metspalu M, Zhivotovsky LA, King RJ, Lin AA, Chow CE, Semino O, Battaglia V, Kutuev I, Järve M, Chaubey G, Ayub Q, Mohyuddin A, Mehdi SQ, Sengupta S, Rogaev EI, Khusnutdinova EK, Pshenichnov A, Balanovsky O, Balanovska E, Jeran N, Augustin DH, Baldovic M, Herrera RJ, Thangaraj K, Singh V, Singh L, Majumder P, Rudan P, Primorac D, Villems R, Kivisild T | display-authors = 6 | title = Separating the post-Glacial coancestry of European and Asian Y chromosomes within haplogroup R1a | journal = European Journal of Human Genetics | volume = 18 | issue = 4 | pages = 479–484 | date = April 2010 | pmid = 19888303 | pmc = 2987245 | doi = 10.1038/ejhg.2009.194 }}</ref> | |||
| In the far north, carriers of the ] arrived to Europe from ], eventually expanding as far as ], though the specific timing of their arrival is uncertain. The most common North European subclade N1c1 is estimated to be around 8,000 years old. There is evidence of human settlement in Finland dating back to 8500 BCE, linked with the ] and its putative ancestor, the ], but the latter is thought to have a European origin. The geographical spread of haplogroup N in Europe is well aligned with the ], whose emergence is commonly dated c. 4200 BCE, and with the distribution of ]. Mitochondrial DNA studies of ], haplogroup ] are consistent with multiple migrations to ] from ]-] region, starting 6,000 to 7,000 years before present.<ref>{{cite journal | vauthors = Ingman M, Gyllensten U | title = A recent genetic link between Sami and the Volga-Ural region of Russia | journal = European Journal of Human Genetics | volume = 15 | issue = 1 | pages = 115–120 | date = January 2007 | pmid = 16985502 | doi = 10.1038/sj.ejhg.5201712 | doi-access = free }}</ref> | |||
| Martin Richards estimated that 11% of European mtDNA is due to immigration in this period. Gene flow from SE to NW Europe seems to have continued in the Neolithic, the percentage significantly declining towards the British Isles. Classical genetics also suggested that the largest admixture to the European Paleolithic/Mesolithic stock was due to the ] of the 7th to 5th millennia BC.<ref>{{cite book |author=Piazza, Alberto; Cavalli-Sforza, L. L.; Menozzi, Paolo |title=The history and geography of human genes |publisher=Princeton University Press |location=Princeton, N.J |year=1994 |isbn=0-691-08750-4 }}</ref> Three main mtDNA gene groups have been identified as contributing Neolithic entrants into Europe: J, T1 and U3 (in that order of importance).<ref>Oppenheimer </ref> With others they amount up to around 20% of the gene pool.<ref> Richards</ref> | |||
| The relationship between roles of European and Asian colonists in the prehistory of Finland is a point of some contention, and some scholars insist that Finns are "predominantly Eastern European and made up of people who trekked north from the Ukrainian refuge during the Ice Age".<ref>{{cite book | chapter-url = http://www.linguistics.fi/julkaisut/SKY2006_1/1FK60.1.9.WIIK.pdf| chapter = Who Are the Finns?| vauthors = Wiik K |access-date=2016-03-16|author-link=Kalevi Wiik | veditors = Suominen M, Arppe A, Airola A, Heinämäki O, Miestamo M, Määttä U, Niemi J, Pitkänen KK, Sinnemäki K | title = A Man of Measure Festschrift in Honour of Fred Karlsson | pages = 97–108 }}</ref> Farther east, the issue is less contentious. Haplogroup N carriers account for a significant part of all non-Slavic ethnic groups in northern ], including 37% of ], 35% of ] (65% according to another study<ref name = "Mirabal_2010" />), 67% of ], as many as 98% of ], 94% of ], and 86% to 94% of ].<ref>{{cite journal | vauthors = Rootsi S, Zhivotovsky LA, Baldovic M, Kayser M, Kutuev IA, Khusainova R, Bermisheva MA, Gubina M, Fedorova SA, Ilumäe AM, Khusnutdinova EK, Voevoda MI, Osipova LP, Stoneking M, Lin AA, Ferak V, Parik J, Kivisild T, Underhill PA, Villems R | display-authors = 6 | title = A counter-clockwise northern route of the Y-chromosome haplogroup N from Southeast Asia towards Europe | journal = European Journal of Human Genetics | volume = 15 | issue = 2 | pages = 204–211 | date = February 2007 | pmid = 17149388 | doi = 10.1038/sj.ejhg.5201748 | doi-access = free }}, Supplemental table 1</ref> | |||
| ] (also known as E-V13) is the most common subclade of E1b1b throughout most of Europe. It is commonly thought to have been a marker of Neolithic migrations, coinciding with the introduction of Agriculture into Europe, from Anatolia or Levant into the ] and Southern ], where it has its highest frequency. However, {{Harvcoltxt|Battaglia et al.|2008}} suggest that it actually arrived into the Balkans from Western Asia during the early ], ahead of the Neolithic. | |||
| The Yamnaya component contains partial ancestry from an Ancient North Eurasian component, a Paleolithic Siberian lineage but closely related to European hunter-gatherers, first identified in ].<ref name="Jones 2015"/><ref>{{Cite journal |last=Yang |first=Melinda A. |date=2022-01-06 |title=A genetic history of migration, diversification, and admixture in Asia |url=http://www.pivotscipub.com/hpgg/2/1/0001 |journal=Human Population Genetics and Genomics |language=en |volume=2 |issue=1 |pages=1–32 |doi=10.47248/hpgg2202010001 |issn=2770-5005|doi-access=free }}</ref> According to Iosif Lazaridis, "the Ancient North Eurasian ancestry is proportionally the smallest component everywhere in Europe, never more than 20 percent, but we find it in nearly every European group we’ve studied."<ref name="Dutchen 2014">{{cite web| vauthors = Dutchen S |title=New Branch Added to European Family Tree|url=http://hms.harvard.edu/news/new-branch-added-european-family-tree|archive-url=https://web.archive.org/web/20141001020113/http://hms.harvard.edu/news/new-branch-added-european-family-tree|archive-date=2014-10-01|publisher=]|access-date=25 November 2015|date=September 17, 2014}}</ref> This genetic component does not come directly from the Mal'ta lineage itself, but a related lineage that separated from the Mal'ta lineage.<ref name="Fu 2016"/> | |||
| E-V13 is a sub-clade of ] (formerly known as E3b, and also known as E-M215, and equivalent to E-M35) which is thought to have originated in the ]<ref name=Semino04>{{Harvcoltxt|Semino et al.|2004}}</ref>. It is by far the most common Y DNA clade in North and Northeast Africa, and is also common throughout the majority of Europe, particularly in the Mediterranean and South Eastern Europe. | |||
| ] in Europe]] | |||
| From the above it can be seen that within a few millennia E1b1b lineages traced a path from an African origin via the Middle East, to Europe where they apparently were present during the incipient Neolithic. {{Harvcoltxt|Underhill and Kivisild|2007}} have remarked that E1b1b seems to represent a late-Pleistocene migration from Africa to Europe over the ] in ], evidence for which does not show up in mitochondrial DNA.<ref>"Y chromosome data show a signal for a separate late-Pleistocene migration from Africa to Europe via Sinai as evidenced through the distribution of haplogroup E3b lineages, which is not manifested in mtDNA haplogroup distributions."{{Harvcoltxt|Underhill and Kivisild|2007|p=547}}</ref> | |||
| Up to a half of the Yamnaya component may have come from a ] strand.<ref name="Jones 2015"/> On November 16, 2015, in a study published in the journal '']'',<ref name="Jones 2015">{{cite journal | vauthors = Jones ER, Gonzalez-Fortes G, Connell S, Siska V, Eriksson A, Martiniano R, McLaughlin RL, Gallego Llorente M, Cassidy LM, Gamba C, Meshveliani T, Bar-Yosef O, Müller W, Belfer-Cohen A, Matskevich Z, Jakeli N, Higham TF, Currat M, Lordkipanidze D, Hofreiter M, Manica A, Pinhasi R, Bradley DG | display-authors = 6 | title = Upper Palaeolithic genomes reveal deep roots of modern Eurasians | journal = Nature Communications | volume = 6 | pages = 8912 | date = November 2015 | pmid = 26567969 | pmc = 4660371 | doi = 10.1038/ncomms9912 | number = 8912 | bibcode = 2015NatCo...6.8912J }}</ref> geneticists announced that they had found a new fourth ancestral "tribe" or "strand" which had contributed to the modern European gene pool. They analysed genomes from two hunter-gatherers from Georgia which were 13,300 and 9,700 years old, and found that these Caucasus hunter-gatherers were probably the source of the farmer-like DNA in the Yamnaya. According to co-author Dr Andrea Manica of the University of Cambridge: "The question of where the Yamnaya come from has been something of a mystery up to now....we can now answer that as we've found that their genetic make-up is a mix of ]s and a population from this pocket of Caucasus hunter-gatherers who weathered much of the last Ice Age in apparent isolation."<ref name="bbcnov16">{{cite news |title=Mystery ancestral 'tribe' revealed |url=https://www.bbc.com/news/science-environment-34832781 |work=BBC News |date=16 November 2015 }}</ref> | |||
| === Bronze and Iron Age migrations === | |||
| {{see|Bronze Age Europe|Iron Age Europe}} | |||
| According to Lazaridis et al. (2016), a population related to the people of the ] ] contributed to roughly half of the ancestry of Yamnaya populations of the Pontic–Caspian steppe. These Iranian Chalcolithic people were a mixture of "the Neolithic people of western Iran, the Levant, and Caucasus Hunter Gatherers."<ref>{{cite bioRxiv|title=The genetic structure of the world's first farmers|biorxiv=10.1101/059311| vauthors = Lazaridis I, Nadel D, Rollefson G, Merrett DC, Rohland N, Mallick S, Fernandes D, Novak M, Gamarra B, Sirak K, Connell S | display-authors = 6 |year=2016}}</ref> | |||
| The ] saw the development of long-distance ]s, particularly along the Atlantic Coast and in the Danube valley. There was migration from ] to ] and ] in this period (and to a lesser extent to mainland Scotland and Ireland). There was also migration from Germany to eastern England. Martin Richards estimated that there was about 4% mtDNA immigration to Europe in the Bronze Age. Oppenheimer could find no genetic evidence for any ] migration to Britain. | |||
| The genetic variations for ] and greater height came with the Yamnaya people.<ref name="Mathieson 2015">{{cite journal | vauthors = Mathieson I, Lazaridis I, Rohland N, Mallick S, Patterson N, Roodenberg SA, Harney E, Stewardson K, Fernandes D, Novak M, Sirak K, Gamba C, Jones ER, Llamas B, Dryomov S, Pickrell J, Arsuaga JL, de Castro JM, Carbonell E, Gerritsen F, Khokhlov A, Kuznetsov P, Lozano M, Meller H, Mochalov O, Moiseyev V, Guerra MA, Roodenberg J, Vergès JM, Krause J, Cooper A, Alt KW, Brown D, Anthony D, Lalueza-Fox C, Haak W, Pinhasi R, Reich D | display-authors = 6 | title = Genome-wide patterns of selection in 230 ancient Eurasians | journal = Nature | volume = 528 | issue = 7583 | pages = 499–503 | date = December 2015 | pmid = 26595274 | pmc = 4918750 | doi = 10.1038/nature16152 | bibcode = 2015Natur.528..499M }}<!--|access-date=25 November 2015--></ref> The derived allele of the ] gene (SNP rs12821256) that is associated with – and likely causal for – ] in Europeans is found in populations with eastern but not ] ancestry, suggesting that its origin is in the ] (ANE) population and may have been spread in Europe by individuals with ]. Consistent with this, the earliest known individual with the derived allele is an ANE individual from the Late Upper Paleolithic ] archaeological complex in central Siberia.<ref name="pmid29466330">{{cite journal | vauthors = Mathieson I, Alpaslan-Roodenberg S, Posth C, Szécsényi-Nagy A, Rohland N, Mallick S, Olalde I, Broomandkhoshbacht N, Candilio F, Cheronet O, Fernandes D, Ferry M, Gamarra B, Fortes GG, Haak W, Harney E, Jones E, Keating D, Krause-Kyora B, Kucukkalipci I, Michel M, Mittnik A, Nägele K, Novak M, Oppenheimer J, Patterson N, Pfrengle S, Sirak K, Stewardson K, Vai S, Alexandrov S, Alt KW, Andreescu R, Antonović D, Ash A, Atanassova N, Bacvarov K, Gusztáv MB, Bocherens H, Bolus M, Boroneanţ A, Boyadzhiev Y, Budnik A, Burmaz J, Chohadzhiev S, Conard NJ, Cottiaux R, Čuka M, Cupillard C, Drucker DG, Elenski N, Francken M, Galabova B, Ganetsovski G, Gély B, Hajdu T, Handzhyiska V, Harvati K, Higham T, Iliev S, Janković I, Karavanić I, Kennett DJ, Komšo D, Kozak A, Labuda D, Lari M, Lazar C, Leppek M, Leshtakov K, Vetro DL, Los D, Lozanov I, Malina M, Martini F, McSweeney K, Meller H, Menđušić M, Mirea P, Moiseyev V, Petrova V, Price TD, Simalcsik A, Sineo L, Šlaus M, Slavchev V, Stanev P, Starović A, Szeniczey T, Talamo S, Teschler-Nicola M, Thevenet C, Valchev I, Valentin F, Vasilyev S, Veljanovska F, Venelinova S, Veselovskaya E, Viola B, Virag C, Zaninović J, Zäuner S, Stockhammer PW, Catalano G, Krauß R, Caramelli D, Zariņa G, Gaydarska B, Lillie M, Nikitin AG, Potekhina I, Papathanasiou A, Borić D, Bonsall C, Krause J, Pinhasi R, Reich D | display-authors = 6 | title = The genomic history of southeastern Europe | journal = Nature | volume = 555 | issue = 7695 | pages = 197–203 | date = March 2018 | pmid = 29466330 | pmc = 6091220 | doi = 10.1038/nature25778 | bibcode = 2018Natur.555..197M }}</ref> | |||
| One theory about the origin of the ] centres around a hypothetical ] people, who are traced, in the ], to somewhere north of the Black Sea at about 4500 BCE. They ], and are considered to have spread their culture and genes across Europe. It has been difficult to identify what these "Kurgan" genes might be, though the ] ] is a proposed marker which would indicate that the physical expansion halted in Germany and only the Kurgan culture and language went further. Another hypothesis — the ] — suggests an origin in Anatolia with a later expansion from eastern Europe. | |||
| === Recent history === | |||
| To what extent Indo-European migrations replaced the ] ] peoples is debated, but a ] has been reached that technology and language transfer played a more important role in this process than actual gene-flow.<ref> See ], '']'', 1st American ed. (New York: Norton, 2001) for an entertaining account of how this consensus was reached. For historical reasons, in the 1980s mtDNA researchers believed that the Indo-European expansion was overwhelmingly a spread of technology and language, not of genes, while those who studied ] believed the opposite. Gradually the mtDNA researchers (Sykes) admitted more physical migration into their scenarios, while the Y folks (Peter Underhill) accepted more technology-copying. Eventually, both groups independently reached a 20% Neolithic - 80% Paleolithic ratio of genetic contribution to today's European population. The mtDNA vs. Y-chromosome discrepancy may be explained by noting that in such conquest-based migrations, a common pattern is of invading foreign males producing offspring with indigenous females, though significant numbers of females of the spreading culture could also arrive with post-conquest settlers. However, where migrations are essentially economic (as most migrations appear to be) it appears equally probable that male family members preceded females into new territory looking for opportunities.</ref> | |||
| {{see|History of Europe}} | |||
| {{see|Demography of the Roman Empire|Migration period|Viking expansion|Slavic expansion|Magyar migration|Muslim conquest of Spain|Turkic expansion|African admixture in Europe|Ottoman wars in Europe|Ostsiedlung|World War II evacuation and expulsion|Population transfer in the Soviet Union|Immigration to Europe}} | |||
| ] | |||
| Expansions of the ] do not appear to have left distinct genetic signatures in Europe. Indeed, Romance-speaking populations in the Balkans, like ], ], ], etc. have been found to genetically resemble neighbouring Greek and South Slavic-speaking peoples rather than modern Italians.<ref>{{cite journal | vauthors = Comas D, Schmid H, Braeuer S, Flaiz C, Busquets A, Calafell F, Bertranpetit J, Scheil HG, Huckenbeck W, Efremovska L, Schmidt H | display-authors = 6 | title = Alu insertion polymorphisms in the Balkans and the origins of the Aromuns | journal = Annals of Human Genetics | volume = 68 | issue = Pt 2 | pages = 120–127 | date = March 2004 | pmid = 15008791 | doi = 10.1046/j.1529-8817.2003.00080.x | s2cid = 21773796 }}</ref><ref>{{cite journal | vauthors = Bosch E, Calafell F, González-Neira A, Flaiz C, Mateu E, Scheil HG, Huckenbeck W, Efremovska L, Mikerezi I, Xirotiris N, Grasa C, Schmidt H, Comas D | display-authors = 6 | title = Paternal and maternal lineages in the Balkans show a homogeneous landscape over linguistic barriers, except for the isolated Aromuns | journal = Annals of Human Genetics | volume = 70 | issue = Pt 4 | pages = 459–487 | date = July 2006 | pmid = 16759179 | doi = 10.1111/j.1469-1809.2005.00251.x | s2cid = 23156886 }}</ref> Steven Bird has speculated that E1b1b1a was spread during the ] through ] and ]n populations from the Balkans into the rest of Europe.<ref name="Bird 2007"/> | |||
| During the Iron Age, Celts are recorded as having moved from ] into northern Italy, Eastern Europe and Anatolia. The relationship between the Celts of Gaul and Spain is unclear as any migration occurred before records exist. | |||
| Concerning the late Roman period of (not only) ] "'']''", some suggestions have been made, at least for Britain, with Y haplogroup I1a being associated with ] immigration in eastern England, and R1a being associated with Norse immigration in northern Scotland.<ref>{{cite journal | vauthors = Capelli C, Redhead N, Abernethy JK, Gratrix F, Wilson JF, Moen T, Hervig T, Richards M, Stumpf MP, Underhill PA, Bradshaw P, Shaha A, Thomas MG, Bradman N, Goldstein DB | display-authors = 6 | title = A Y chromosome census of the British Isles | journal = Current Biology | volume = 13 | issue = 11 | pages = 979–984 | date = May 2003 | pmid = 12781138 | doi = 10.1016/S0960-9822(03)00373-7 | doi-access = free | hdl = 20.500.11820/8acb01f3-a7c1-45f5-89de-b796266d651e | hdl-access = free }} also at {{citation |url=http://www.ucl.ac.uk/tcga/tcgapdf/capelli-CB-03.pdf |title=030705U491 |access-date=2011-06-01}}</ref> | |||
| ====North African admixture==== | |||
| ==Genetics of modern European populations== | |||
| The Y haplogroup ] (E-M81) is seen as a marker of Northern African migration into Southern Europe, at least some of which may have happened in recent millennia. | |||
| {{see|Ethnic groups in Europe}} | |||
| ===Patrilineal studies=== | |||
| In Europe, ] (E-M81) is found everywhere but mostly in the ], where it is more common than E-M78 unlike in the rest of Europe<ref>{{Harvcoltxt|Adams et al.|2008}}, shows an average frequency of 4% in the Iberian Peninsula with frequencies reaching 9% in ], 10% in Western] and Northwest ], .</ref> at an average frequency of 4-5.6%, with frequencies reaching 9% in ], 10% in Western ] and Northwest ] and 13 % in ]<ref>{{Harvcoltxt|Flores et al.|2005}}, {{Harvcoltxt|Beleza et al.|2006}}, {{Harvcoltxt|Adams et al.|2008}}</ref> | |||
| There are four main Y-chromosome DNA ]s that account for most of Europe's ].<ref name="Semino_2000">{{cite journal | vauthors = Semino O, Passarino G, Oefner PJ, Lin AA, Arbuzova S, Beckman LE, De Benedictis G, Francalacci P, Kouvatsi A, Limborska S, Marcikiae M, Mika A, Mika B, Primorac D, Santachiara-Benerecetti AS, Cavalli-Sforza LL, Underhill PA | display-authors = 6 | title = The genetic legacy of Paleolithic Homo sapiens sapiens in extant Europeans: a Y chromosome perspective | journal = Science | volume = 290 | issue = 5494 | pages = 1155–1159 | date = November 2000 | pmid = 11073453 | doi = 10.1126/science.290.5494.1155 | bibcode = 2000Sci...290.1155S }} Note: Haplogroup names are different in this article. For example: ] is referred as M170</ref> | |||
| In a study of ] Y-chromosome lineages, {{Harvcoltxt|Gonçalves et al.|2005}} revealed that "The mtDNA and Y data indicate that the Berber presence in that region dates prior to the Moorish expansion in 711 AD... Our data indicate that male Berbers, unlike sub-Saharan immigrants, constituted a long-lasting and continuous community in the country". | |||
| * Haplogroup ] is common in Europe, particularly in ], with the ] being the most common among Western Europeans.<ref name=balaresque>{{cite journal | vauthors = Balaresque P, Bowden GR, Adams SM, Leung HY, King TE, Rosser ZH, Goodwin J, Moisan JP, Richard C, Millward A, Demaine AG, Barbujani G, Previderè C, Wilson IJ, Tyler-Smith C, Jobling MA | display-authors = 6 | title = A predominantly neolithic origin for European paternal lineages | journal = PLOS Biology | volume = 8 | issue = 1 | pages = e1000285 | date = January 2010 | pmid = 20087410 | pmc = 2799514 | doi = 10.1371/journal.pbio.1000285 | doi-access = free }}</ref><ref name=Myres2010>{{cite journal | vauthors = Myres NM, Rootsi S, Lin AA, Järve M, King RJ, Kutuev I, Cabrera VM, Khusnutdinova EK, Pshenichnov A, Yunusbayev B, Balanovsky O, Balanovska E, Rudan P, Baldovic M, Herrera RJ, Chiaroni J, Di Cristofaro J, Villems R, Kivisild T, Underhill PA | display-authors = 6 | title = A major Y-chromosome haplogroup R1b Holocene era founder effect in Central and Western Europe | journal = European Journal of Human Genetics | volume = 19 | issue = 1 | pages = 95–101 | date = January 2011 | pmid = 20736979 | pmc = 3039512 | doi = 10.1038/ejhg.2010.146 }}</ref><ref name=Cruciani2010>{{cite journal | vauthors = Cruciani F, Trombetta B, Antonelli C, Pascone R, Valesini G, Scalzi V, Vona G, Melegh B, Zagradisnik B, Assum G, Efremov GD, Sellitto D, Scozzari R | display-authors = 6 | title = Strong intra- and inter-continental differentiation revealed by Y chromosome SNPs M269, U106 and U152 | journal = Forensic Science International. Genetics | volume = 5 | issue = 3 | pages = e49–e52 | date = June 2011 | pmid = 20732840 | doi = 10.1016/j.fsigen.2010.07.006 | hdl = 11573/226727 }}</ref> Nearly all of this R1b in Europe is in the form of the R1b1a2 (2011 name) (R-M269) sub-clade, specifically within the R-L23 sub-sub-clade whereas R1b found in ], ] and ] tends to be in other clades. It has also been pointed out that ] types are present in Europe and are particularly notable in some areas such as Sardinia and Armenia.<ref>{{cite journal | vauthors = Morelli L, Contu D, Santoni F, Whalen MB, Francalacci P, Cucca F | title = A comparison of Y-chromosome variation in Sardinia and Anatolia is more consistent with cultural rather than demic diffusion of agriculture | journal = PLOS ONE | volume = 5 | issue = 4 | pages = e10419 | date = April 2010 | pmid = 20454687 | pmc = 2861676 | doi = 10.1371/journal.pone.0010419 | doi-access = free | bibcode = 2010PLoSO...510419M }}</ref> Haplogroup R1b frequencies vary from highs in western Europe in a steadily decreasing cline with growing distance from the Atlantic: 80–90% (], ], ], ], ]) around 70–80% in Spain, Britain and France and around 40–60% in parts of ], and northern Italy. It drops outside this area and is around 30% or less in areas such as southern Italy, ], the Balkans and ]. R1b remains the most common clade as one moves east to Germany, while farther east, in Poland, R1a is more common (see below).<ref name="Kayser_2005">{{cite journal | vauthors = Kayser M, Lao O, Anslinger K, Augustin C, Bargel G, Edelmann J, Elias S, Heinrich M, Henke J, Henke L, Hohoff C, Illing A, Jonkisz A, Kuzniar P, Lebioda A, Lessig R, Lewicki S, Maciejewska A, Monies DM, Pawłowski R, Poetsch M, Schmid D, Schmidt U, Schneider PM, Stradmann-Bellinghausen B, Szibor R, Wegener R, Wozniak M, Zoledziewska M, Roewer L, Dobosz T, Ploski R | display-authors = 6 | title = Significant genetic differentiation between Poland and Germany follows present-day political borders, as revealed by Y-chromosome analysis | journal = Human Genetics | volume = 117 | issue = 5 | pages = 428–443 | date = September 2005 | pmid = 15959808 | doi = 10.1007/s00439-005-1333-9 | s2cid = 11066186 }} A copy can be found here {{cite web |url=http://dirkschweitzer.net/E3b-papers/HG-05-428-Poland-Germany.pdf |title=Significant genetic differentiation between Poland and Germany follows present-day political borders, as revealed by Y-chromosome analysis |access-date=2008-11-02 |url-status=dead |archive-url=https://web.archive.org/web/20090304100323/http://dirkschweitzer.net/E3b-papers/HG-05-428-Poland-Germany.pdf |archive-date=2009-03-04 }}.</ref> In ], R1b drops behind R1a in the area in and around Hungary and Serbia but is more common both to the south and north of this region.<ref name="Peričic_2005" /> R1b in Western Europe is dominated by at least two sub-clades, R-U106, which is distributed from the east side of the ] into northern and central Europe (with a strong presence in England) and R-P312, which is most common west of the Rhine, including the ].<ref name=Myres2010/><ref name=Cruciani2010/> | |||
| * Haplogroup ], almost entirely in the R1a1a sub-clade, is prevalent in much of ] and ] (also in ] and ]). For example, there is a sharp increase in R1a1 and decrease in R1b1b2 as one goes east from Germany to Poland.<ref name="Kayser_2005"/> It also has a substantial presence in Scandinavia (particularly Norway).<ref name="Bowden_2008">{{cite journal | vauthors = Bowden GR, Balaresque P, King TE, Hansen Z, Lee AC, Pergl-Wilson G, Hurley E, Roberts SJ, Waite P, Jesch J, Jones AL, Thomas MG, Harding SE, Jobling MA | title = Excavating past population structures by surname-based sampling: the genetic legacy of the Vikings in northwest England | journal = Molecular Biology and Evolution | volume = 25 | issue = 2 | pages = 301–9 | date = February 2008 | pmid = 18032405 | pmc = 2628767 | doi = 10.1093/molbev/msm255 }}</ref><ref name="Dupuy_2021">{{cite journal | vauthors = Lall GM, Larmuseau MH, Wetton JH, Batini C, Hallast P, Huszar TI, Zadik D, Aase S, Baker T, Balaresque P, Bodmer W, Børglum AD, de Knijff P, Dunn H, Harding SE, Løvvik H, Dupuy BM, Pamjav H, Tillmar AO, Tomaszewski M, Tyler-Smith C, Verdugo MP, Winney B, Vohra P, Story J, King TE, Jobling MA | display-authors = 6 | title = Subdividing Y-chromosome haplogroup R1a1 reveals Norse Viking dispersal lineages in Britain | journal = European Journal of Human Genetics | volume = 29 | issue = 3 | pages = 512–523 | date = March 2021 | pmid = 33139852 | pmc = 7940619 | doi = 10.1038/s41431-020-00747-z }}</ref> In the Baltic countries R1a frequencies decrease from Lithuania (45%) to Estonia (around 30%).<ref name="Kasperaviciūte_2004">{{cite journal | vauthors = Kasperaviciūte D, Kucinskas V, Stoneking M | title = Y chromosome and mitochondrial DNA variation in Lithuanians | journal = Annals of Human Genetics | volume = 68 | issue = Pt 5 | pages = 438–52 | date = September 2004 | pmid = 15469421 | doi = 10.1046/j.1529-8817.2003.00119.x | s2cid = 26562505 }}</ref> | |||
| * ] is found in the form of various sub-clades throughout Europe and is found at highest frequencies in the ] as I1 (], ], ], ]) and in the ] as I2a (] 65%,<ref name = "Mirabal_2010">{{cite journal | vauthors = Mirabal S, Varljen T, Gayden T, Regueiro M, Vujovic S, Popovic D, Djuric M, Stojkovic O, Herrera RJ | display-authors = 6 | title = Human Y-chromosome short tandem repeats: a tale of acculturation and migrations as mechanisms for the diffusion of agriculture in the Balkan Peninsula | journal = American Journal of Physical Anthropology | volume = 142 | issue = 3 | pages = 380–390 | date = July 2010 | pmid = 20091845 | doi = 10.1002/ajpa.21235 }}</ref> ] and ]). I1 is also frequent in ], ] and ], while I2a is frequent also in ], ]/], ] and ]. This clade is found at its highest expression by far in Europe and may have been there since before the ].<ref name=rootsi>{{cite journal | vauthors = Rootsi S, Magri C, Kivisild T, Benuzzi G, Help H, Bermisheva M, Kutuev I, Barać L, Pericić M, Balanovsky O, Pshenichnov A, Dion D, Grobei M, Zhivotovsky LA, Battaglia V, Achilli A, Al-Zahery N, Parik J, King R, Cinnioğlu C, Khusnutdinova E, Rudan P, Balanovska E, Scheffrahn W, Simonescu M, Brehm A, Goncalves R, Rosa A, Moisan JP, Chaventre A, Ferak V, Füredi S, Oefner PJ, Shen P, Beckman L, Mikerezi I, Terzić R, Primorac D, Cambon-Thomsen A, Krumina A, Torroni A, Underhill PA, Santachiara-Benerecetti AS, Villems R, Semino O | display-authors = 6 | title = Phylogeography of Y-chromosome haplogroup I reveals distinct domains of prehistoric gene flow in europe | journal = American Journal of Human Genetics | volume = 75 | issue = 1 | pages = 128–137 | date = July 2004 | pmid = 15162323 | pmc = 1181996 | doi = 10.1086/422196 | url = http://www.familytreedna.com/pdf/DNA.RootsiHaplogroupISpread.pdf | access-date = 2009-07-04 | url-status = dead | archive-url = https://web.archive.org/web/20090624135411/http://www.familytreedna.com/pdf/DNA.RootsiHaplogroupISpread.pdf | archive-date = 2009-06-24 }}</ref> | |||
| * Haplogroup ] (formerly known as E3b) was part of a migration of Neolithic farmers from the Middle East, which carried E1b1b at low to medium frequency and was introduced into Neolithic Middle Easterners throughout genetic drift from a migration from Africa into the Middle East associated with the ]. It is believed to have first appeared in Northeast Africa approximately 26,000 years ago and dispersed to North Africa and the Near East during the late Paleolithic and Mesolithic periods. E1b1b lineages are closely linked to the diffusion of Afroasiatic languages. Although present throughout Europe, it peaks in the southern ] amongst ] and their neighbors. It is also common in Italy and the Iberian peninsula at lower frequency. Haplogroup ], mainly in the form of its E1b1b1a2 (E-V13) sub-clade, reaches frequencies above 47% around the area of ].<ref name="Peričic_2005">{{cite journal | vauthors = Pericić M, Lauc LB, Klarić IM, Rootsi S, Janićijevic B, Rudan I, Terzić R, Colak I, Kvesić A, Popović D, Sijacki A, Behluli I, Dordevic D, Efremovska L, Bajec DD, Stefanović BD, Villems R, Rudan P | display-authors = 6 | title = High-resolution phylogenetic analysis of southeastern Europe traces major episodes of paternal gene flow among Slavic populations | journal = Molecular Biology and Evolution | volume = 22 | issue = 10 | pages = 1964–1975 | date = October 2005 | pmid = 15944443 | doi = 10.1093/molbev/msi185 | doi-access = free }}</ref> This clade is thought to have arrived in Europe from western Asia either in the later Mesolithic,<ref name="Battaglia_2008">{{cite journal | vauthors = Battaglia V, Fornarino S, Al-Zahery N, Olivieri A, Pala M, Myres NM, King RJ, Rootsi S, Marjanovic D, Primorac D, Hadziselimovic R, Vidovic S, Drobnic K, Durmishi N, Torroni A, Santachiara-Benerecetti AS, Underhill PA, Semino O | display-authors = 6 | title = Y-chromosomal evidence of the cultural diffusion of agriculture in Southeast Europe | journal = European Journal of Human Genetics | volume = 17 | issue = 6 | pages = 820–830 | date = June 2009 | pmid = 19107149 | pmc = 2947100 | doi = 10.1038/ejhg.2008.249 }}</ref> or the Neolithic.<ref name="cruciani2004">{{cite journal | vauthors = Cruciani F, La Fratta R, Santolamazza P, Sellitto D, Pascone R, Moral P, Watson E, Guida V, Colomb EB, Zaharova B, Lavinha J, Vona G, Aman R, Cali F, Akar N, Richards M, Torroni A, Novelletto A, Scozzari R | display-authors = 6 | title = Phylogeographic analysis of haplogroup E3b (E-M215) y chromosomes reveals multiple migratory events within and out of Africa | journal = American Journal of Human Genetics | volume = 74 | issue = 5 | pages = 1014–1022 | date = May 2004 | pmid = 15042509 | pmc = 1181964 | doi = 10.1086/386294 }}</ref> North Africa subclade E-M81 is also present in Sicily and Andalusia. | |||
| Putting aside small enclaves, there are also several haplogroups apart from the above four that are less prominent or most common only in certain areas of Europe. | |||
| According to one recent study (by Adams ''et al.'', December 2008) that analysed 1140 unrelated Y-chromosome samples in Iberia "mean North African admixture is 10.6%, with wide geographical variation, ranging from zero in Gascony to 21.7% in Northwest Castile".<ref>, Adams et al. 2008</ref><ref>, ], December 4, 2008 </ref> | |||
| * ], a common haplogroup among European Neolithic farmers, is common in most parts of Europe at a low frequency, reaching peaks above 70% around ] and among the ] (although living in Asia they border the eastern perimeter of Europe), up to 10% in Sardinia, 12% in Corsica and Uppsala (Sweden), 11% in the Balkans and Portugal, 10% in Spain and 9% in European Russia. This clade is also found in the Near East. | |||
| * ], is common only in the northeast of Europe and in the form of its N1c1 sub-clade reaches frequencies of approximately 60% among Finns and approximately 40% among Estonians, Latvians, and Lithuanians. | |||
| * ], in various sub-clades (J2a, J2b), is found in levels of around 15–30% in the Balkans (particularly ]) and ]. Haplogroup J2 is frequent in ] and the ].<ref name="Semino_2004">{{cite journal | vauthors = Semino O, Magri C, Benuzzi G, Lin AA, Al-Zahery N, Battaglia V, Maccioni L, Triantaphyllidis C, Shen P, Oefner PJ, Zhivotovsky LA, King R, Torroni A, Cavalli-Sforza LL, Underhill PA, Santachiara-Benerecetti AS | display-authors = 6 | title = Origin, diffusion, and differentiation of Y-chromosome haplogroups E and J: inferences on the neolithization of Europe and later migratory events in the Mediterranean area | journal = American Journal of Human Genetics | volume = 74 | issue = 5 | pages = 1023–1034 | date = May 2004 | pmid = 15069642 | pmc = 1181965 | doi = 10.1086/386295 }}</ref> | |||
| === Matrilineal studies === | |||
| Apart from E-M81, other related haplogroups within the E-M35 clade are also seen as showing immigration from Northern Africa. Cruciani ''et al.'' (2007) using 6,501 unrelated Y-chromosome samples from 81 populations found that: "Considering both these E-M78 sub-haplogroups (E-V12, E-V22, E-V65) and the E-M81 haplogroup, the contribution of northern African lineages to the entire male gene pool of ] (barring Pasiegos), continental ] and ] can be estimated as 5.6%, 3.6%, and 6.6%, respectively."<ref>{{cite journal |author=Cruciani F, La Fratta R, Trombetta B, ''et al.'' |title=Tracing past human male movements in northern/eastern Africa and western Eurasia: new clues from Y-chromosomal haplogroups E-M78 and J-M12 |journal=Mol. Biol. Evol. |volume=24 |issue=6 |pages=1300–11 |year=2007 |month=June |pmid=17351267 |doi=10.1093/molbev/msm049 |url=http://mbe.oxfordjournals.org/cgi/content/full/24/6/1300}} See page 1307</ref> | |||
| There have been a number of studies about the ] (mtDNA) in Europe. In contrast to Y DNA haplogroups, mtDNA haplogroups did not show as much geographical patterning, but were more evenly ubiquitous. Apart from the outlying Saami, all Europeans are characterised by the predominance of haplogroups H, U and T. The lack of observable geographic structuring of mtDNA may be due to socio-cultural factors, namely the phenomena of ] and ].<ref name = "Rosser_2000" /> | |||
| Genetic studies suggest some maternal gene flow to eastern Europe from eastern Asia or southern Siberia 13,000 – 6,600 years ].<ref name=po2012dmd>{{cite journal | vauthors = Derenko M, Malyarchuk B, Denisova G, Perkova M, Rogalla U, Grzybowski T, Khusnutdinova E, Dambueva I, Zakharov I | display-authors = 6 | title = Complete mitochondrial DNA analysis of eastern Eurasian haplogroups rarely found in populations of northern Asia and eastern Europe | journal = PLOS ONE | volume = 7 | issue = 2 | pages = e32179 | year = 2012 | pmid = 22363811 | pmc = 3283723 | doi = 10.1371/journal.pone.0032179 | doi-access = free | bibcode = 2012PLoSO...732179D }}</ref> Analysis of Neolithic skeletons in the ] found a high frequency of eastern Asian mtDNA haplogroups, some of which survive in modern eastern European populations.<ref name=po2012dmd/> Maternal gene flow to Europe from sub-Saharan Africa began as early as 11,000 years BP, although the majority of lineages, approximately 65%, are estimated to have arrived more recently, including during the Romanization period, the Arab conquests of southern Europe, and during the Atlantic slave trade.<ref name=cerezo>{{cite journal | vauthors = Cerezo M, Achilli A, Olivieri A, Perego UA, Gómez-Carballa A, Brisighelli F, Lancioni H, Woodward SR, López-Soto M, Carracedo A, Capelli C, Torroni A, Salas A | display-authors = 6 | title = Reconstructing ancient mitochondrial DNA links between Africa and Europe | journal = Genome Research | volume = 22 | issue = 5 | pages = 821–826 | date = May 2012 | pmid = 22454235 | pmc = 3337428 | doi = 10.1101/gr.134452.111 }}</ref> | |||
| === European population sub-structure === | |||
| Genetic studies on Iberian populations also show that North African ] sequences (]) and sub-Saharan sequences (]), although present at only low levels, are still at much higher levels than those generally observed elsewhere in Europe. <ref>"Haplogroup U6 is present at frequencies ranging from 0 to 7% in the various Iberian populations, with an average of 1.8%. Given that the frequency of U6 in NW Africa is 10%, the mtDNA contribution of NW Africa to Iberia can be estimated at 18%. This is larger than the contribution estimated with Y-chromosomal lineages (7%) (Bosch et al. 2001)."</ref><ref name=Pereira05>{{cite journal |author=Pereira L, Cunha C, Alves C, Amorim A |title=African female heritage in Iberia: a reassessment of mtDNA lineage distribution in present times |journal=Hum. Biol. |volume=77 |issue=2 |pages=213–29 |year=2005 |month=April |pmid=16201138 |quote=Although the absolute value of observed U6 frequency in Iberia is low, it reveals a considerable North African female contribution, if we keep in mind that haplogroup U6 is not very common in North Africa itself and virtually absent in the rest of Europe. Indeed, because the range of variation in western North Africa is 4-28%, the estimated minimum input is 8.54%}}</ref><ref>"Our results clearly reinforce, extend, and clarify the preliminary clues of an "important mtDNA contribution from northwest Africa into the Iberian Peninsula" (Côrte-Real et al., 1996; Rando et al., 1998; Flores et al., 2000a; Rocha et al., 1999)(...) Our own data allow us to make minimal estimates of the maternal African pre-Neolithic, Neolithic, and/or recent slave trade input into Iberia. For the former, we consider only the mean value of the U6 frequency in northern African populations, excluding Saharans, Tuareg, and Mauritanians (16%), as the pre-Neolithic frequency in that area, and the present frequency in the whole Iberian Peninsula (2.3%) as the result of the northwest African gene flow at that time. The value obtained (14%) could be as high as 35% using the data of Corte-Real et al. (1996), or 27% with our north Portugal sample." </ref> Haplotype U6 have also been detected in ] at very low levels. It happens also to be a characteristic genetic marker of the Saami populations of Northern Scandinavia.<ref name=Pereira05/> It is difficult to ascertain that U6's presence is the consequence of Islam's expansion into Europe during the ], particularly because it is more frequent in the north of the Iberian Peninsula rather than in the south. In smaller numbers it is also attested too in the ], again at their northern and western borders. It may be a trace of a prehistoric neolithic/megalithic expansion along the Atlantic coasts from North Africa, perhaps in conjunction with seaborne trade. One subclade of U6 is particularly common among ] as a result of native ] (proto-Berber) ancestry. | |||
| Genetically, Europe is relatively homogeneous, but distinct sub-population patterns of various types of genetic markers have been found,<ref name = "Cavalli-Sforza_1993">{{cite book | vauthors = Cavalli-Sforza LL, Menozzi P, Piazza A |author-link1=Luigi Cavalli-Sforza|title=The History and Geography of Human Genes |publisher=] |year=1993|isbn=978-0-691-08750-4|url=https://books.google.com/books?id=FrwNcwKaUKoC|access-date=2009-07-22}}</ref> particularly along a southeast–northwest cline.<ref name = "Lao_2008" /> For example, Cavalli-Sforza's principal component analyses revealed five major clinal patterns throughout Europe, and similar patterns have continued to be found in more recent studies.<ref name = "Cavalli-Sforza_1993" />{{rp|291–296}} | |||
| # A cline of genes with highest frequencies in the ], spreading to lowest levels northwest. Cavalli-Sforza originally described this as faithfully reflecting the spread of agriculture in Neolithic times. This has been the general tendency in interpretation of all genes with this pattern. | |||
| # A cline of genes with highest frequencies among ] and ] in the extreme north east, and spreading to lowest frequencies in the south west. | |||
| # A cline of genes with highest frequencies in the area of the lower ] and ] rivers in ], and spreading to lowest frequencies in Spain, ], ] and the areas inhabited by ] in the extreme north of ]. Cavalli-Sforza associated this with the spread of Indo-European languages, which he links in turn to a "secondary expansion" after the spread of agriculture, associated with animal grazing. | |||
| # A cline of genes with highest frequencies in the ] and Southern Italy, spreading to lowest levels in Britain and the Basque country. Cavalli-Sforza associates this with "the Greek expansion, which reached its peak in historical times around 1000 and 500 BCE but which certainly began earlier". | |||
| # A cline of genes with highest frequencies in the ], and lower levels beyond the area of Iberia and ]. In perhaps the most well-known conclusion from Cavalli-Sforza, this weakest of the five patterns was described as isolated remnants of the pre-Neolithic population of Europe, "who at least partially withstood the expansion of the cultivators". It corresponds roughly to the geographical spread of ] blood types. In particular, the conclusion that the Basques are a genetic isolate has become widely discussed, but also a controversial conclusion. | |||
| He also created a phylogenetic tree to analyse the internal relationships among Europeans. He found four major 'outliers'- ], ], ] and ];<ref>Cavalli-Sforza, Luca; Menozzi, Paolo; Piazza, Alberto (1994). ''The History and Geography of Human Genes''. Princeton University Press, p. 272</ref> a result he attributed to their relative isolation (note: the Icelanders and the Sardinians speak ], while the other two groups do not). ] and ] represented a second group of less extreme outliers. The remaining populations clustered into several groups : "]", "]", "south-western Europeans", "]ns" and "eastern Europeans".<ref name = "Cavalli-Sforza_1993" />{{rp|268}} | |||
| ==== Sub Saharan Admixture admixture==== | |||
| A study conducted in May of 2009<ref>, Nelis et al. 2009</ref> researching 19 populations from Europe using 270,000 SNPs highlighted the genetic diversity of European populations corresponding to the northwest to southeast gradient and distinguished "four several distinct regions" within Europe: | |||
| {{see|Sub-Saharan DNA admixture in Europe|}} | |||
| * Finland, showing the greatest distance to the rest of Europeans. | |||
| * the ] (], ] and ]), western ] and eastern ]. | |||
| * Central and Western Europe. | |||
| * ], due to the alps acting as a great genetic barrier. | |||
| In this study, barrier analysis revealed "genetic barriers" between Finland, Italy and other countries and that barriers could also be demonstrated within Finland (between Helsinki and Kuusamo) and Italy (between northern and southern part, Fst=0.0050). Fst (]) was found to correlate considerably with geographic distances ranging from ≤0.0010 for neighbouring populations to 0.0200–0.0230 for Southern Italy and Finland. For comparisons, pair-wise Fst of non-European samples were as follows: Europeans – Africans (Yoruba) 0.1530; Europeans – Chinese 0.1100; Africans (Yoruba) – Chinese 0.1900.<ref>{{Cite journal |last1=Nelis |first1=Mari |last2=Esko |first2=Tõnu |last3=Mägi |first3=Reedik |last4=Zimprich |first4=Fritz |last5=Zimprich |first5=Alexander |last6=Toncheva |first6=Draga |last7=Karachanak |first7=Sena |last8=Piskáčková |first8=Tereza |last9=Balaščák |first9=Ivan |last10=Peltonen |first10=Leena |last11=Jakkula |first11=Eveliina |last12=Rehnström |first12=Karola |last13=Lathrop |first13=Mark |last14=Heath |first14=Simon |last15=Galan |first15=Pilar |date=2009-05-08 |title=Genetic Structure of Europeans: A View from the North–East |journal=PLOS ONE |language=en |volume=4 |issue=5 |pages=e5472 |doi=10.1371/journal.pone.0005472 |issn=1932-6203 |doi-access=free}}</ref> | |||
| Sub-Saharan African Y-chromosomes are much less common in Europe, for the reasons discussed above. The small presence of the Haplogroups E(xE3b) (i.e. clades of E other than E3b) and Haplogroup A in Europe is attributable to the slave trade, the Moor invasion of Spain or prehistoric migrations.<ref>{{cite journal |author=Capelli C, Onofri V, Brisighelli F, ''et al.'' |title=Moors and Saracens in Europe: estimating the medieval North African male legacy in southern Europe |journal=Eur. J. Hum. Genet. |volume=17 |issue=6 |pages=848–52 |year=2009 |month=June |pmid=19156170 |doi=10.1038/ejhg.2008.258 }}</ref><ref>Sanchez et al. (2005). "High frequencies of Y chromosome lineages characterized by E3b1, DYS19-11, DYS392-12 in Somali males". European Journal of Human Genetics; 13:856–866</ref> Haplotype A has been detected in ]<ref name="king"/> Portugal (3%), France (2.5% in a very small sample), Germany (2%), Sardinia (1.6%), ] (0.78%), ] (0.45%), ] (0.42%), ] (0.27%) Cyprus and Turkey.<ref name="king"/>. By contrast, ] have about 5% ] sub-Saharan admixture.<ref>{{cite journal |author=Cruciani F, La Fratta R, Santolamazza P, ''et al.'' |title=Phylogeographic analysis of haplogroup E3b (E-M215) y chromosomes reveals multiple migratory events within and out of Africa |journal=Am. J. Hum. Genet. |volume=74 |issue=5 |pages=1014–22 |year=2004 |month=May |pmid=15042509 |pmc=1181964 |doi=10.1086/386294 }}<br/>{{cite journal |author=Flores C, Maca-Meyer N, González AM, ''et al.'' |title=Reduced genetic structure of the Iberian peninsula revealed by Y-chromosome analysis: implications for population demography |journal=Eur. J. Hum. Genet. |volume=12 |issue=10 |pages=855–63 |year=2004 |month=October |pmid=15280900 |doi=10.1038/sj.ejhg.5201225 }}<br/>, ,<br/>{{cite journal |author=Rosser ZH, Zerjal T, Hurles ME, ''et al.'' |title=Y-chromosomal diversity in Europe is clinal and influenced primarily by geography, rather than by language |journal=Am. J. Hum. Genet. |volume=67 |issue=6 |pages=1526–43 |year=2000 |month=December |pmid=11078479 |pmc=1287948 |doi=10.1086/316890 }}<br/>{{cite journal |author=Semino O, Magri C, Benuzzi G, ''et al.'' |title=Origin, diffusion, and differentiation of Y-chromosome haplogroups E and J: inferences on the neolithization of Europe and later migratory events in the Mediterranean area |journal=Am. J. Hum. Genet. |volume=74 |issue=5 |pages=1023–34 |year=2004 |month=May |pmid=15069642 |pmc=1181965 |doi=10.1086/386295 }}<br/>{{cite journal |author=Di Giacomo F, Luca F, Anagnou N, ''et al.'' |title=Clinal patterns of human Y chromosomal diversity in continental Italy and Greece are dominated by drift and founder effects |journal=Mol. Phylogenet. Evol. |volume=28 |issue=3 |pages=387–95 |year=2003 |month=September |pmid=12927125 }} | |||
| '''Bosch et al. 2001''' '''</ref> | |||
| A study by Chao Tian in August 2009 extended the analysis of European population genetic structure to include additional southern European groups and levantine populations (], ]...) from the Near-East. This study determined autosomal Fst between 18 population groups and concluded that, in general, genetic distances corresponded to geographical relationships with smaller values between population groups with origins in neighbouring countries/regions (for example, ]/]: Fst=0.0010, ]/]: Fst=0.0057) compared with those from very different regions in Europe (for example ]/]: Fst=0.0087, ]/]: Fst=0.0108).<ref name="European population genetic substru">{{cite journal | vauthors = Tian C, Kosoy R, Nassir R, Lee A, Villoslada P, Klareskog L, Hammarström L, Garchon HJ, Pulver AE, Ransom M, Gregersen PK, Seldin MF | display-authors = 6 | title = European population genetic substructure: further definition of ancestry informative markers for distinguishing among diverse European ethnic groups | journal = Molecular Medicine | volume = 15 | issue = 11–12 | pages = 371–383 | year = 2009 | pmid = 19707526 | pmc = 2730349 | doi = 10.2119/molmed.2009.00094 }}</ref> | |||
| African Mitochondrial haplogroups are distributed along a west-to-east cline and a south-to-north cline. The highest frequencies are observed in the Iberian Peninsula. | |||
| ==== Autosomal DNA ==== | |||
| Malyarchuk et al. identified 8 African haplogroups in Russians, Czechs, Slovaks and Polish populations. These haplogroups included L1b, L2a, L3b, L3d and M1, of which L1b, L3b1, L3d appeared to be of West African origin. Haplogroup L2a1a was identified as most likely having entered Europe about 10,000 years ago, possibly through the Iberian Peninsula. <ref>{{cite journal|year=2008|last=Malyarchuk et al.|doi=10.1038/ejhg.2008.70|url=http://www.nature.com/ejhg/journal/v16/n9/abs/ejhg200870a.html|title=Reconstructing the phylogeny of African mitochondrial DNA lineages in Slavs}}</ref> | |||
| Seldin (2006) used over 5,000 autosomal SNPs. It showed "a consistent and reproducible distinction between ‘northern’ and ‘southern’ ] groups". Most individual participants with ]an ancestry (], ], ], ]), and ] have >85% membership in the southern population; and most northern, western, central, and eastern Europeans (], ], ], ], and ]) have >90% in the northern population group. Many of the participants in this study were American citizens who self-identified with different European ethnicities based on self-reported familial pedigree.<ref>{{cite journal | vauthors = Seldin MF, Shigeta R, Villoslada P, Selmi C, Tuomilehto J, Silva G, Belmont JW, Klareskog L, Gregersen PK | display-authors = 6 | title = European population substructure: clustering of northern and southern populations | journal = PLOS Genetics | volume = 2 | issue = 9 | pages = e143 | date = September 2006 | pmid = 17044734 | pmc = 1564423 | doi = 10.1371/journal.pgen.0020143 | doi-access = free }}</ref> | |||
| A similar study in 2007 using samples predominantly from Europe found that the most important genetic differentiation in Europe occurs on a line from the north to the south-east (] to the Balkans), with another east–west axis of differentiation across Europe. Its findings were consistent with earlier results based on mtDNA and Y-chromosomal DNA that support the theory that modern Iberians (Spanish and Portuguese) hold the most ancient European genetic ancestry, as well as separating Basques and Sami from other European populations.<ref name="sitesled2007">{{cite journal | vauthors = Bauchet M, McEvoy B, Pearson LN, Quillen EE, Sarkisian T, Hovhannesyan K, Deka R, Bradley DG, Shriver MD | display-authors = 6 | title = Measuring European population stratification with microarray genotype data | journal = American Journal of Human Genetics | volume = 80 | issue = 5 | pages = 948–956 | date = May 2007 | pmid = 17436249 | pmc = 1852743 | doi = 10.1086/513477 }}</ref> | |||
| ==== Uralic, Central, and East Asian admixture ==== | |||
| It suggested that the ] and Irish cluster with other Northern and Eastern Europeans such as ] and ], while some Basque and Italian individuals also clustered with Northern Europeans. Despite these stratifications, it noted that "there is low apparent diversity in Europe with the entire continent-wide samples only marginally more dispersed than single population samples elsewhere in the world".<ref name="sitesled2007"/> | |||
| Central Asian Y Chromosomes are somewhat common in European populations. Tat-C (haplogroup 16) is a Y-chromosome lineage that originated in Central Asia<ref>{{cite journal |author=Lell JT, Sukernik RI, Starikovskaya YB, ''et al.'' |title=The dual origin and Siberian affinities of Native American Y chromosomes |journal=Am. J. Hum. Genet. |volume=70 |issue=1 |pages=192–206 |year=2002 |month=January |pmid=11731934 |pmc=384887 |doi=10.1086/338457 }}</ref> and likely spread to Northeastern Europe with male Uralic hunter/gatherer migrations occurring over the last 4000 years. <ref> "The network of Tat-C and DYS7C haplotypes revealed that the ancestral Tat-C haplotype (7C) was found only in southern Middle Siberia, indicating that this Y-chromosome lineage arose in that region. Moreover, the limited microsatellite diversity and resulting compact nature of the network indicates that the Tat-C lineage arose relatively recently (Zerjal et al. 1997). The absence of the Tat-C haplogroup in the Americas, with the exception of a single Navajo (Karafet et al. 1999), along with its high frequency in both northern Europe and northeastern Siberia, indicates that the Tat-C lineage was disseminated from central Asia by both westward and eastward male migrations, the eastward migration reaching Chukotka after the Bering Land Bridge was submerged. Both the M45 and Tat-C haplogroups have been found in Europe, indicating both ancient and recent central Asian influences. However, neither of these major Middle Siberian Y-chromosome lineages appears to have been greatly influenced by the paternal gene pool of Han Chinese or other East Asian populations (Su et al. 1999)."</ref>. | |||
| Today it's found In Northern and Northeast Europe in low to high frequencies. It is found in Finland (55%), European Russia (14%), Ukraine (11%), Lithuania (47%), Estonia (37)%, Sweden (8%), Norway, (6%), Poland (4%), Germany (3%), Slovakia (3%), Denmark (2%), and Belarus (2%). <ref>{{cite journal |author=Kittles RA, Perola M, Peltonen L, ''et al.'' |title=Dual origins of Finns revealed by Y chromosome haplotype variation |journal=Am. J. Hum. Genet. |volume=62 |issue=5 |pages=1171–9 |year=1998 |month=May |pmid=9545401 |pmc=1377088 |doi=10.1086/301831 |url=http://www.cell.com/AJHG/abstract/S0002-9297(07)61539-0}}</ref><ref>{{cite journal |author=Passarino G, Cavalleri GL, Lin AA, Cavalli-Sforza LL, Børresen-Dale AL, Underhill PA |title=Different genetic components in the Norwegian population revealed by the analysis of mtDNA and Y chromosome polymorphisms |journal=Eur. J. Hum. Genet. |volume=10 |issue=9 |pages=521–9 |year=2002 |month=September |pmid=12173029 |doi=10.1038/sj.ejhg.5200834 |url=http://www.nature.com/ejhg/journal/v10/n9/full/5200834a.html}}</ref> | |||
| In 2008, two international research teams published analyses of large-scale genotyping of large samples of Europeans, using over 300,000 autosomal SNPs. With the exception of usual isolates such as ], ] and ], the European population lacked sharp discontinuities (clustering) as previous studies have found (see Seldin ''et al.'' 2006 and Bauchet ''et al.'' 2007<ref name="sitesled2007"/>), although there was a discernible south to north gradient. Overall, they found only a low level of genetic differentiation between subpopulations, and differences which did exist were characterised by a strong continent-wide correlation between geographic and genetic distance. In addition, they found that diversity was greatest in southern Europe due a larger effective population size and/or ] from southern to northern Europe.<ref name = "Lao_2008" /> The researchers take this observation to imply that genetically, Europeans are not distributed into discrete populations.<ref>{{cite journal | vauthors = Novembre J, Johnson T, Bryc K, Kutalik Z, Boyko AR, Auton A, Indap A, King KS, Bergmann S, Nelson MR, Stephens M, Bustamante CD | display-authors = 6 | title = Genes mirror geography within Europe | journal = Nature | volume = 456 | issue = 7218 | pages = 98–101 | date = November 2008 | pmid = 18758442 | pmc = 2735096 | doi = 10.1038/nature07331 | first8 = KS | first12 = CD | first11 = M | first5 = AR | first9 = S | bibcode = 2008Natur.456...98N | first10 = MR | first6 = A | first7 = A }}</ref><ref name = "Lao_2008">{{cite journal | vauthors = Lao O, Lu TT, Nothnagel M, Junge O, Freitag-Wolf S, Caliebe A, Balascakova M, Bertranpetit J, Bindoff LA, Comas D, Holmlund G, Kouvatsi A, Macek M, Mollet I, Parson W, Palo J, Ploski R, Sajantila A, Tagliabracci A, Gether U, Werge T, Rivadeneira F, Hofman A, Uitterlinden AG, Gieger C, Wichmann HE, Rüther A, Schreiber S, Becker C, Nürnberg P, Nelson MR, Krawczak M, Kayser M | display-authors = 6 | title = Correlation between genetic and geographic structure in Europe | journal = Current Biology | volume = 18 | issue = 16 | pages = 1241–1248 | date = August 2008 | pmid = 18691889 | doi = 10.1016/j.cub.2008.07.049 | s2cid = 16945780 | doi-access = free }}</ref> | |||
| ==Immigration in more recent historical periods== | |||
| === Bronze and Iron Age migrations === | |||
| {{see|Bronze Age Europe|Iron Age Europe}} | |||
| Two whole-genome studies of the two Eastern European populations in Ukraine (] from ]) and Russia (]ns from ]) showed genomic diversity, which has not been represented in the previous genomic surveys, as studies in Europe are mostly biased towards the populations in the western part of the continent.<ref>{{cite journal | vauthors = Oleksyk TK, Wolfsberger WW, Weber AM, Shchubelka K, Oleksyk OT, Levchuk O, Patrus A, Lazar N, Castro-Marquez SO, Hasynets Y, Boldyzhar P, Neymet M, Urbanovych A, Stakhovska V, Malyar K, Chervyakova S, Podoroha O, Kovalchuk N, Rodriguez-Flores JL, Zhou W, Medley S, Battistuzzi F, Liu R, Hou Y, Chen S, Yang H, Yeager M, Dean M, Mills RE, Smolanka V | display-authors = 6 | title = Genome diversity in Ukraine | journal = GigaScience | volume = 10 | issue = 1 | date = January 2021 | pmid = 33438729 | pmc = 7804371 | doi = 10.1093/gigascience/giaa159 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Zhernakova DV, Brukhin V, Malov S, Oleksyk TK, Koepfli KP, Zhuk A, Dobrynin P, Kliver S, Cherkasov N, Tamazian G, Rotkevich M, Krasheninnikova K, Evsyukov I, Sidorov S, Gorbunova A, Chernyaeva E, Shevchenko A, Kolchanova S, Komissarov A, Simonov S, Antonik A, Logachev A, Polev DE, Pavlova OA, Glotov AS, Ulantsev V, Noskova E, Davydova TK, Sivtseva TM, Limborska S, Balanovsky O, Osakovsky V, Novozhilov A, Puzyrev V, O'Brien SJ | display-authors = 6 | title = Genome-wide sequence analyses of ethnic populations across Russia | journal = Genomics | volume = 112 | issue = 1 | pages = 442–458 | date = January 2020 | pmid = 30902755 | pmc =4644275 | doi = 10.1186/s13742-015-0095-0 | doi-access = free }}</ref> Within ], ], who live in the northeastern regions and are part of the ] language family that also includes ], form a pole of genetic diversity that is distinct from other populations, and characterized by a higher European hunter-gatherer (WHG) and Ancient North Eurasian ancestry.<ref name=plosone130307>{{cite journal | vauthors = Khrunin AV, Khokhrin DV, Filippova IN, Esko T, Nelis M, Bebyakova NA, Bolotova NL, Klovins J, Nikitina-Zake L, Rehnström K, Ripatti S, Schreiber S, Franke A, Macek M, Krulišová V, Lubinski J, Metspalu A, Limborska SA | display-authors = 6 | title = A genome-wide analysis of populations from European Russia reveals a new pole of genetic diversity in northern Europe | journal = PLOS ONE | volume = 8 | issue = 3 | pages = e58552 | date = March 7, 2013 | pmid = 23505534 | pmc = 3591355 | doi = 10.1371/journal.pone.0058552 | doi-access = free | bibcode = 2013PLoSO...858552K }}</ref><ref name="The Arrival of Siberian Ancestry Co">{{cite journal | vauthors = Saag L, Laneman M, Varul L, Malve M, Valk H, Razzak MA, Shirobokov IG, Khartanovich VI, Mikhaylova ER, Kushniarevich A, Scheib CL, Solnik A, Reisberg T, Parik J, Saag L, Metspalu E, Rootsi S, Montinaro F, Remm M, Mägi R, D'Atanasio E, Crema ER, Díez-Del-Molino D, Thomas MG, Kriiska A, Kivisild T, Villems R, Lang V, Metspalu M, Tambets K | display-authors = 6 | title = The Arrival of Siberian Ancestry Connecting the Eastern Baltic to Uralic Speakers further East | journal = Current Biology | volume = 29 | issue = 10 | pages = 1701–1711.e16 | date = May 2019 | pmid = 31080083 | pmc = 6544527 | doi = 10.1016/j.cub.2019.04.026 }}</ref> | |||
| The ] saw the development of long-distance ]s, particularly along the Atlantic Coast and in the Danube valley. There was migration from ] to ] and ] in this period (and to a lesser extent to mainland Scotland and Ireland). There was also migration from Germany to eastern England. Martin Richards estimated that there was about 4% mtDNA immigration to Europe in the Bronze Age. Oppenheimer could find no genetic evidence for any ] migration to Britain. | |||
| According to geneticist ], based on ] that his laboratory sequenced in 2016, Europeans descend from a mixture of four West-Eurasian ancestral components, namely WHG (western hunter-gatherers), EHG, Neolithic farmers from the Levant/Anatolia as well as from Neolithic farmers from Iran (often summarized as "EEF"; early European farmers), in varying degrees.<ref>{{cite journal | vauthors = Lazaridis I, Nadel D, Rollefson G, Merrett DC, Rohland N, Mallick S, Fernandes D, Novak M, Gamarra B, Sirak K, Connell S, Stewardson K, Harney E, Fu Q, Gonzalez-Fortes G, Jones ER, Roodenberg SA, Lengyel G, Bocquentin F, Gasparian B, Monge JM, Gregg M, Eshed V, Mizrahi AS, Meiklejohn C, Gerritsen F, Bejenaru L, Blüher M, Campbell A, Cavalleri G, Comas D, Froguel P, Gilbert E, Kerr SM, Kovacs P, Krause J, McGettigan D, Merrigan M, Merriwether DA, O'Reilly S, Richards MB, Semino O, Shamoon-Pour M, Stefanescu G, Stumvoll M, Tönjes A, Torroni A, Wilson JF, Yengo L, Hovhannisyan NA, Patterson N, Pinhasi R, Reich D | display-authors = 6 | title = Genomic insights into the origin of farming in the ancient Near East | journal = Nature | volume = 536 | issue = 7617 | pages = 419–424 | date = August 2016 | pmid = 27459054 | doi = 10.1038/nature19310 | pmc = 5003663 | bibcode = 2016Natur.536..419L | quote = bottom-left: Western Hunter Gatherers (WHG), top-left: Eastern Hunter Gatherers (EHG), bottom-right: Neolithic Levant and Natufians, top-right: Neolithic Iran. This suggests the hypothesis that diverse ancient West Eurasians can be modelled as mixtures of as few as four streams of ancestry related to these population }}</ref><ref>{{cite journal |display-authors=6 |vauthors=Yang MA, Gao X, Theunert C, Tong H, Aximu-Petri A, Nickel B, Slatkin M, Meyer M, Pääbo S, Kelso J, Fu Q |date=October 2017 |title=40,000-Year-Old Individual from Asia Provides Insight into Early Population Structure in Eurasia |journal=Current Biology |volume=27 |issue=20 |pages=3202–3208.e9 |doi=10.1016/j.cub.2017.09.030 |pmc=6592271 |pmid=29033327 |quote=}}</ref> | |||
| One theory about the origin of the ] centres around a hypothetical ] people, who are traced, in the ], to somewhere north of the Black Sea at about 4500 BCE. They ], and are considered to have spread their culture and genes across Europe. It has been difficult to identify what these "Kurgan" genes might be, though the ] ] is a proposed marker which would indicate that the physical expansion halted in Germany and only the Kurgan culture and language went further. Another hypothesis — the ] — suggests an origin in Anatolia with a later expansion from eastern Europe. | |||
| Siberian geneflow is found among several Uralic-speaking European ethnic groups. This Siberian component is itself a composition of Ancient North Eurasian and East Asian-related ancestry from Eastern Siberia, maximized among ] and ] or ]. The spread of this ancestry is linked by some geneticists to the dispersal of ], others however maintain that the Uralic languages spread prior to the arrival of Siberian geneflow, which is a secondary source of diversity within Uralic-speaking populations.<ref>{{Cite journal |last1=Lamnidis |first1=Thiseas C. |last2=Majander |first2=Kerttu |last3=Jeong |first3=Choongwon |last4=Salmela |first4=Elina |last5=Wessman |first5=Anna |last6=Moiseyev |first6=Vyacheslav |last7=Khartanovich |first7=Valery |last8=Balanovsky |first8=Oleg |last9=Ongyerth |first9=Matthias |last10=Weihmann |first10=Antje |last11=Sajantila |first11=Antti |last12=Kelso |first12=Janet |last13=Pääbo |first13=Svante |last14=Onkamo |first14=Päivi |last15=Haak |first15=Wolfgang |date=2018-11-27 |title=Ancient Fennoscandian genomes reveal origin and spread of Siberian ancestry in Europe |journal=Nature Communications |volume=9 |issue=1 |pages=5018 |doi=10.1038/s41467-018-07483-5 |issn=2041-1723 |pmc=6258758 |pmid=30479341|bibcode=2018NatCo...9.5018L }}</ref><ref>{{Cite journal |last1=Santos |first1=Patrícia |last2=Gonzàlez-Fortes |first2=Gloria |last3=Trucchi |first3=Emiliano |last4=Ceolin |first4=Andrea |last5=Cordoni |first5=Guido |last6=Guardiano |first6=Cristina |last7=Longobardi |first7=Giuseppe |last8=Barbujani |first8=Guido |date=December 2020 |title=More Rule than Exception: Parallel Evidence of Ancient Migrations in Grammars and Genomes of Finno-Ugric Speakers |journal=Genes |language=en |volume=11 |issue=12 |pages=1491 |doi=10.3390/genes11121491 |pmid=33322364 |pmc=7763979 |issn=2073-4425|doi-access=free }}</ref> Genetic data points to a ]n ] origin of the observed Siberian geneflow among Uralic-speaking groups. Western Siberian hunter-gatherers were characterized by high ] ancestry and lower amounts of Eastern Siberian admixture. Genetic data on ] or ], found among "''Western Turkic speakers, like Chuvash and Volga Tatar, the East Asian component was detected only in low amounts (~ 5%)''".<ref>{{Cite journal |last=Wong |first=Emily H.M. |date=2015 |title=Reconstructing genetic history of Siberian and Northeastern European populations |journal=Genome Research |volume=27 |issue=1 |pages=1–14 |doi=10.1101/gr.202945.115 |pmid=27965293 |pmc=5204334}}</ref><ref>{{Cite journal |last1=Triska |first1=Petr |last2=Chekanov |first2=Nikolay |last3=Stepanov |first3=Vadim |last4=Khusnutdinova |first4=Elza K. |last5=Kumar |first5=Ganesh Prasad Arun |last6=Akhmetova |first6=Vita |last7=Babalyan |first7=Konstantin |last8=Boulygina |first8=Eugenia |last9=Kharkov |first9=Vladimir |last10=Gubina |first10=Marina |last11=Khidiyatova |first11=Irina |last12=Khitrinskaya |first12=Irina |last13=Khrameeva |first13=Ekaterina E. |last14=Khusainova |first14=Rita |last15=Konovalova |first15=Natalia |date=2017-12-28 |title=Between Lake Baikal and the Baltic Sea: genomic history of the gateway to Europe |journal=BMC Genetics |volume=18 |issue=1 |pages=110 |doi=10.1186/s12863-017-0578-3 |issn=1471-2156 |pmc=5751809 |pmid=29297395 |quote=Comparative analysis of modern and ancient genomes suggests that Western Siberians have more Ancient North European ancestry (represented by Mal’ta) than other populations of the Russian Federation. |doi-access=free }}</ref> | |||
| To what extent Indo-European migrations replaced the ] ] peoples is debated, but a ] has been reached that technology and language transfer played a more important role in this process than actual gene-flow.<ref> See ], '']'', 1st American ed. (New York: Norton, 2001) for an entertaining account of how this consensus was reached. For historical reasons, in the 1980s mtDNA researchers believed that the Indo-European expansion was overwhelmingly a spread of technology and language, not of genes, while those who studied ] believed the opposite. Gradually the mtDNA researchers (Sykes) admitted more physical migration into their scenarios, while the Y folks (Peter Underhill) accepted more technology-copying. Eventually, both groups independently reached a 20% Neolithic - 80% Paleolithic ratio of genetic contribution to today's European population. The mtDNA vs. Y-chromosome discrepancy may be explained by noting that in such conquest-based migrations, a common pattern is of invading foreign males producing offspring with indigenous females, though significant numbers of females of the spreading culture could also arrive with post-conquest settlers. However, where migrations are essentially economic (as most migrations appear to be) it appears equally probable that male family members preceded females into new territory looking for opportunities.</ref> | |||
| ] ancestry is found at low frequency among some Europeans, such as ] (2.5 ± 1%), ] (3.8 ± 1%), ] (0.7 ± 0.8%) and ] (0.7 ± 0.8%). Finns and Russians have more than 12% East Asian ancestry, deriving from historic intermarriages with Mongolian populations.<ref>{{Cite journal |last1=Qin |first1=Pengfei |last2=Zhou |first2=Ying |last3=Lou |first3=Haiyi |last4=Lu |first4=Dongsheng |last5=Yang |first5=Xiong |last6=Wang |first6=Yuchen |last7=Jin |first7=Li |last8=Chung |first8=Yeun-Jun |last9=Xu |first9=Shuhua |date=2015-04-02 |title=Quantitating and Dating Recent Gene Flow between European and East Asian Populations |journal=Scientific Reports |language=en |volume=5 |issue=1 |pages=9500 |doi=10.1038/srep09500 |pmid=25833680 |pmc=4382708 |bibcode=2015NatSR...5E9500Q |issn=2045-2322}} "The gene flow from EAS to Finnish and Northeastern Russian populations could be dated back 64.2 ± 1.1 and 45.2 ± 1.3 generations, or 1,862 and 1,311 years, respectively."</ref> But a 2017 study finds no evidence of Asian admixture among Russians, except for ] residents and ] in Siberia.<ref>{{Cite journal |last1=Triska |first1=Petr |last2=Chekanov |first2=Nikolay |last3=Stepanov |first3=Vadim |last4=Khusnutdinova |first4=Elza K. |display-authors=3 |date=2017 |title=Between Lake Baikal and the Baltic Sea: genomic history of the gateway to Europe |journal=BMC Genetics |volume=18 |issue=1 |page=110 |doi=10.1186/s12863-017-0578-3 |pmid=29297395 |doi-access=free|pmc=5751809 }}</ref>The ], a ] in Belarus, have significant East Eurasian ancestry, making up one-third of their genome.<ref name="srep30197">{{cite journal |last1=Pankratov |first1=Vasili |last2=Litvinov |first2=Sergei |last3=Kassian |first3=Alexei |last4=Shulhin |first4=Dzmitry |last5=Tchebotarev |first5=Lieve |last6=Yunusbayev |first6=Bayazit |last7=Möls |first7=Märt |last8=Sahakyan |first8=Hovhannes |last9=Yepiskoposyan |first9=Levon |last10=Rootsi |first10=Siiri |last11=Metspalu |first11=Ene |last12=Golubenko |first12=Maria |last13=Ekomasova |first13=Natalia |last14=Akhatova |first14=Farida |last15=Khusnutdinova |first15=Elza |date=25 July 2016 |title=East Eurasian ancestry in the middle of Europe: genetic footprints of Steppe nomads in the genomes of Belarusian Lipka Tatars |journal=Scientific Reports |language=en |volume=6 |pages=30197 |bibcode=2016NatSR...630197P |doi=10.1038/srep30197 |pmc=4958967 |pmid=27453128 |last16=Heyer |first16=Evelyne |last17=Endicott |first17=Phillip |last18=Derenko |first18=Miroslava |last19=Malyarchuk |first19=Boris |last20=Metspalu |first20=Mait |last21=Davydenko |first21=Oleg |last22=Villems |first22=Richard |last23=Kushniarevich |first23=Alena}}{{Creative Commons text attribution notice|cc=by4|from this source=yes}}</ref> | |||
| During the Iron Age, Celts are recorded as having moved from ] into northern Italy, Eastern Europe and Anatolia. The relationship between the Celts of Gaul and Spain is unclear as any migration occurred before records exist. | |||
| Like other Eurasian populations, Mesolithic, Neolithic or Bronze Age ancestries are not homogenously distributed in European populations. But WHG-related ancestries are highest in present-day individuals from the Baltic States, Belarus, Poland and Russia whilst EHG-related ancestries are highest in Finland and Estonia. Steppe-related ancestries are found in high levels in northern Europe, peaking in Ireland, Iceland, Norway and Sweden, but decrease further south, especially in southern Europe, where Neolithic Anatolian-related farmer ancestries dominate.<ref>{{Cite journal |last1=Irving-Pease |first1=Evan K. |last2=Refoyo-Martínez |first2=Alba |last3=Barrie |first3=William |last4=Ingason |first4=Andrés |display-authors=3 |date=2024 |title=The selection landscape and genetic legacy of ancient Eurasians |url=https://www.nature.com/articles/s41586-023-06705-1#Sec11 |journal=Nature |volume=625 |issue=7994 |pages=312–320 |doi=10.1038/s41586-023-06705-1 |via=Nature.com}}</ref> | |||
| ====Roman Period admixture ==== | |||
| During the period of the ], historical sources show that there were many movements of people around Europe, both within and outside the Empire. These included army personnel and administrators as well as private citizens. However, compared with the total population, these movements seem mostly to have been small. No genetic information on these migrations appears to exist other than a person with a rare Yorkshire surname of African ancestry (Y Hg A1).<ref name="king">{{cite journal|title=Africans in Yorkshire? |last= King et al.|year=2007|url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2590664}}</ref> | |||
| ==== Autosomal genetic distances (Fst) based on SNPs (2009) ==== | |||
| == Inferences from ancient DNA == | |||
| The genetic history of Europe has mostly been reconstructed from the modern populations of Europe, assuming genetic continuity. This is because of availability of data. However, a small number of ancient mtDNA analyses are available from both the historical and prehistorical periods. These have been summarised by Ellen Levy-Coffman in the ''Journal of Genetic Genealogy''. There are some large differences in the frequencies of occurrence of the various haplogroups compared wth the modern population. | |||
| The genetic distance between populations is often measured by ] (Fst), based on genetic polymorphism data, such as ] or ]. Fst is a special case of ], the concept developed in the 1920s by ]. Fst is simply the correlation of randomly chosen alleles within the same sub-population relative to that found in the entire population. It is often expressed as the proportion of genetic diversity due to allele frequency differences among populations. | |||
| For example, ], while presently rare (0.18%-0.3%), occurred in as many as 25% of ] Europeans. <ref>{{cite journal |author=Haak W, Forster P, Bramanti B, ''et al.'' |title=Ancient DNA from the first European farmers in 7500-year-old Neolithic sites |journal=Science |volume=310 |issue=5750 |pages=1016–8 |year=2005 |month=November |pmid=16284177 |doi=10.1126/science.1118725 }}</ref><ref>{{cite journal |author=Balter M |title=Evolution. Ancient DNA yields clues to the puzzle of European origins |journal=Science |volume=310 |issue=5750 |pages=964–5 |year=2005 |month=November |pmid=16284157 |doi=10.1126/science.310.5750.964 }}</ref>. The cause of this reduction is unknown. | |||
| The values range from 0 to 1. A zero value implies that the two populations are panmictic, that they are interbreeding freely. A value of one would imply that the two populations are completely separate. The greater the Fst value, the greater the genetic distance. Essentially, these low Fst values suggest that the majority of genetic variation is at the level of individuals within the same population group (~ 85%); whilst belonging to a different population group within same ‘race’/ continent, and even to different racial/ continental groups added a much smaller degree of variation (3–8%; 6–11%, respectively). | |||
| She concludes that the genetic profile of Europe has undergone significant transformation over time and that the modern population is not a living fossil of the ancient one. However, the very small sample sizes of the ancient DNA are a problem and more data is needed. | |||
| {|class="wikitable" style="text-align:center; width:80%;" | |||
| == Footnotes == | |||
| |+ Intra-European/Mediterranean ] based on 3,500 ] using the Weir and Cockerham algorithm<ref name="European population genetic substru"/> | |||
| {{reflist|2}} | |||
| |- | |||
| ! scope=col | | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| |- | |||
| ! scope=row |Italian Americans | |||
| | | |||
| |0.0064 | |||
| |0.0064 | |||
| |0.0057 | |||
| |0.0010 | |||
| |0.0029 | |||
| |0.0088 | |||
| |0.0048 | |||
| |0.0000 | |||
| |0.0040 | |||
| |0.0067 | |||
| |- | |||
| ! scope=row |Palestinians | |||
| |0.0064 | |||
| | | |||
| |0.0191 | |||
| |0.0064 | |||
| |0.0101 | |||
| |0.0136 | |||
| |0.0202 | |||
| |0.0170 | |||
| |0.0057 | |||
| |0.0093 | |||
| |0.0108 | |||
| |- | |||
| ! scope=row |Swedes | |||
| |0.0064 | |||
| |0.0191 | |||
| | | |||
| |0.0167 | |||
| |0.0040 | |||
| |0.0007 | |||
| |0.0030 | |||
| |0.0020 | |||
| |0.0084 | |||
| |0.0120 | |||
| |0.0117 | |||
| |- | |||
| ! scope=row |Druzes | |||
| |0.0057 | |||
| |0.0064 | |||
| |0.0167 | |||
| | | |||
| |0.0096 | |||
| |0.0121 | |||
| |0.0194 | |||
| |0.0154 | |||
| |0.0052 | |||
| |0.0088 | |||
| |0.0092 | |||
| |- | |||
| ! scope=row |Spaniards | |||
| |0.0010 | |||
| |0.0101 | |||
| |0.0040 | |||
| |0.0096 | |||
| | | |||
| |0.0015 | |||
| |0.0070 | |||
| |0.0037 | |||
| |0.0035 | |||
| |0.0056 | |||
| |0.0090 | |||
| |- | |||
| ! scope=row |Germans | |||
| |0.0029 | |||
| |0.0136 | |||
| |0.0007 | |||
| |0.0121 | |||
| |0.0015 | |||
| | | |||
| |0.0030 | |||
| |0.0010 | |||
| |0.0039 | |||
| |0.0072 | |||
| |0.0089 | |||
| |- | |||
| ! scope=row |Russians | |||
| |0.0088 | |||
| |0.0202 | |||
| |0.0030 | |||
| |0.0194 | |||
| |0.0070 | |||
| |0.0030 | |||
| | | |||
| |0.0038 | |||
| |0.0108 | |||
| |0.0137 | |||
| |0.0120 | |||
| |- | |||
| ! scope=row |Irish | |||
| |0.0048 | |||
| |0.0170 | |||
| |0.0020 | |||
| |0.0154 | |||
| |0.0037 | |||
| |0.0010 | |||
| |0.0038 | |||
| | | |||
| |0.0067 | |||
| |0.0109 | |||
| |0.0110 | |||
| |- | |||
| ! scope=row |Greek Americans | |||
| |0.0000 | |||
| |0.0057 | |||
| |0.0084 | |||
| |0.0052 | |||
| |0.0035 | |||
| |0.0039 | |||
| |0.0108 | |||
| |0.0067 | |||
| | | |||
| |0.0042 | |||
| |0.0054 | |||
| |- | |||
| ! scope=row |Ashkenazi Jews | |||
| |0.0040 | |||
| |0.0093 | |||
| |0.0120 | |||
| |0.0088 | |||
| |0.0056 | |||
| |0.0072 | |||
| |0.0137 | |||
| |0.0109 | |||
| |0.0042 | |||
| | | |||
| |0.0107 | |||
| |- | |||
| ! scope=row |Circassians | |||
| |0.0067 | |||
| |0.0108 | |||
| |0.0117 | |||
| |0.0092 | |||
| |0.0090 | |||
| |0.0089 | |||
| |0.0120 | |||
| |0.0110 | |||
| |0.0054 | |||
| |0.0107 | |||
| | | |||
| |} | |||
| {|class="wikitable" style="text-align:center; width:80%;" | |||
| |+ European Population Genetic Substructure based on SNPs<ref name="nelis">{{cite journal | vauthors = Nelis M, Esko T, Mägi R, Zimprich F, Zimprich A, Toncheva D, Karachanak S, Piskácková T, Balascák I, Peltonen L, Jakkula E, Rehnström K, Lathrop M, Heath S, Galan P, Schreiber S, Meitinger T, Pfeufer A, Wichmann HE, Melegh B, Polgár N, Toniolo D, Gasparini P, D'Adamo P, Klovins J, Nikitina-Zake L, Kucinskas V, Kasnauskiene J, Lubinski J, Debniak T, Limborska S, Khrunin A, Estivill X, Rabionet R, Marsal S, Julià A, Antonarakis SE, Deutsch S, Borel C, Attar H, Gagnebin M, Macek M, Krawczak M, Remm M, Metspalu A | display-authors = 6 | title = Genetic structure of Europeans: a view from the North-East | journal = PLOS ONE | volume = 4 | issue = 5 | pages = e5472 | date = 8 May 2009 | pmid = 19424496 | pmc = 2675054 | doi = 10.1371/journal.pone.0005472 | doi-access = free | bibcode = 2009PLoSO...4.5472N }}</ref><ref name="FleischerNelis2009"/> | |||
| |- | |||
| ! scope=col | | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] (]) | |||
| ! scope=col |] (]) | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |] | |||
| ! scope=col |CEU | |||
| |- | |||
| ! scope=row |Austria | |||
| | | |||
| |1.14 | |||
| |1.08 | |||
| |1.58 | |||
| |2.24 | |||
| |3.30 | |||
| |1.16 | |||
| |1.10 | |||
| |1.04 | |||
| |1.04 | |||
| |1.49 | |||
| |1.79 | |||
| |1.85 | |||
| |1.70 | |||
| |1.19 | |||
| |1.47 | |||
| |1.41 | |||
| |1.21 | |||
| |1.19 | |||
| |1.12 | |||
| |Austria | |||
| |- | |||
| ! scope=row |Bulgaria | |||
| |1.14 | |||
| | | |||
| |1.21 | |||
| |1.70 | |||
| |2.19 | |||
| |2.91 | |||
| |1.22 | |||
| |1.32 | |||
| |1.19 | |||
| |1.10 | |||
| |1.32 | |||
| |1.38 | |||
| |1.86 | |||
| |1.73 | |||
| |1.29 | |||
| |1.53 | |||
| |1.30 | |||
| |1.47 | |||
| |1.13 | |||
| |1.29 | |||
| |Bulgaria | |||
| |- | |||
| ! scope=row |Czech Republic | |||
| |1.08 | |||
| |1.21 | |||
| | | |||
| |1.42 | |||
| |2.20 | |||
| |3.26 | |||
| |1.35 | |||
| |1.15 | |||
| |1.16 | |||
| |1.06 | |||
| |1.69 | |||
| |2.04 | |||
| |1.62 | |||
| |1.48 | |||
| |1.09 | |||
| |1.27 | |||
| |1.63 | |||
| |1.26 | |||
| |1.37 | |||
| |1.21 | |||
| |Czech Republic | |||
| |- | |||
| ! scope=row |Estonia | |||
| |1.58 | |||
| |1.70 | |||
| |1.42 | |||
| | | |||
| |1.71 | |||
| |2.80 | |||
| |2.08 | |||
| |1.53 | |||
| |1.70 | |||
| |1.41 | |||
| |2.42 | |||
| |2.93 | |||
| |1.24 | |||
| |1.28 | |||
| |1.17 | |||
| |1.21 | |||
| |2.54 | |||
| |1.49 | |||
| |2.16 | |||
| |1.59 | |||
| |Estonia | |||
| |- | |||
| ! scope=row |Finland (Helsinki) | |||
| |2.24 | |||
| |2.19 | |||
| |2.20 | |||
| |1.71 | |||
| | | |||
| |1.86 | |||
| |2.69 | |||
| |2.17 | |||
| |2.35 | |||
| |1.87 | |||
| |2.82 | |||
| |3.37 | |||
| |2.31 | |||
| |2.33 | |||
| |1.75 | |||
| |2.10 | |||
| |3.14 | |||
| |1.89 | |||
| |2.77 | |||
| |1.99 | |||
| |Finland (Helsinki) | |||
| |- | |||
| ! scope=row |Finland (Kuusamo) | |||
| |3.30 | |||
| |2.91 | |||
| |3.26 | |||
| |2.80 | |||
| |1.86 | |||
| | | |||
| |3.72 | |||
| |3.27 | |||
| |3.46 | |||
| |2.68 | |||
| |3.64 | |||
| |4.18 | |||
| |3.33 | |||
| |3.37 | |||
| |2.49 | |||
| |3.16 | |||
| |4.21 | |||
| |2.87 | |||
| |3.83 | |||
| |2.89 | |||
| |Finland (Kuusamo) | |||
| |- | |||
| ! scope=row |France | |||
| |1.16 | |||
| |1.22 | |||
| |1.35 | |||
| |2.08 | |||
| |2.69 | |||
| |3.72 | |||
| | | |||
| |1.25 | |||
| |1.12 | |||
| |1.16 | |||
| |1.38 | |||
| |1.68 | |||
| |2.40 | |||
| |2.20 | |||
| |1.44 | |||
| |1.94 | |||
| |1.13 | |||
| |1.38 | |||
| |1.10 | |||
| |1.13 | |||
| |France | |||
| |- | |||
| ! scope=row |Northern Germany | |||
| |1.10 | |||
| |1.32 | |||
| |1.15 | |||
| |1.53 | |||
| |2.17 | |||
| |3.27 | |||
| |1.25 | |||
| | | |||
| |1.08 | |||
| |1.11 | |||
| |1.72 | |||
| |2.14 | |||
| |1.84 | |||
| |1.66 | |||
| |1.18 | |||
| |1.49 | |||
| |1.62 | |||
| |1.12 | |||
| |1.36 | |||
| |1.06 | |||
| |Northern Germany | |||
| |- | |||
| ! scope=row |Southern Germany | |||
| |1.04 | |||
| |1.19 | |||
| |1.16 | |||
| |1.70 | |||
| |2.35 | |||
| |3.46 | |||
| |1.12 | |||
| |1.08 | |||
| | | |||
| |1.08 | |||
| |1.53 | |||
| |1.85 | |||
| |1.20 | |||
| |1.84 | |||
| |1.23 | |||
| |1.58 | |||
| |1.40 | |||
| |1.21 | |||
| |1.17 | |||
| |1.07 | |||
| |Southern Germany | |||
| |- | |||
| ! scope=row |Hungary | |||
| |1.04 | |||
| |1.10 | |||
| |1.06 | |||
| |1.41 | |||
| |1.87 | |||
| |2.68 | |||
| |1.16 | |||
| |1.11 | |||
| |1.08 | |||
| | | |||
| |1.42 | |||
| |1.63 | |||
| |1.58 | |||
| |1.46 | |||
| |1.14 | |||
| |1.28 | |||
| |1.32 | |||
| |1.22 | |||
| |1.16 | |||
| |1.13 | |||
| |Hungary | |||
| |- | |||
| ! scope=row |Northern Italy | |||
| |1.49 | |||
| |1.32 | |||
| |1.69 | |||
| |2.42 | |||
| |2.82 | |||
| |3.64 | |||
| |1.38 | |||
| |1.72 | |||
| |1.53 | |||
| |1.42 | |||
| | | |||
| |1.54 | |||
| |2.64 | |||
| |2.48 | |||
| |1.75 | |||
| |2.24 | |||
| |1.42 | |||
| |1.86 | |||
| |1.36 | |||
| |1.56 | |||
| |Northern Italy | |||
| |- | |||
| ! scope=row |Southern Italy | |||
| |1.79 | |||
| |1.38 | |||
| |2.04 | |||
| |2.93 | |||
| |3.37 | |||
| |4.18 | |||
| |1.68 | |||
| |2.14 | |||
| |1.85 | |||
| |1.63 | |||
| |1.54 | |||
| | | |||
| |3.14 | |||
| |2.96 | |||
| |1.99 | |||
| |2.68 | |||
| |1.67 | |||
| |2.28 | |||
| |1.54 | |||
| |1.84 | |||
| |Southern Italy | |||
| |- | |||
| ! scope=row |Latvia | |||
| |1.85 | |||
| |1.86 | |||
| |1.62 | |||
| |1.24 | |||
| |2.31 | |||
| |3.33 | |||
| |2.40 | |||
| |1.84 | |||
| |1.20 | |||
| |1.58 | |||
| |2.64 | |||
| |3.14 | |||
| | | |||
| |1.20 | |||
| |1.26 | |||
| |1.84 | |||
| |2.82 | |||
| |1.89 | |||
| |2.52 | |||
| |1.87 | |||
| |Latvia | |||
| |- | |||
| ! scope=row |Lithuania | |||
| |1.70 | |||
| |1.73 | |||
| |1.48 | |||
| |1.28 | |||
| |2.33 | |||
| |3.37 | |||
| |2.20 | |||
| |1.66 | |||
| |1.84 | |||
| |1.46 | |||
| |2.48 | |||
| |2.96 | |||
| |1.20 | |||
| | | |||
| |1.20 | |||
| |1.26 | |||
| |2.62 | |||
| |1.74 | |||
| |2.29 | |||
| |1.74 | |||
| |Lithuania | |||
| |- | |||
| ! scope=row |Poland | |||
| |1.19 | |||
| |1.29 | |||
| |1.09 | |||
| |1.17 | |||
| |1.75 | |||
| |2.49 | |||
| |1.44 | |||
| |1.18 | |||
| |1.23 | |||
| |1.14 | |||
| |1.75 | |||
| |1.99 | |||
| |1.26 | |||
| |1.20 | |||
| | | |||
| |1.18 | |||
| |1.66 | |||
| |1.30 | |||
| |1.46 | |||
| |1.28 | |||
| |Poland | |||
| |- | |||
| ! scope=row |Russia | |||
| |1.47 | |||
| |1.53 | |||
| |1.27 | |||
| |1.21 | |||
| |2.10 | |||
| |3.16 | |||
| |1.94 | |||
| |1.49 | |||
| |1.58 | |||
| |1.28 | |||
| |2.24 | |||
| |2.68 | |||
| |1.84 | |||
| |1.26 | |||
| |1.18 | |||
| | | |||
| |2.32 | |||
| |1.59 | |||
| |1.20 | |||
| |1.56 | |||
| |Russia | |||
| |- | |||
| ! scope=row |Spain | |||
| |1.41 | |||
| |1.30 | |||
| |1.63 | |||
| |2.54 | |||
| |3.14 | |||
| |4.21 | |||
| |1.13 | |||
| |1.62 | |||
| |1.40 | |||
| |1.32 | |||
| |1.42 | |||
| |1.67 | |||
| |2.82 | |||
| |2.62 | |||
| |1.66 | |||
| |2.32 | |||
| | | |||
| |1.73 | |||
| |1.16 | |||
| |1.34 | |||
| |Spain | |||
| |- | |||
| ! scope=row |Sweden | |||
| |1.21 | |||
| |1.47 | |||
| |1.26 | |||
| |1.49 | |||
| |1.89 | |||
| |2.87 | |||
| |1.38 | |||
| |1.12 | |||
| |1.21 | |||
| |1.22 | |||
| |1.86 | |||
| |2.28 | |||
| |1.89 | |||
| |1.74 | |||
| |1.30 | |||
| |1.59 | |||
| |1.73 | |||
| | | |||
| |1.50 | |||
| |1.09 | |||
| |Sweden | |||
| |- | |||
| ! scope=row |Switzerland | |||
| |1.19 | |||
| |1.13 | |||
| |1.37 | |||
| |2.16 | |||
| |2.77 | |||
| |3.83 | |||
| |1.10 | |||
| |1.36 | |||
| |1.17 | |||
| |1.16 | |||
| |1.36 | |||
| |1.54 | |||
| |2.52 | |||
| |2.29 | |||
| |1.46 | |||
| |1.20 | |||
| |1.16 | |||
| |1.50 | |||
| | | |||
| |1.21 | |||
| |Switzerland | |||
| |- | |||
| ! scope=row |CEU | |||
| |1.12 | |||
| |1.29 | |||
| |1.21 | |||
| |1.59 | |||
| |1.99 | |||
| |2.89 | |||
| |1.13 | |||
| |1.06 | |||
| |1.07 | |||
| |1.13 | |||
| |1.56 | |||
| |1.84 | |||
| |1.87 | |||
| |1.74 | |||
| |1.28 | |||
| |1.56 | |||
| |1.34 | |||
| |1.09 | |||
| |1.21 | |||
| | | |||
| |CEU | |||
| |- | |||
| ! scope=col | | |||
| ! scope=col |Austria | |||
| ! scope=col |Bulgaria | |||
| ! scope=col |Czech Republic | |||
| ! scope=col |Estonia | |||
| ! scope=col |Finland (Helsinki) | |||
| ! scope=col |Finland (Kuusamo) | |||
| ! scope=col |France | |||
| ! scope=col |Northern Germany | |||
| ! scope=col |Southern Germany | |||
| ! scope=col |Hungary | |||
| ! scope=col |Northern Italy | |||
| ! scope=col |Southern Italy | |||
| ! scope=col |Latvia | |||
| ! scope=col |Lithuania | |||
| ! scope=col |Poland | |||
| ! scope=col |Russia | |||
| ! scope=col |Spain | |||
| ! scope=col |Sweden | |||
| ! scope=col |Switzerland | |||
| ! scope=col |CEU | |||
| |- | |||
| |} | |||
| CEU – Utah residents with ancestry from Northern and Western Europe. | |||
| == |
== History of research == | ||
| {{Further|Population genetics}} | |||
| === Classical genetic markers (by proxy) === | |||
| *{{Citation|author=Adams et al.|title=The Genetic Legacy of Religious Diversity and Intolerance: Paternal Lineages of Christians, Jews, and Muslims in the Iberian Peninsula| journal=The American Journal of Human Genetics | year=2008 |doi=10.1016/j.ajhg.2008.11.007 | volume=83|url=http://www.cell.com/AJHG/abstract/S0002-9297%2808%2900592-2|pages=725}} | |||
| *{{Citation | |||
| | last =Arredi et al. | |||
| | first = | |||
| | author-link = | |||
| | last2 = | |||
| | first2 = | |||
| | author2-link = | |||
| | title = A Predominantly Neolithic Origin for Y-Chromosomal DNA Variation in North Africa | |||
| | journal =] | |||
| | volume = 75 | |||
| | issue = | |||
| | pages = 338–345 | |||
| | date = | |||
| | year = 2004 | |||
| | url = http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1216069 | |||
| | doi = 10.1086/423147 | |||
| | id = }} | |||
| *{{Citation| author=Battaglia et al. | year=2008 | title=Y-chromosomal evidence of the cultural diffusion of agriculture in southeast Europe | journal=European Journal of Human Genetics | doi=10.1038/ejhg.2008.249}} | |||
| *{{Citation | |||
| | last = Beleza et al. | |||
| | first = | |||
| | author-link = | |||
| | last2 = | |||
| | first2 = | |||
| | author2-link = | |||
| | title = Micro-Phylogeographic and Demographic History of Portuguese Male Lineages | |||
| | journal = Annals of Human Genetics | |||
| | volume = 70 | |||
| | issue = | |||
| | pages = 181–194 | |||
| | date = | |||
| | year = 2005 | |||
| | url = http://www3.interscience.wiley.com/cgi-bin/fulltext/118548798/PDFSTART | |||
| | doi = 10.1111/j.1529-8817.2005.00221.x | |||
| | id = }} | |||
| *{{Citation | |||
| | last = Bosch et al. | |||
| | first = | |||
| | author-link = | |||
| | last2 = | |||
| | first2 = | |||
| | author2-link = | |||
| | title = High-resolution analysis of human Y-chromosome variation shows a sharp discontinuity and limited gene flow between north-western Africa and the Iberian Peninsula | |||
| | journal = Am J Hum Genet | |||
| | volume = 68 | |||
| | issue = | |||
| | pages = 1019–1029 | |||
| | date = | |||
| | year = 2001 | |||
| | url = http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=11254456 | |||
| | doi = 10.1086/319521 | |||
| | id = }} | |||
| *{{Citation | last = Capelli et al. | title = A Y Chromosome Census of the British Isles | journal = Current Biology| volume = 13| issue =11 | pages = 979–84| date = | year = 2003| url = http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VRT-48PV5SH-12&_user=10&_coverDate=05%2F27%2F2003&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=0eb0c8ff85bde2ebc2ef136619f57e7a| doi = 10.1016/S0960-9822(03)00373-7| id = }} also at | |||
| *{{Citation| author=Capelli et al.| year=2009| title=Moors and Saracens in Europe: estimating the medieval North African male legacy in southern Europe| journal=European Journal of Human Genetics| doi=10.1038/ejhg.2008.258}} | |||
| *{{Citation | |||
| | last = Cruciani et al. | |||
| | first = | |||
| | author-link = | |||
| | last2 = | |||
| | first2 = | |||
| | author2-link = | |||
| | title = A Back Migration from Asia to Sub-Saharan Africa Is Supported by High-Resolution Analysis of Human Y-Chromosome Haplotypes | |||
| | journal = ] | |||
| | volume = 70 | |||
| | issue = | |||
| | pages = 1197–1214 | |||
| | date = | |||
| | year = 2002 | |||
| | url = http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B8JDD-4RH3CKT-C-J&_cdi=43612&_user=10&_coverDate=05%2F31%2F2002&_sk=%23TOC%2343612%232002%23999299994%23677124%23FLA%23display%23Volume_70,_Issue_5,_Pages_i-ii,_1077-1388_(May_2002)%23tagged%23Volume%23first%3D70%23Issue%23first%3D5%23date%23(May_2002)%23&view=c&_gw=y&wchp=dGLbVzz-zSkzS&md5=49fa407673a5d86db8983413c144248a&ie=/sdarticle.pdf | |||
| | doi = 10.1086/340257 | |||
| | id = }} | |||
| *{{Citation | |||
| | last = Cruciani et al. | |||
| | first = | |||
| | author-link = | |||
| | last2 = | |||
| | first2 = | |||
| | author2-link = | |||
| | title = Phylogeographic Analysis of Haplogroup E3b (E-M215) Y Chromosomes Reveals Multiple Migratory Events Within and Out Of Africa | |||
| | journal = ] | |||
| | volume = 74 | |||
| | issue = | |||
| | pages = 1014–1022 | |||
| | date = | |||
| | year = 2004 | |||
| | url = http://www.familytreedna.com/pdf/hape3b.pdf | |||
| | doi = 10.1086/386294 | |||
| | id = }} | |||
| *{{Citation | |||
| | last = Cruciani et al. | |||
| | first = | |||
| | author-link = | |||
| | last2 = | |||
| | first2 = | |||
| | author2-link = | |||
| | title = Molecular Dissection of the Y Chromosome Haplogroup E-M78 (E3b1a): A Posteriori Evaluation of a Microsatellite-Network-Based Approach Through Six New Biallelic Markers | |||
| | journal = Human Mutation | |||
| | volume = 27 | |||
| | issue = | |||
| | pages = 831 | |||
| | date = | |||
| | year = 2006 | |||
| | url = http://www3.interscience.wiley.com/homepages/38515/pdf/916.pdf | |||
| | doi = 10.1002/humu.9445 | |||
| | id = }} | |||
| *{{Citation | |||
| | last = Cruciani et al. | |||
| | first = | |||
| | author-link = | |||
| | last2 = | |||
| | first2 = | |||
| | author2-link = | |||
| | title = Tracing Past Human Male Movements in Northern/Eastern Africa and Western Eurasia: New Clues from Y-Chromosomal Haplogroups E-M78 and J-M12 | |||
| | journal = Molecular Biology and Evolution | |||
| | volume = 24 | |||
| | issue = | |||
| | pages = 1300–1311 | |||
| | date = | |||
| | year = 2007 | |||
| | url = http://mbe.oxfordjournals.org/cgi/reprint/24/6/1300 | |||
| | doi = 10.1093/molbev/msm049 | |||
| | id = }} Also see . | |||
| *{{Citation|author=Di Gaetano et al.|year=2008|title=Differential Greek and northern African migrations to Sicily are supported by genetic evidence from the Y chromosome| journal=European Journal of Human Genetics}} | |||
| *{{Citation | author=Flores et al. | title=Reduced genetic structure of the Iberian peninsula revealed by Y-chromosome analysis: implications for population demography | url=http://hpgl.stanford.edu/publications/EJHG_2004_v12_p855.pdf | year=2004 | journal=European Journal of Human Genetics | volume=12 | pages=855–863 | doi=10.1038/sj.ejhg.5201225}} | |||
| *{{Citation| author=Francalacci et al. | year=2003 | title=Peopling of Three Mediterranean Islands (Corsica, Sardinia, and Sicily) Inferred by Y-Chromosome Biallelic Variability|journal=American Journal of Physical Anthropology | volume=121| page=270–279| doi=10.1002/ajpa.10265}} | |||
| *{{Citation | last=Gonçalves et al. | year=2005 | title=Y-chromosome Lineages from Portugal, Madeira and Açores Record Elements of Sephardim and Berber Ancestry | journal=Annals of Human Genetics | volume=69 | pages=443–454 | url=http://www3.interscience.wiley.com/cgi-bin/fulltext/118682163/PDFSTART| doi=10.1046/j.1529-8817.2005.00161.x}} Also http://wysinger.homestead.com/portugal.pdf | |||
| *{{Citation|title=Congruent distribution of Neolithic painted pottery and ceramic figurines with Y-chromosome lineages|journal=Antiquity|year=2002|volume=76|pages=707–14|author=King and Underhill }} | |||
| *{{cite journal |author=Pericić M, Lauc LB, Klarić IM, ''et al.'' |title=High-resolution phylogenetic analysis of southeastern Europe traces major episodes of paternal gene flow among Slavic populations |journal=Mol. Biol. Evol. |volume=22 |issue=10 |pages=1964–75 |year=2005 |month=October |pmid=15944443 |doi=10.1093/molbev/msi185 |url=http://mbe.oxfordjournals.org/cgi/content/full/22/10/1964 }} | |||
| *{{Citation | |||
| | last = Rosser et al. | |||
| | first = | |||
| | author-link = | |||
| | last2 = | |||
| | first2 = | |||
| | author2-link = | |||
| | title = Y-Chromosomal Diversity in Europe Is Clinal and Influenced Primarily by Geography, Rather than by Language | |||
| | journal = ] | |||
| | volume = 67 | |||
| | issue = | |||
| | pages = 1526–1543. | |||
| | date = | |||
| | year = 2000 | |||
| | url = http://www.ajhg.org/AJHG/abstract/S0002-9297(07)63221-2 | |||
| | doi = 10.1086/316890 | |||
| | id = }} | |||
| *{{Citation|author=Scozzari et al.| year=2001 | journal=Human Immunology | volume=62 | title=Human Y-Chromosome Variation in the Western Mediterranean Area: Implications for the Peopling of the Region|url=http://evolutsioon.ut.ee/publications/Scozzari2001.pdf|doi=10.1016/S0198-8859(01)00286-5|pages=871|unused_data=|[pages=871–884}} | |||
| *{{cite journal |author=Semino O, Passarino G, Oefner PJ, ''et al.'' |title=The genetic legacy of Paleolithic Homo sapiens sapiens in extant Europeans: a Y chromosome perspective |journal=Science |volume=290 |issue=5494 |pages=1155–9 |year=2000 |month=November |pmid=11073453 |url=http://hpgl.stanford.edu/publications/Science_2000_v290_p1155.pdf |doi=10.1126/science.290.5494.1155}}. | |||
| *{{Citation | author=Semino et al. | year=2002 | title=Ethiopians and Khoisan share the deepest clades of the human Y-chromosome phylogeny | periodical=Am J Hum Genet | volume= 70 | pages=265–8 | url=http://hpgl.stanford.edu/publications/AJHG_2002_v70_p265-268.pdf | doi=10.1086/338306}} | |||
| *{{Citation | |||
| |url=http://books.google.com/books?id=tJkyAAAACAAJ&dq=%22Blood+of+the+Isles%22&ei=fbIKR9r0FqOOpwLs0YDTCA | |||
| |title=Blood of the Isles: Exploring the Genetic Roots of Our Tribal History | |||
| |first=Bryan | |||
| |last=Sykes | |||
| |year=2006 | |||
| |publisher=Bantam | |||
| |isbn=0593056523}} | |||
| *{{Citation | author=Underhill et al. | year=2000 | title=Y chromosome sequence variation and the history of human populations | periodical=Nat Genet | volume=26 | pages=358–361 | url=http://www.nature.com/ng/journal/v26/n3/full/ng1100_358.html | doi=10.1038/81685}} | |||
| *{{Citation | author=Underhill et al. | year=2001 | title=The phylogeography of Y chromosome binary haplotypes and the origins of modern human populations | periodical=Ann Hum Genet | volume=65 | pages=43–62 | url=http://hpgl.stanford.edu/publications/AHG_2001_v65_p43.pdf | doi=10.1046/j.1469-1809.2001.6510043.x}} | |||
| *{{Citation|author=Underhill | year=2002| title=Inference of Neolithic Population Histories using Y-chromosome Haplotypes| editor=Bellwood and Renfrew| booktitle=Examining the farming/language dispersal hypothesis, McDonald Institute for Archaeological Research|isbn=1-902937-20-1|publisher=McDonald Institute for Archaeological Research|location=Cambridge}} | |||
| *{{Citation | title=Use of Y Chromosome and Mitochondrial DNA Population Structure in Tracing Human Migrations| journal=Annu. Rev. Genet. | year=2007 |volume=41| pages=539–64 | doi=10.1146/annurev.genet.41.110306.130407 | author=Underhill and Kivisild}} | |||
| One of the first scholars to perform genetic studies was ]. He used classical genetic markers to analyse DNA by proxy. This method studies differences in the frequencies of particular allelic traits, namely ] from proteins found within ] (such as the ], Rhesus blood antigens, ], ], ] ]s, among others). Subsequently, his team calculated ] between populations, based on the principle that two populations that share similar frequencies of a trait are more closely related than populations that have more divergent frequencies of the trait.<ref name = "Cavalli-Sforza_1993" />{{rp|51}} | |||
| *Luca Cavalli-Sforza http://www.pnas.org/cgi/content/full/94/15/7719 Genes, peoples, and languages - Cavalli-Sforza 94 (15): 7719 - Proceedings of the National Academy of Sciences | |||
| *Perlès C, Monthel G ( 2001) The Early Neolithic in Greece: The First Farming Communities in Europe. Cambridge University Press, Cambridge. | |||
| From this, he constructed ]s that showed genetic distances diagrammatically. His team also performed ], which is good at analysing ] with minimal loss of information. The information that is lost can be partly restored by generating a second principal component, and so on.<ref name = "Cavalli-Sforza_1993" />{{rp|39}} In turn, the information from each individual principal component ('''PC''') can be presented graphically in ''synthetic maps''. These maps show peaks and troughs, which represent populations whose ] take extreme values compared to others in the studied area.<ref name="Cavalli-Sforza_1993"/>{{rp|51}} | |||
| *Runnels C (2003) The origins of the Greek Neolithic: a personal view, in Ammerman and Biagi (2003 eds). | |||
| Peaks and troughs usually connected by smooth gradients are called ]. Genetic clines can be generated by adaptation to environment (]), continuous ] between two initially different populations or a demographic expansion into a scarcely populated environment, with little initial ] with existing populations.<ref name = "Arredi_2007">{{cite book| vauthors = Arredi B, Poloni ES, Tyler-Smith C |chapter=The Peopling of Europe |title=Anthropological Genetics: Theory, Methods and Applications |year=2007 |publisher=] |isbn=978-0-521-54697-3}}</ref>{{rp|390}} Cavalli-Sforza connected these gradients with postulated pre-historical population movements, based on archaeological and linguistic theories. However, given that the time depths of such patterns are not known, "associating them with particular demographic events is usually speculative".<ref name = "Rosser_2000" /> | |||
| === Direct DNA analysis === | |||
| {{Further |Genetic drift |Founder effect |Population bottleneck}} | |||
| Studies using direct DNA analysis are now abundant and may use ], the non-recombining portion of the Y chromosome (NRY), or even autosomal DNA. MtDNA and NRY DNA share some similar features, which have made them particularly useful in genetic anthropology. These properties include the direct, unaltered inheritance of mtDNA and NRY DNA from mother to offspring and father to son, respectively, without the 'scrambling' effects of ]. We also presume that these genetic loci are not affected by natural selection and that the major process responsible for changes in ]s has been mutation (which can be calculated).<ref name = "Milisauskas_2002" />{{rp|58}} | |||
| The smaller ] of the NRY and mtDNA enhances the consequences of drift and founder effect, relative to the autosomes, making NRY and mtDNA variation a potentially sensitive index of population composition.<ref name = "Rosser_2000" /><ref name = "Richards_2000" /><ref name="Semino_2000" /> These biologically plausible assumptions are not concrete; Rosser suggests that climatic conditions may affect the fertility of certain lineages.<ref name = "Rosser_2000" /> | |||
| The underlying ] used by the geneticists is more questionable. They often use different mutation rates and studies frequently arrive at vastly different conclusions.<ref name = "Rosser_2000" /> NRY and mtDNA may be so susceptible to drift that some ancient patterns may have become obscured. Another assumption is that population genealogies are approximated by ]. ] points out that this only holds if population groups develop from a genetically monomorphic set of founders. Barbujani argues that there is no reason to believe that Europe was colonised by monomorphic populations. This would result in an overestimation of haplogroup age, thus falsely extending the demographic history of Europe into the ] rather than the ] era.<ref name = "Barbujani_2001">{{cite journal | vauthors = Barbujani G, Bertorelle G | title = Genetics and the population history of Europe | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 98 | issue = 1 | pages = 22–25 | date = January 2001 | pmid = 11136246 | pmc = 33353 | doi = 10.1073/pnas.98.1.22 | doi-access = free | bibcode = 2001PNAS...98...22B }}</ref> Greater certainty about chronology may be obtained from studies of ancient DNA (see below), but so far these have been comparatively few. | |||
| Whereas ] and mtDNA haplogroups represent but a small component of a person's DNA pool, ] has the advantage of containing hundreds of thousands of examinable genetic loci, thus giving a more complete picture of genetic composition. Descent relationships can only be determined on a statistical basis, because autosomal DNA undergoes recombination. A single chromosome can record a history for each gene. Autosomal studies are much more reliable for showing the relationships between existing populations, but do not offer the possibilities for unravelling their histories in the same way as mtDNA and NRY DNA studies promise, despite their many complications. | |||
| Genetic studies operate on numerous assumptions and suffer from methodological limitations, such as ] and confounding phenomena like ], foundation and bottleneck effects cause large errors, particularly in haplogroup studies. No matter how accurate the methodology, conclusions derived from such studies are compiled on the basis of how the author envisages their data fits with established archaeological or linguistic theories.{{citation needed|date=February 2021}} | |||
| == See also == | == See also == | ||
| {{ |
{{Portal|Europe|Evolutionary biology|History}} | ||
| {{col-float}} | |||
| *] | |||
| ;General | |||
| *] | |||
| * ] | |||
| *] | |||
| * ] | |||
| *] | |||
| * ] | |||
| *] | |||
| * ] | |||
| *] | |||
| *] | * ] | ||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| {{col-float-break}} | |||
| ;Genetics by European group | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| {{col-float-end}} | |||
| == References == | |||
| === Inline citations === | |||
| {{Reflist}} | |||
| ===Sources referenced=== | |||
| {{refbegin|2}} | |||
| * {{cite journal | vauthors = Adams SM, Bosch E, Balaresque PL, Ballereau SJ, Lee AC, Arroyo E, López-Parra AM, Aler M, Grifo MS, Brion M, Carracedo A, Lavinha J, Martínez-Jarreta B, Quintana-Murci L, Picornell A, Ramon M, Skorecki K, Behar DM, Calafell F, Jobling MA | display-authors = 6 | title = The genetic legacy of religious diversity and intolerance: paternal lineages of Christians, Jews, and Muslims in the Iberian Peninsula | journal = American Journal of Human Genetics | volume = 83 | issue = 6 | pages = 725–736 | date = December 2008 | pmid = 19061982 | pmc = 2668061 | doi = 10.1016/j.ajhg.2008.11.007 }} | |||
| * {{cite journal | vauthors = Arredi B, Poloni ES, Paracchini S, Zerjal T, Fathallah DM, Makrelouf M, Pascali VL, Novelletto A, Tyler-Smith C | display-authors = 6 | title = A predominantly neolithic origin for Y-chromosomal DNA variation in North Africa | journal = American Journal of Human Genetics | volume = 75 | issue = 2 | pages = 338–345 | date = August 2004 | pmid = 15202071 | pmc = 1216069 | doi = 10.1086/423147 }} | |||
| * {{cite journal | vauthors = Auton A, Bryc K, Boyko AR, Lohmueller KE, Novembre J, Reynolds A, Indap A, Wright MH, Degenhardt JD, Gutenkunst RN, King KS, Nelson MR, Bustamante CD | display-authors = 6 | title = Global distribution of genomic diversity underscores rich complex history of continental human populations | journal = Genome Research | volume = 19 | issue = 5 | pages = 795–803 | date = May 2009 | pmid = 19218534 | pmc = 2675968 | doi = 10.1101/gr.088898.108 }} | |||
| * {{cite journal | vauthors = Battaglia V, Fornarino S, Al-Zahery N, Olivieri A, Pala M, Myres NM, King RJ, Rootsi S, Marjanovic D, Primorac D, Hadziselimovic R, Vidovic S, Drobnic K, Durmishi N, Torroni A, Santachiara-Benerecetti AS, Underhill PA, Semino O | display-authors = 6 | title = Y-chromosomal evidence of the cultural diffusion of agriculture in Southeast Europe | journal = European Journal of Human Genetics | volume = 17 | issue = 6 | pages = 820–830 | date = June 2009 | pmid = 19107149 | pmc = 2947100 | doi = 10.1038/ejhg.2008.249 }} | |||
| * {{cite journal | vauthors = Bauchet M, McEvoy B, Pearson LN, Quillen EE, Sarkisian T, Hovhannesyan K, Deka R, Bradley DG, Shriver MD | display-authors = 6 | title = Measuring European population stratification with microarray genotype data | journal = American Journal of Human Genetics | volume = 80 | issue = 5 | pages = 948–956 | date = May 2007 | pmid = 17436249 | pmc = 1852743 | doi = 10.1086/513477 }} | |||
| * {{cite journal | vauthors = Beleza S, Gusmão L, Lopes A, Alves C, Gomes I, Giouzeli M, Calafell F, Carracedo A, Amorim A | display-authors = 6 | title = Micro-phylogeographic and demographic history of Portuguese male lineages | journal = Annals of Human Genetics | volume = 70 | issue = Pt 2 | pages = 181–194 | date = March 2006 | pmid = 16626329 | doi = 10.1111/j.1529-8817.2005.00221.x | s2cid = 4652154 }}{{dead link|date=May 2021|bot=medic}}{{cbignore|bot=medic}} | |||
| * {{cite journal | vauthors = Bosch E, Calafell F, Comas D, Oefner PJ, Underhill PA, Bertranpetit J | title = High-resolution analysis of human Y-chromosome variation shows a sharp discontinuity and limited gene flow between northwestern Africa and the Iberian Peninsula | journal = American Journal of Human Genetics | volume = 68 | issue = 4 | pages = 1019–1029 | date = April 2001 | pmid = 11254456 | pmc = 1275654 | doi = 10.1086/319521 }} | |||
| * {{cite journal | vauthors = Brace CL, Seguchi N, Quintyn CB, Fox SC, Nelson AR, Manolis SK, Qifeng P | title = The questionable contribution of the Neolithic and the Bronze Age to European craniofacial form | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 103 | issue = 1 | pages = 242–247 | date = January 2006 | pmid = 16371462 | pmc = 1325007 | doi = 10.1073/pnas.0509801102 | doi-access = free | bibcode = 2006PNAS..103..242B }} | |||
| * {{cite journal | vauthors = Capelli C, Onofri V, Brisighelli F, Boschi I, Scarnicci F, Masullo M, Ferri G, Tofanelli S, Tagliabracci A, Gusmao L, Amorim A, Gatto F, Kirin M, Merlitti D, Brion M, Verea AB, Romano V, Cali F, Pascali V | display-authors = 6 | title = Moors and Saracens in Europe: estimating the medieval North African male legacy in southern Europe | journal = European Journal of Human Genetics | volume = 17 | issue = 6 | pages = 848–852 | date = June 2009 | pmid = 19156170 | pmc = 2947089 | doi = 10.1038/ejhg.2008.258 }} | |||
| * {{cite journal | vauthors = Cavalli-Sforza LL | title = Genes, peoples, and languages | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 94 | issue = 15 | pages = 7719–7724 | date = July 1997 | pmid = 9223254 | pmc = 33682 | doi = 10.1073/pnas.94.15.7719 | bibcode = 1997PNAS...94.7719C | doi-access = free | author-link = Luigi Cavalli-Sforza }} | |||
| * {{cite journal | vauthors = Cruciani F, Santolamazza P, Shen P, Macaulay V, Moral P, Olckers A, Modiano D, Holmes S, Destro-Bisol G, Coia V, Wallace DC, Oefner PJ, Torroni A, Cavalli-Sforza LL, Scozzari R, Underhill PA | display-authors = 6 | title = A back migration from Asia to sub-Saharan Africa is supported by high-resolution analysis of human Y-chromosome haplotypes | journal = American Journal of Human Genetics | volume = 70 | issue = 5 | pages = 1197–1214 | date = May 2002 | pmid = 11910562 | pmc = 447595 | doi = 10.1086/340257 }} | |||
| * {{cite journal | vauthors = Cruciani F, La Fratta R, Santolamazza P, Sellitto D, Pascone R, Moral P, Watson E, Guida V, Colomb EB, Zaharova B, Lavinha J, Vona G, Aman R, Cali F, Akar N, Richards M, Torroni A, Novelletto A, Scozzari R | display-authors = 6 | title = Phylogeographic analysis of haplogroup E3b (E-M215) y chromosomes reveals multiple migratory events within and out of Africa | journal = American Journal of Human Genetics | volume = 74 | issue = 5 | pages = 1014–1022 | date = May 2004 | pmid = 15042509 | pmc = 1181964 | doi = 10.1086/386294 }} | |||
| * {{cite journal | vauthors = Cruciani F, La Fratta R, Torroni A, Underhill PA, Scozzari R | title = Molecular dissection of the Y chromosome haplogroup E-M78 (E3b1a): a posteriori evaluation of a microsatellite-network-based approach through six new biallelic markers | journal = Human Mutation | volume = 27 | issue = 8 | pages = 831–832 | date = August 2006 | pmid = 16835895 | doi = 10.1002/humu.9445 | s2cid = 26886757 | doi-access = free }} | |||
| * {{cite journal | vauthors = Di Gaetano C, Cerutti N, Crobu F, Robino C, Inturri S, Gino S, Guarrera S, Underhill PA, King RJ, Romano V, Cali F, Gasparini M, Matullo G, Salerno A, Torre C, Piazza A | display-authors = 6 | title = Differential Greek and northern African migrations to Sicily are supported by genetic evidence from the Y chromosome | journal = European Journal of Human Genetics | volume = 17 | issue = 1 | pages = 91–99 | date = January 2009 | pmid = 18685561 | pmc = 2985948 | doi = 10.1038/ejhg.2008.120 }} | |||
| * {{cite journal | vauthors = Flores C, Maca-Meyer N, González AM, Oefner PJ, Shen P, Pérez JA, Rojas A, Larruga JM, Underhill PA | display-authors = 6 | title = Reduced genetic structure of the Iberian peninsula revealed by Y-chromosome analysis: implications for population demography | journal = European Journal of Human Genetics | volume = 12 | issue = 10 | pages = 855–863 | date = October 2004 | pmid = 15280900 | doi = 10.1038/sj.ejhg.5201225 | s2cid = 16765118 | doi-access = free }} | |||
| * {{cite journal | vauthors = Francalacci P, Morelli L, Underhill PA, Lillie AS, Passarino G, Useli A, Madeddu R, Paoli G, Tofanelli S, Calò CM, Ghiani ME, Varesi L, Memmi M, Vona G, Lin AA, Oefner P, Cavalli-Sforza LL | display-authors = 6 | title = Peopling of three Mediterranean islands (Corsica, Sardinia, and Sicily) inferred by Y-chromosome biallelic variability | journal = American Journal of Physical Anthropology | volume = 121 | issue = 3 | pages = 270–279 | date = July 2003 | pmid = 12772214 | doi = 10.1002/ajpa.10265 }} | |||
| * {{cite journal | vauthors = Gonçalves R, Freitas A, Branco M, Rosa A, Fernandes AT, Zhivotovsky LA, Underhill PA, Kivisild T, Brehm A | display-authors = 6 | title = Y-chromosome lineages from Portugal, Madeira and Açores record elements of Sephardim and Berber ancestry | journal = Annals of Human Genetics | volume = 69 | issue = Pt 4 | pages = 443–454 | date = July 2005 | pmid = 15996172 | doi = 10.1111/j.1529-8817.2005.00161.x | hdl = 10400.13/3018 | s2cid = 3229760 | hdl-access = free }}{{dead link|date=May 2021|bot=medic}}{{cbignore|bot=medic}} | |||
| * {{cite journal | vauthors = Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T | title = A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications | journal = Human Mutation | volume = 29 | issue = 5 | pages = 648–658 | date = May 2008 | pmid = 18286470 | doi = 10.1002/humu.20695 | s2cid = 12489449 | doi-access = free }} | |||
| * {{cite journal|title=Congruent distribution of Neolithic painted pottery and ceramic figurines with Y-chromosome lineages|journal=]|year=2002|volume=76|issue=293|pages=707–14| vauthors = King R, Underhill PA |doi=10.1017/s0003598x00091158|s2cid=160359661 }} | |||
| * {{cite journal | vauthors = Lao O, Lu TT, Nothnagel M, Junge O, Freitag-Wolf S, Caliebe A, Balascakova M, Bertranpetit J, Bindoff LA, Comas D, Holmlund G, Kouvatsi A, Macek M, Mollet I, Parson W, Palo J, Ploski R, Sajantila A, Tagliabracci A, Gether U, Werge T, Rivadeneira F, Hofman A, Uitterlinden AG, Gieger C, Wichmann HE, Rüther A, Schreiber S, Becker C, Nürnberg P, Nelson MR, Krawczak M, Kayser M | display-authors = 6 | title = Correlation between genetic and geographic structure in Europe | journal = Current Biology | volume = 18 | issue = 16 | pages = 1241–1248 | date = August 2008 | pmid = 18691889 | doi = 10.1016/j.cub.2008.07.049 | s2cid = 16945780 | doi-access = free }} | |||
| * {{cite journal | vauthors = Pericić M, Lauc LB, Klarić IM, Rootsi S, Janićijevic B, Rudan I, Terzić R, Colak I, Kvesić A, Popović D, Sijacki A, Behluli I, Dordevic D, Efremovska L, Bajec DD, Stefanović BD, Villems R, Rudan P | display-authors = 6 | title = High-resolution phylogenetic analysis of southeastern Europe traces major episodes of paternal gene flow among Slavic populations | journal = Molecular Biology and Evolution | volume = 22 | issue = 10 | pages = 1964–1975 | date = October 2005 | pmid = 15944443 | doi = 10.1093/molbev/msi185 | doi-access = free }}{{dead link|date=May 2021|bot=medic}}{{cbignore|bot=medic}} | |||
| * {{cite journal | vauthors = Ricaut FX, Waelkens M | title = Cranial discrete traits in a Byzantine population and eastern Mediterranean population movements | journal = Human Biology | volume = 80 | issue = 5 | pages = 535–564 | date = October 2008 | pmid = 19341322 | doi = 10.3378/1534-6617-80.5.535 | s2cid = 25142338 }} | |||
| * {{cite journal | vauthors = Scozzari R, Cruciani F, Pangrazio A, Santolamazza P, Vona G, Moral P, Latini V, Varesi L, Memmi MM, Romano V, De Leo G, Gennarelli M, Jaruzelska J, Villems R, Parik J, Macaulay V, Torroni A | display-authors = 6 | title = Human Y-chromosome variation in the western Mediterranean area: implications for the peopling of the region | journal = Human Immunology | volume = 62 | issue = 9 | pages = 871–884 | date = September 2001 | pmid = 11543889 | doi = 10.1016/S0198-8859(01)00286-5 | citeseerx = 10.1.1.408.4857 }} | |||
| * {{cite journal | vauthors = Semino O, Passarino G, Oefner PJ, Lin AA, Arbuzova S, Beckman LE, De Benedictis G, Francalacci P, Kouvatsi A, Limborska S, Marcikiae M, Mika A, Mika B, Primorac D, Santachiara-Benerecetti AS, Cavalli-Sforza LL, Underhill PA | display-authors = 6 | title = The genetic legacy of Paleolithic Homo sapiens sapiens in extant Europeans: a Y chromosome perspective | journal = Science | volume = 290 | issue = 5494 | pages = 1155–1159 | date = November 2000 | pmid = 11073453 | doi = 10.1126/science.290.5494.1155 | url = http://hpgl.stanford.edu/publications/Science_2000_v290_p1155.pdf | url-status = dead | access-date = 2009-07-22 | bibcode = 2000Sci...290.1155S | archive-url = https://web.archive.org/web/20080307035541/http://hpgl.stanford.edu/publications/Science_2000_v290_p1155.pdf | archive-date = 2008-03-07 }}. | |||
| * {{cite journal | vauthors = Semino O, Santachiara-Benerecetti AS, Falaschi F, Cavalli-Sforza LL, Underhill PA | title = Ethiopians and Khoisan share the deepest clades of the human Y-chromosome phylogeny | journal = American Journal of Human Genetics | volume = 70 | issue = 1 | pages = 265–268 | date = January 2002 | pmid = 11719903 | pmc = 384897 | doi = 10.1086/338306 | url = http://hpgl.stanford.edu/publications/AJHG_2002_v70_p265-268.pdf | url-status = dead | access-date = 2009-07-22 | archive-url = https://web.archive.org/web/20060315210510/http://hpgl.stanford.edu/publications/AJHG_2002_v70_p265-268.pdf | archive-date = 2006-03-15 }} | |||
| * {{cite book | url = https://books.google.com/books?id=tJkyAAAACAAJ&q=%22Blood+of+the+Isles%22 | title = Blood of the Isles: Exploring the Genetic Roots of Our Tribal History | vauthors = Sykes B | year = 2006 | publisher = Bantam | isbn = 978-0-593-05652-3 | access-date = 2009-07-22 }} | |||
| * {{cite journal | vauthors = Underhill PA, Shen P, Lin AA, Jin L, Passarino G, Yang WH, Kauffman E, Bonné-Tamir B, Bertranpetit J, Francalacci P, Ibrahim M, Jenkins T, Kidd JR, Mehdi SQ, Seielstad MT, Wells RS, Piazza A, Davis RW, Feldman MW, Cavalli-Sforza LL, Oefner PJ | display-authors = 6 | title = Y chromosome sequence variation and the history of human populations | journal = Nature Genetics | volume = 26 | issue = 3 | pages = 358–361 | date = November 2000 | pmid = 11062480 | doi = 10.1038/81685 | s2cid = 12893406 }} | |||
| * {{cite journal | vauthors = Underhill PA, Passarino G, Lin AA, Shen P, Mirazón Lahr M, Foley RA, Oefner PJ, Cavalli-Sforza LL | display-authors = 6 | title = The phylogeography of Y chromosome binary haplotypes and the origins of modern human populations | journal = Annals of Human Genetics | volume = 65 | issue = Pt 1 | pages = 43–62 | date = January 2001 | pmid = 11415522 | doi = 10.1046/j.1469-1809.2001.6510043.x | s2cid = 9441236 | doi-access = free }} | |||
| * {{Cite book| vauthors = Underhill PA |year=2002 |chapter=Inference of Neolithic Population Histories using Y-chromosome Haplotypes|editor=Bellwood and Renfrew |title=Examining the farming/language dispersal hypothesis, McDonald Institute for Archaeological Research|isbn=978-1-902937-20-5|publisher=McDonald Institute for Archaeological Research|location=Cambridge}} | |||
| * Perlès C, Monthel G ( 2001) The Early Neolithic in Greece: The First Farming Communities in Europe. ], Cambridge. | |||
| * Runnels C (2003) The origins of the Greek Neolithic: a personal view, in Ammerman and Biagi (2003 eds). | |||
| {{refend}} | |||
| <!--===Unannotated references=== | |||
| * Nature Article - 日本語要約 - The genetic history of Ice Age Europe - Qiaomei Fu, Cosimo Posth, Mateja Hajdinjak, Martin Petr, Swapan Mallick, Daniel Fernandes, Anja Furtwängler, Wolfgang Haak, Matthias Meyer, Alissa Mittnik, Birgit Nickel, Alexander Peltzer, Nadin Rohland, Viviane Slon, Sahra Talamo, Iosif Lazaridis, Mark Lipson, Iain Mathieson, Stephan Schiffels, Pontus Skoglund, Anatoly P. Derevianko, Nikolai Drozdov, Vyacheslav Slavinsky, Alexander Tsybankov, Renata Grifoni Cremonesi, Francesco Mallegni, Bernard Gély, Eligio Vacca, Manuel R. González Morales, Lawrence G. Straus, Christine Neugebauer-Maresch, Maria Teschler-Nicola, Silviu Constantin, Oana Teodora Moldovan, Stefano Benazzi, Marco Peresani, Donato Coppola, Martina Lari, Stefano Ricci, Annamaria Ronchitelli, Frédérique Valentin, Corinne Thevenet, Kurt Wehrberger, Dan Grigorescu, Hélène Rougier, Isabelle Crevecoeur, Damien Flas, Patrick Semal, Marcello A. Mannino, Christophe Cupillard, Hervé Bocherens, Nicholas J. Conard, Katerina Harvati, Vyacheslav Moiseyev, Dorothée G. Drucker, Jiří Svoboda, Michael P. Richards, David Caramelli, Ron Pinhasi, Janet Kelso, Nick Patterson, Johannes Krause, Svante Pääbo & David Reich – Nature 534, 200–205 (09 June 2016) doi:10.1038/nature17993 Received 18 December 2015, Accepted 12 April 2016, Published online 02 May 2016 (in particular RE: Epigravettian R1b find) | |||
| * {{cite journal | vauthors = Fu Q, Posth C, Hajdinjak M, Petr M, Mallick S, Fernandes D, Furtwängler A, Haak W, Meyer M, Mittnik A, Nickel B, Peltzer A, Rohland N, Slon V, Talamo S, Lazaridis I, Lipson M, Mathieson I, Schiffels S, Skoglund P, Derevianko AP, Drozdov N, Slavinsky V, Tsybankov A, Cremonesi RG, Mallegni F, Gély B, Vacca E, Morales MR, Straus LG, Neugebauer-Maresch C, Teschler-Nicola M, Constantin S, Moldovan OT, Benazzi S, Peresani M, Coppola D, Lari M, Ricci S, Ronchitelli A, Valentin F, Thevenet C, Wehrberger K, Grigorescu D, Rougier H, Crevecoeur I, Flas D, Semal P, Mannino MA, Cupillard C, Bocherens H, Conard NJ, Harvati K, Moiseyev V, Drucker DG, Svoboda J, Richards MP, Caramelli D, Pinhasi R, Kelso J, Patterson N, Krause J, Pääbo S, Reich D | display-authors = 6 | title = The genetic history of Ice Age Europe | journal = Nature | volume = 534 | issue = 7606 | pages = 200–5 | date = June 2016 | pmid = 27135931 | pmc = 4943878 | doi = 10.1038/nature17993 | url = | bibcode = 2016Natur.534..200F | hdl = 10211.3/198594 }} | |||
| * https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4943878/ | |||
| --> | |||
| == Further reading == | |||
| * Rodríguez-Varela, Ricardo et al. "The genetic history of Scandinavia from the Roman Iron Age to the present". In: ''Cell''. Volume 186, Issue 1, 5 January 2023, Pages 32–46.e19. {{doi|10.1016/j.cell.2022.11.024}} | |||
| * Skourtanioti, E., Ringbauer, H., Gnecchi Ruscone, G.A. et al. "Ancient DNA reveals admixture history and endogamy in the prehistoric Aegean". In: ''Nature Ecology & Evolution'' (2023). https://doi.org/10.1038/s41559-022-01952-3 | |||
| == External links == | == External links == | ||
| {{Commons category}} | |||
| * | |||
| * {{cite web|url=http://admixturemap.paintmychromosomes.com/|title=A genetic atlas of human admixture history|year=2014}} | |||
| * | |||
| * | |||
| * | |||
| * | |||
| *. | |||
| * | |||
| * | |||
| {{Human genetics}} | |||
| ] | |||
| {{History of Europe}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 00:36, 8 December 2024
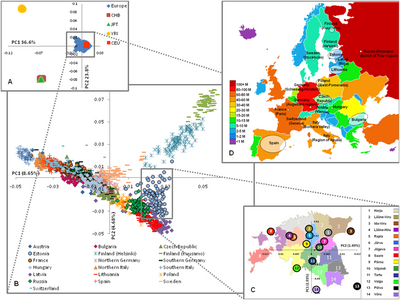
The genetic history of Europe includes information around the formation, ethnogenesis, and other DNA-specific information about populations indigenous, or living in Europe.
European early modern human (EEMH) lineages between 40 and 26 ka (Aurignacian) were still part of a large Western Eurasian "meta-population", related to Central and Western Asian populations. Divergence into genetically distinct sub-populations within Western Eurasia is a result of increased selection pressure and founder effects during the Last Glacial Maximum (LGM, Gravettian).
By the end of the LGM, after 20 ka, A Western European lineage, dubbed west European hunter-gatherer (WHG) emerged from the Solutrean refugium during the European Mesolithic. These mesolithic hunter-gatherer cultures are subsequently replaced in the Neolithic Revolution as a result of the arrival of Early European Farmer (EEF) lineages derived from mesolithic populations of West Asia (Anatolia and the Caucasus). In the European Bronze Age, there were again substantial population replacements in parts of Europe by the intrusion of Western Steppe Herder (WSH) lineages from the Pontic–Caspian steppes, arising from admixture between Eastern Hunter Gatherers (EHG) and peoples related to Near Easterners. These Bronze Age population replacements are associated with the Bell Beaker and Corded Ware cultures archaeologically and with the Indo-European expansion linguistically.
As a result of the population movements during the Mesolithic to Bronze Age, modern European populations are distinguished by differences in WHG, EEF and Ancient North Eurasian (ANE) ancestry. Admixture rates varied geographically; in the late Neolithic, WHG ancestry in farmers in Hungary was at around 10%, in Germany around 25% and in Iberia as high as 50%. The contribution of EEF is more significant in Mediterranean Europe, and declines towards northern and northeastern Europe, where WHG ancestry is stronger; the Sardinians are considered to be the closest European group to the population of the EEF.
Ethnogenesis of the modern ethnic groups of Europe in the historical period is associated with numerous admixture events, primarily those associated with the Migration period and the decline of the Roman Empire, associated with the Germanic, Norse, and Slavic expansions
Research into the genetic history of Europe became possible in the second half of the 20th century, but did not yield results with high resolution before the 1990s. In the 1990s, preliminary results became possible, but they remained mostly limited to studies of mitochondrial and Y-chromosomal lineages. Autosomal DNA became more easily accessible in the 2000s, and since the mid-2010s, results of previously unattainable resolution, many of them based on full-genome analysis of ancient DNA, have been published at an accelerated pace.
Prehistory
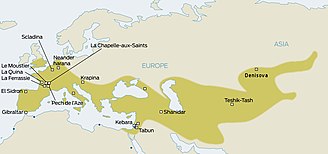 Distribution of the Neanderthals, and main sites
Distribution of the Neanderthals, and main sites
Due to natural selection, the percentage of Neanderthal DNA in ancient Europeans gradually decreased over time. From 45,000 BP to 7,000 BP, the percentage dropped from around 3–6% to 2%. The removal of Neanderthal-derived alleles occurred more frequently around genes than other parts of the genome.
Palaeolithic
Further information: Peopling of Europe, Archaic human admixture with modern humans § Neanderthals, and Paleolithic EuropeNeanderthals inhabited much of Europe and western Asia from as far back as 130,000 years ago. They existed in Europe as late as 30,000 years ago. They were eventually replaced by anatomically modern humans (AMH; sometimes known as Cro-Magnons), who began to appear in Europe circa 40,000 years ago. Given that the two hominid species likely coexisted in Europe, anthropologists have long wondered whether the two interacted. The question was resolved only in 2010, when it was established that Eurasian populations exhibit Neanderthal admixture, estimated at 1.5–2.1% on average. The question now became whether this admixture had taken place in Europe, or rather in the Levant, prior to AMH migration into Europe.
There has also been speculation about the inheritance of specific genes from Neanderthals. For example, one MAPT locus 17q21.3 which is split into deep genetic lineages H1 and H2. Since the H2 lineage seems restricted to European populations, several authors had argued for inheritance from Neanderthals beginning in 2005. However the preliminary results from the sequencing of the full Neanderthal Genome at that time (2009), failed to uncover evidence of interbreeding between Neanderthals and modern humans. By 2010, findings by Svante Pääbo (Max Planck Institute for Evolutionary Anthropology at Leipzig, Germany), Richard E. Green (University of California, Santa Cruz), and David Reich (Harvard Medical School), comparing the genetic material from the bones of three Neanderthals with that from five modern humans, did show a relationship between Neanderthals and modern people outside Africa.
Upper Paleolithic
Main articles: Upper Paleolithic and Early European modern humans
It is thought that modern humans began to inhabit Europe during the Upper Paleolithic about 40,000 years ago. Some evidence shows the spread of the Aurignacian culture.
From a purely patrilineal, Y-chromosome perspective, it appears that Haplogroup C1a2, F and K2a may be those with the oldest presence in Europe. They have been found in some of the oldest human remains sequenced from the paleolithic era. However, other haplogroups are far more common among modern European males, because of later demographic changes.
Currently the oldest sample of Haplogroup I (M170), which is now relatively common and widespread within Europe, has been found to be Krems WA3 from Lower Austria dating back to about 30–31,000 ybp. At about this time, an Upper Palaeolithic culture also appeared, known as the Gravettian.
Earlier research into Y-DNA had instead focused on haplogroup R1 (M173): the most populous lineage among living European males; R1 was also believed to have emerged ~ 40,000 BP in Central Asia. However, it is now estimated that R1 emerged substantially more recently: a 2008 study dated the most recent common ancestor of haplogroup IJ to 38,500 and haplogroup R1 to 18,000 BP. This suggested that haplogroup IJ colonists formed the first wave and haplogroup R1 arrived much later.
Thus the genetic data suggests that, at least from the perspective of patrilineal ancestry, separate groups of modern humans took two routes into Europe: from the Middle East via the Balkans and another from Central Asia via the Eurasian Steppe, to the north of the Black Sea.
Martin Richards et al. found that 15–40% of extant mtDNA lineages trace back to the Palaeolithic migrations (depending on whether one allows for multiple founder events). MtDNA haplogroup U5, dated to be ~ 40–50 kYa, arrived during the first early upper Palaeolithic colonisation. Individually, it accounts for 5–15% of total mtDNA lineages. Middle U.P. movements are marked by the haplogroups HV, I and U4. HV split into Pre-V (around 26,000 years old) and the larger branch H, both of which spread over Europe, possibly via Gravettian contacts.
Haplogroup H accounts for about half the gene lines in Europe, with many subgroups. The above mtDNA lineages or their precursors, are most likely to have arrived into Europe via the Middle East. This contrasts with Y DNA evidence, whereby some 50%-plus of male lineages are characterised by the R1 superfamily, which is of possible central Asian origin. Ornella Semino postulates that these differences "may be due in part to the apparent more recent molecular age of Y chromosomes relative to other loci, suggesting more rapid replacement of previous Y chromosomes. Gender-based differential migratory demographic behaviors will also influence the observed patterns of mtDNA and Y variation".
Last Glacial Maximum
Main article: Early European modern humans Further information: Last Glacial Maximum and Last Glacial Maximum refugia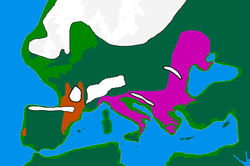
Solutrean and Proto-Solutrean Cultures Epi-Gravettian Culture
The Last Glacial Maximum ("LGM") started c. 30 ka BCE, at the end of MIS 3, leading to a depopulation of Northern Europe. According to the classical model, people took refuge in climatic sanctuaries (or refugia) as follows:
- Northern Iberia and Southwest France, together making up the "Franco-Cantabrian" refugium
- The Balkans
- Ukraine and more generally the northern coast of the Black Sea
- Italy.
This event decreased the overall genetic diversity in Europe, a "result of drift, consistent with an inferred population bottleneck during the Last Glacial Maximum". As the glaciers receded from about 16,000–13,000 years ago, Europe began to be slowly repopulated by people from refugia, leaving genetic signatures.
Some Y haplogroup I clades appear to have diverged from their parental haplogroups sometime during or shortly after the LGM.
Cinnioglu sees evidence for the existence of an Anatolian refuge, which also harboured Hg R1b1b2. Today, R1b dominates the y chromosome landscape of western Europe, including the British Isles, suggesting that there could have been large population composition changes based on migrations after the LGM.
Semino, Passarino and Pericic place the origins of haplogroup R1a within the Ukrainian ice-age refuge. Its current distribution in eastern Europe and parts of Scandinavia are in part reflective of a re-peopling of Europe from the southern Russian/Ukrainian steppes after the Late Glacial Maximum.
From an mtDNA perspective, Richards et al. found that the majority of mtDNA diversity in Europe is accounted for by post-glacial re-expansions during the late upper Palaeolithic/ Mesolithic. "The regional analyses lend some support to the suggestion that much of western and central Europe was repopulated largely from the southwest when the climate improved. The lineages involved include much of the most common haplogroup, H, as well as much of K, T, W, and X." The study could not determine whether there were new migrations of mtDNA lineages from the near east during this period; a significant input was deemed unlikely.
The alternative model of more refugees was discussed by Bilton et al.
From a study of 51 individuals, researchers were able to identify five separate genetic clusters of ancient Eurasians during the LGM: the Věstonice Cluster (34,000–26,000 years ago), associated with the Gravettian culture; the Mal'ta Cluster (24,000–17,000), associated with the Mal'ta-Buret' culture, the El Mirón Cluster (19,000–14,000 years ago), associated with the Magdalenian culture; the Villabruna Cluster (14,000–7,000 years ago) and the Satsurblia cluster (13,000 to 10,000 years ago).
From around 37,000 years ago, all ancient Europeans began to share some ancestry with modern Europeans. This founding population is represented by GoyetQ116-1, a 35,000 year old specimen from Belgium. This lineage disappears from the record and is not found again until 19,000 BP in Spain at El Mirón, which shows strong affinities to GoyetQ116-1. During this interval, the distinct Věstonice Cluster is predominant in Europe, even at Goyet. The re-expansion of the El Mirón Cluster coincided with warming temperatures following the retreat of the glaciers during the Last Glacial Maximum. From 37,000 to 14,000 years ago, the population of Europe consisted of an isolated population descended from a founding population that didn't interbreed significantly with other populations.
Mesolithic
Further information: Mesolithic Europe, Western hunter-gatherer, and Caucasus hunter-gathererMesolithic (post-LGM) populations had diverged significantly due to their relative isolation over several millennia, to the harsh selection pressures during the LGM, and to the founder effects caused by the rapid expansion from LGM refugia in the beginning Mesolithic. By the end of the LGM, around 19 to 11 ka, the familiar varieties of Eurasian phenotypes had emerged. However, the lineage of Mesolithic hunter-gatherers of Western Europe (WHG) does not survive as a majority contribution in any modern population. They were most likely blue eyed, and retained the dark skin pigmentation of pre-LGM EEMH. The HERC2 and OCA2 variations for blue eyes are derived from the WHG lineage were also found in the Yamnaya people.
Around 14,000 years ago, the Villabruna Cluster shifted away from GoyetQ116-1 affinity and started to show more affinity with the Near East, a shift which coincided with the warming temperatures of the Bølling-Allerød interstadial. This genetic shift shows that Near East populations had probably already begun moving into Europe during the end of the Upper Paleolithic, about 6,000 years earlier than previously thought, before the introduction of farming. A few specimens from the Villabruna Cluster also show genetic affinities for East Asians that are derived from gene flow. The HERC2 variation for blue eyes first appears around 13,000 to 14,000 years ago in Italy and the Caucasus. The light skin pigmentation characteristic of modern Europeans is estimated to have spread across Europe in a "selective sweep" during the Mesolithic (19 to 11 ka). The associated TYRP1 SLC24A5 and SLC45A2 alleles emerge around 19 ka, still during the LGM, most likely in the Caucasus.
Neolithic
Further information: Neolithic Europe, Neolithic Revolution, Early European Farmers, and Holocene

A big cline in genetic variation that has long been recognised in Europe seems to show important dispersals from the direction of the Middle East. This has often been linked to the spread of farming technology during the Neolithic, which has been argued to be one of the most important periods in determining modern European genetic diversity.
The Neolithic started with the introduction of farming, beginning in SE Europe approximately 10,000–3000 BCE, and extending into NW Europe between 4500 and 1700 BCE. During this era, the Neolithic Revolution led to drastic economic as well as socio-cultural changes in Europe and this is also thought to have had a big effect on Europe's genetic diversity, especially concerning genetic lineages entering Europe from the Middle East into the Balkans. There were several phases of this period:
- In a late European Mesolithic prelude to the Neolithic, it appears that Near Eastern peoples from areas that already had farming, and who also had sea-faring technology, had a transient presence in Greece (for example at Franchthi Cave).
- There is consensus that agricultural technology and the main breeds of animals and plants which are farmed entered Europe from somewhere in the area of the Fertile Crescent and specifically the Levant region from the Sinai to Southern Anatolia. (Less certainly, this agricultural revolution is sometimes argued to have in turn been partly triggered by movements of people and technology coming across the Sinai from Africa.) For more see Fertile Crescent: Cosmopolitan diffusion.
- A later stage of the Neolithic, the so-called Pottery Neolithic, saw an introduction of pottery into the Levant, Balkans and Southern Italy (it had been present in the area of modern Sudan for some time before it is found in the Eastern Mediterranean, but it is thought to have developed independently), and this may have also been a period of cultural transfer from the Levant into the Balkans.
An important issue regarding the genetic impact of neolithic technologies in Europe is the manner by which they were transferred into Europe. Farming was introduced by a significant migration of farmers from the Near East (Cavalli-Sforza's biological demic diffusion model) or a "cultural diffusion" or a combination of the two, and population geneticists have tried to clarify whether any genetic signatures of Near Eastern origin correspond to the expansion routes postulated by the archaeological evidence.
Martin Richards estimated that only 11% of European mtDNA is due to immigration in this period, suggesting that farming was spread primarily due to being adopted by indigenous Mesolithic populations, rather than due to immigration from Near East. Gene flow from SE to NW Europe seems to have continued in the Neolithic, the percentage significantly declining towards the British Isles. Classical genetics also suggested that the largest admixture to the European Paleolithic/Mesolithic stock was due to the Neolithic revolution of the 7th to 5th millennia BCE. Three main mtDNA gene groups have been identified as contributing Neolithic entrants into Europe: J, T1 and U3 (in that order of importance). With others, they amount up to around 20% of the gene pool.
In 2000, Semino's study on Y DNA revealed the presence of haplotypes belonging to the large clade E1b1b1 (E-M35). These were predominantly found in the southern Balkans, southern Italy and parts of Iberia. Semino connected this pattern, along with J haplogroup subclades, to be the Y-DNA component of Cavalli-Sforza's Neolithic demic-diffusion of farmers from the Near East. Rosser et al. rather saw it as a (direct) 'North African component' in European genealogy, although they did not propose a timing and mechanism to account for it. also described E1b1b as representing a late-Pleistocene migration from Africa to Europe over the Sinai Peninsula in Egypt, evidence for which does not show up in mitochondrial DNA.
Concerning timing the distribution and diversity of V13 however, Battaglia proposed an earlier movement whereby the E-M78* lineage ancestral to all modern E-V13 men moved rapidly out of a Southern Egyptian homeland and arrived in Europe with only Mesolithic technologies. They then suggest that the E-V13 sub-clade of E-M78 only expanded subsequently as native Balkan 'foragers-cum-farmers' adopted Neolithic technologies from the Near East. They propose that the first major dispersal of E-V13 from the Balkans may have been in the direction of the Adriatic Sea with the Neolithic Impressed Ware culture often referred to as Impressa or Cardial, rather propose that the main route of E-V13 spread was along the Vardar-Morava-Danube river 'highway' system.
In contrast to Battaglia, Cruciani tentatively suggested (i) a different point where the V13 mutation happened on its way from Egypt to the Balkans via the Middle East, and (ii) a later dispersal time. The authors proposed that the V13 mutation first appeared in western Asia, where it is found in low but significant frequencies, whence it entered the Balkans sometime after 11 kYa. It later experienced a rapid dispersal which he dated to c. 5300 years ago in Europe, coinciding with the Balkan Bronze Age. Like Peričic et al. they consider that "the dispersion of the E-V13 and J-M12 haplogroups seems to have mainly followed the river waterways connecting the southern Balkans to north-central Europe".
More recently, Lacan announced that a 7000-year-old skeleton in a Neolithic context in a Spanish funeral cave, was an E-V13 man. (The other specimens tested from the same site were in haplogroup G2a, which has been found in Neolithic contexts throughout Europe.) Using 7 STR markers, this specimen was identified as being similar to modern individuals tested in Albania, Bosnia, Greece, Corsica, and Provence. The authors therefore proposed that, whether or not the modern distribution of E-V13 of today is a result of more recent events, E-V13 was already in Europe within the Neolithic, carried by early farmers from the Eastern Mediterranean to the Western Mediterranean, much earlier than the Bronze Age. This supports the proposals of Battaglia et al. rather than Cruciani et al. at least concerning earliest European dispersals, but E-V13 may have dispersed more than once. Even more recent than the Bronze Age, it has also been proposed that modern E-V13's modern distribution in Europe is at least partly caused by Roman era movements of people. (See below.)
The migration of Neolithic farmers into Europe brought along several new adaptations. The variation for light skin colour was introduced to Europe by the neolithic farmers. After the arrival of the neolithic farmers, a SLC22A4 mutation was selected for, a mutation which probably arose to deal with ergothioneine deficiency but increases the risk of ulcerative colitis, coeliac disease, and irritable bowel syndrome.
Bronze Age
Further information: Bronze Age EuropeThe Bronze Age saw the development of long-distance trading networks, particularly along the Atlantic Coast and in the Danube valley. There was migration from Norway to Orkney and Shetland in this period (and to a lesser extent to mainland Scotland and Ireland). There was also migration from Germany to eastern England. Martin Richards estimated that there was about 4% mtDNA immigration to Europe in the Bronze Age.

Another theory about the origin of the Indo-European language centres around a hypothetical Proto-Indo-European people, who, according to the Kurgan hypothesis, can be traced to north of the Black and Caspian Seas at about 4500 BCE. They domesticated the horse and possibly invented the wooden disk wheel, and are considered to have spread their culture and genes across Europe. The Y haplogroup R1a is a proposed marker of these "Kurgan" genes, as is the Y Haplogroup R1b, although these haplogroups as a whole may be much older than the language family.
In the far north, carriers of the Y-haplogroup N arrived to Europe from Siberia, eventually expanding as far as Finland, though the specific timing of their arrival is uncertain. The most common North European subclade N1c1 is estimated to be around 8,000 years old. There is evidence of human settlement in Finland dating back to 8500 BCE, linked with the Kunda culture and its putative ancestor, the Swiderian culture, but the latter is thought to have a European origin. The geographical spread of haplogroup N in Europe is well aligned with the Pit–Comb Ware culture, whose emergence is commonly dated c. 4200 BCE, and with the distribution of Uralic languages. Mitochondrial DNA studies of Sami people, haplogroup U5 are consistent with multiple migrations to Scandinavia from Volga-Ural region, starting 6,000 to 7,000 years before present.
The relationship between roles of European and Asian colonists in the prehistory of Finland is a point of some contention, and some scholars insist that Finns are "predominantly Eastern European and made up of people who trekked north from the Ukrainian refuge during the Ice Age". Farther east, the issue is less contentious. Haplogroup N carriers account for a significant part of all non-Slavic ethnic groups in northern Russia, including 37% of Karelians, 35% of Komi people (65% according to another study), 67% of Mari people, as many as 98% of Nenets people, 94% of Nganasans, and 86% to 94% of Yakuts.
The Yamnaya component contains partial ancestry from an Ancient North Eurasian component, a Paleolithic Siberian lineage but closely related to European hunter-gatherers, first identified in Mal'ta. According to Iosif Lazaridis, "the Ancient North Eurasian ancestry is proportionally the smallest component everywhere in Europe, never more than 20 percent, but we find it in nearly every European group we’ve studied." This genetic component does not come directly from the Mal'ta lineage itself, but a related lineage that separated from the Mal'ta lineage.
Up to a half of the Yamnaya component may have come from a Caucasus hunter-gatherer strand. On November 16, 2015, in a study published in the journal Nature Communications, geneticists announced that they had found a new fourth ancestral "tribe" or "strand" which had contributed to the modern European gene pool. They analysed genomes from two hunter-gatherers from Georgia which were 13,300 and 9,700 years old, and found that these Caucasus hunter-gatherers were probably the source of the farmer-like DNA in the Yamnaya. According to co-author Dr Andrea Manica of the University of Cambridge: "The question of where the Yamnaya come from has been something of a mystery up to now....we can now answer that as we've found that their genetic make-up is a mix of Eastern European hunter-gatherers and a population from this pocket of Caucasus hunter-gatherers who weathered much of the last Ice Age in apparent isolation."
According to Lazaridis et al. (2016), a population related to the people of the Chalcolithic Iran contributed to roughly half of the ancestry of Yamnaya populations of the Pontic–Caspian steppe. These Iranian Chalcolithic people were a mixture of "the Neolithic people of western Iran, the Levant, and Caucasus Hunter Gatherers."
The genetic variations for lactase persistence and greater height came with the Yamnaya people. The derived allele of the KITLG gene (SNP rs12821256) that is associated with – and likely causal for – blond hair in Europeans is found in populations with eastern but not western hunter-gatherers ancestry, suggesting that its origin is in the Ancient North Eurasian (ANE) population and may have been spread in Europe by individuals with steppe ancestry. Consistent with this, the earliest known individual with the derived allele is an ANE individual from the Late Upper Paleolithic Afontova Gora archaeological complex in central Siberia.
Recent history
Further information: History of Europe Further information: Demography of the Roman Empire, Migration period, Viking expansion, Slavic expansion, Magyar migration, Muslim conquest of Spain, Turkic expansion, African admixture in Europe, Ottoman wars in Europe, Ostsiedlung, World War II evacuation and expulsion, Population transfer in the Soviet Union, and Immigration to Europe
Expansions of the Roman Empire do not appear to have left distinct genetic signatures in Europe. Indeed, Romance-speaking populations in the Balkans, like Romanians, Aromanians, Moldovans, etc. have been found to genetically resemble neighbouring Greek and South Slavic-speaking peoples rather than modern Italians. Steven Bird has speculated that E1b1b1a was spread during the Roman era through Thracian and Dacian populations from the Balkans into the rest of Europe.
Concerning the late Roman period of (not only) Germanic "Völkerwanderung", some suggestions have been made, at least for Britain, with Y haplogroup I1a being associated with Anglo-Saxon immigration in eastern England, and R1a being associated with Norse immigration in northern Scotland.
Genetics of modern European populations
Further information: Ethnic groups in EuropePatrilineal studies
There are four main Y-chromosome DNA haplogroups that account for most of Europe's patrilineal descent.
- Haplogroup R1b is common in Europe, particularly in Western Europe, with the R1b1a1a2 being the most common among Western Europeans. Nearly all of this R1b in Europe is in the form of the R1b1a2 (2011 name) (R-M269) sub-clade, specifically within the R-L23 sub-sub-clade whereas R1b found in Central Asia, western Asia and Africa tends to be in other clades. It has also been pointed out that outlier types are present in Europe and are particularly notable in some areas such as Sardinia and Armenia. Haplogroup R1b frequencies vary from highs in western Europe in a steadily decreasing cline with growing distance from the Atlantic: 80–90% (Welsh, Basque, Irish, Scots, Bretons) around 70–80% in Spain, Britain and France and around 40–60% in parts of eastern Germany, and northern Italy. It drops outside this area and is around 30% or less in areas such as southern Italy, Poland, the Balkans and Cyprus. R1b remains the most common clade as one moves east to Germany, while farther east, in Poland, R1a is more common (see below). In Southeast Europe, R1b drops behind R1a in the area in and around Hungary and Serbia but is more common both to the south and north of this region. R1b in Western Europe is dominated by at least two sub-clades, R-U106, which is distributed from the east side of the Rhine into northern and central Europe (with a strong presence in England) and R-P312, which is most common west of the Rhine, including the British Isles.
- Haplogroup R1a, almost entirely in the R1a1a sub-clade, is prevalent in much of Eastern and Central Europe (also in South and Central Asia). For example, there is a sharp increase in R1a1 and decrease in R1b1b2 as one goes east from Germany to Poland. It also has a substantial presence in Scandinavia (particularly Norway). In the Baltic countries R1a frequencies decrease from Lithuania (45%) to Estonia (around 30%).
- Haplogroup I is found in the form of various sub-clades throughout Europe and is found at highest frequencies in the Nordic countries as I1 (Norway, Denmark, Sweden, Finland) and in the Balkan Peninsula as I2a (Bosnia and Herzegovina 65%, Croatia and Serbia). I1 is also frequent in Germany, Great Britain and Netherlands, while I2a is frequent also in Sardinia, Romania/Moldova, Bulgaria and Ukraine. This clade is found at its highest expression by far in Europe and may have been there since before the LGM.
- Haplogroup E1b1b (formerly known as E3b) was part of a migration of Neolithic farmers from the Middle East, which carried E1b1b at low to medium frequency and was introduced into Neolithic Middle Easterners throughout genetic drift from a migration from Africa into the Middle East associated with the Afroasiatic languages. It is believed to have first appeared in Northeast Africa approximately 26,000 years ago and dispersed to North Africa and the Near East during the late Paleolithic and Mesolithic periods. E1b1b lineages are closely linked to the diffusion of Afroasiatic languages. Although present throughout Europe, it peaks in the southern Balkan region amongst Albanians and their neighbors. It is also common in Italy and the Iberian peninsula at lower frequency. Haplogroup E1b1b1, mainly in the form of its E1b1b1a2 (E-V13) sub-clade, reaches frequencies above 47% around the area of Kosovo. This clade is thought to have arrived in Europe from western Asia either in the later Mesolithic, or the Neolithic. North Africa subclade E-M81 is also present in Sicily and Andalusia.
Putting aside small enclaves, there are also several haplogroups apart from the above four that are less prominent or most common only in certain areas of Europe.
- Haplogroup G, a common haplogroup among European Neolithic farmers, is common in most parts of Europe at a low frequency, reaching peaks above 70% around Georgia and among the Madjars (although living in Asia they border the eastern perimeter of Europe), up to 10% in Sardinia, 12% in Corsica and Uppsala (Sweden), 11% in the Balkans and Portugal, 10% in Spain and 9% in European Russia. This clade is also found in the Near East.
- Haplogroup N, is common only in the northeast of Europe and in the form of its N1c1 sub-clade reaches frequencies of approximately 60% among Finns and approximately 40% among Estonians, Latvians, and Lithuanians.
- Haplogroup J2, in various sub-clades (J2a, J2b), is found in levels of around 15–30% in the Balkans (particularly Greece) and Italy. Haplogroup J2 is frequent in Western Asia and the Eastern Mediterranean.
Matrilineal studies
There have been a number of studies about the mitochondrial DNA haplogroups (mtDNA) in Europe. In contrast to Y DNA haplogroups, mtDNA haplogroups did not show as much geographical patterning, but were more evenly ubiquitous. Apart from the outlying Saami, all Europeans are characterised by the predominance of haplogroups H, U and T. The lack of observable geographic structuring of mtDNA may be due to socio-cultural factors, namely the phenomena of polygyny and patrilocality.
Genetic studies suggest some maternal gene flow to eastern Europe from eastern Asia or southern Siberia 13,000 – 6,600 years BP. Analysis of Neolithic skeletons in the Great Hungarian Plain found a high frequency of eastern Asian mtDNA haplogroups, some of which survive in modern eastern European populations. Maternal gene flow to Europe from sub-Saharan Africa began as early as 11,000 years BP, although the majority of lineages, approximately 65%, are estimated to have arrived more recently, including during the Romanization period, the Arab conquests of southern Europe, and during the Atlantic slave trade.
European population sub-structure
Genetically, Europe is relatively homogeneous, but distinct sub-population patterns of various types of genetic markers have been found, particularly along a southeast–northwest cline. For example, Cavalli-Sforza's principal component analyses revealed five major clinal patterns throughout Europe, and similar patterns have continued to be found in more recent studies.
- A cline of genes with highest frequencies in the Middle East, spreading to lowest levels northwest. Cavalli-Sforza originally described this as faithfully reflecting the spread of agriculture in Neolithic times. This has been the general tendency in interpretation of all genes with this pattern.
- A cline of genes with highest frequencies among Finnish and Sami in the extreme north east, and spreading to lowest frequencies in the south west.
- A cline of genes with highest frequencies in the area of the lower Don and Volga rivers in southern Russia, and spreading to lowest frequencies in Spain, Southern Italy, Greece and the areas inhabited by Saami speakers in the extreme north of Scandinavia. Cavalli-Sforza associated this with the spread of Indo-European languages, which he links in turn to a "secondary expansion" after the spread of agriculture, associated with animal grazing.
- A cline of genes with highest frequencies in the Balkans and Southern Italy, spreading to lowest levels in Britain and the Basque country. Cavalli-Sforza associates this with "the Greek expansion, which reached its peak in historical times around 1000 and 500 BCE but which certainly began earlier".
- A cline of genes with highest frequencies in the Basque country, and lower levels beyond the area of Iberia and Southern France. In perhaps the most well-known conclusion from Cavalli-Sforza, this weakest of the five patterns was described as isolated remnants of the pre-Neolithic population of Europe, "who at least partially withstood the expansion of the cultivators". It corresponds roughly to the geographical spread of rhesus negative blood types. In particular, the conclusion that the Basques are a genetic isolate has become widely discussed, but also a controversial conclusion.
He also created a phylogenetic tree to analyse the internal relationships among Europeans. He found four major 'outliers'- Basques, Sami, Sardinians and Icelanders; a result he attributed to their relative isolation (note: the Icelanders and the Sardinians speak Indo-European languages, while the other two groups do not). Greeks and Yugoslavs represented a second group of less extreme outliers. The remaining populations clustered into several groups : "Celtic", "Germanic", "south-western Europeans", "Scandinavians" and "eastern Europeans".
A study conducted in May of 2009 researching 19 populations from Europe using 270,000 SNPs highlighted the genetic diversity of European populations corresponding to the northwest to southeast gradient and distinguished "four several distinct regions" within Europe:
- Finland, showing the greatest distance to the rest of Europeans.
- the Baltic region (Estonia, Latvia and Lithuania), western Russia and eastern Poland.
- Central and Western Europe.
- Italy, due to the alps acting as a great genetic barrier.
In this study, barrier analysis revealed "genetic barriers" between Finland, Italy and other countries and that barriers could also be demonstrated within Finland (between Helsinki and Kuusamo) and Italy (between northern and southern part, Fst=0.0050). Fst (Fixation index) was found to correlate considerably with geographic distances ranging from ≤0.0010 for neighbouring populations to 0.0200–0.0230 for Southern Italy and Finland. For comparisons, pair-wise Fst of non-European samples were as follows: Europeans – Africans (Yoruba) 0.1530; Europeans – Chinese 0.1100; Africans (Yoruba) – Chinese 0.1900.
A study by Chao Tian in August 2009 extended the analysis of European population genetic structure to include additional southern European groups and levantine populations (Palestinians, Druzes...) from the Near-East. This study determined autosomal Fst between 18 population groups and concluded that, in general, genetic distances corresponded to geographical relationships with smaller values between population groups with origins in neighbouring countries/regions (for example, Greeks/Tuscans: Fst=0.0010, Greeks/Palestinians: Fst=0.0057) compared with those from very different regions in Europe (for example Greeks/Swedish: Fst=0.0087, Greeks/Russians: Fst=0.0108).
Autosomal DNA
Seldin (2006) used over 5,000 autosomal SNPs. It showed "a consistent and reproducible distinction between ‘northern’ and ‘southern’ European population groups". Most individual participants with southern European ancestry (Italians, Greeks, Portuguese, Spaniards), and Ashkenazi Jews have >85% membership in the southern population; and most northern, western, central, and eastern Europeans (Swedes, English, Irish, Germans, and Ukrainians) have >90% in the northern population group. Many of the participants in this study were American citizens who self-identified with different European ethnicities based on self-reported familial pedigree.
A similar study in 2007 using samples predominantly from Europe found that the most important genetic differentiation in Europe occurs on a line from the north to the south-east (northern Europe to the Balkans), with another east–west axis of differentiation across Europe. Its findings were consistent with earlier results based on mtDNA and Y-chromosomal DNA that support the theory that modern Iberians (Spanish and Portuguese) hold the most ancient European genetic ancestry, as well as separating Basques and Sami from other European populations.
It suggested that the English and Irish cluster with other Northern and Eastern Europeans such as Germans and Poles, while some Basque and Italian individuals also clustered with Northern Europeans. Despite these stratifications, it noted that "there is low apparent diversity in Europe with the entire continent-wide samples only marginally more dispersed than single population samples elsewhere in the world".
In 2008, two international research teams published analyses of large-scale genotyping of large samples of Europeans, using over 300,000 autosomal SNPs. With the exception of usual isolates such as Basques, Finns and Sardinians, the European population lacked sharp discontinuities (clustering) as previous studies have found (see Seldin et al. 2006 and Bauchet et al. 2007), although there was a discernible south to north gradient. Overall, they found only a low level of genetic differentiation between subpopulations, and differences which did exist were characterised by a strong continent-wide correlation between geographic and genetic distance. In addition, they found that diversity was greatest in southern Europe due a larger effective population size and/or population expansion from southern to northern Europe. The researchers take this observation to imply that genetically, Europeans are not distributed into discrete populations.
Two whole-genome studies of the two Eastern European populations in Ukraine (Ukrainians from Ukraine) and Russia (Russians from Russia) showed genomic diversity, which has not been represented in the previous genomic surveys, as studies in Europe are mostly biased towards the populations in the western part of the continent. Within Russia, Komi people, who live in the northeastern regions and are part of the Uralic language family that also includes Finns, form a pole of genetic diversity that is distinct from other populations, and characterized by a higher European hunter-gatherer (WHG) and Ancient North Eurasian ancestry.
According to geneticist David Reich, based on ancient human genomes that his laboratory sequenced in 2016, Europeans descend from a mixture of four West-Eurasian ancestral components, namely WHG (western hunter-gatherers), EHG, Neolithic farmers from the Levant/Anatolia as well as from Neolithic farmers from Iran (often summarized as "EEF"; early European farmers), in varying degrees.
Siberian geneflow is found among several Uralic-speaking European ethnic groups. This Siberian component is itself a composition of Ancient North Eurasian and East Asian-related ancestry from Eastern Siberia, maximized among Evenks and Evens or Nganasans. The spread of this ancestry is linked by some geneticists to the dispersal of Uralic languages, others however maintain that the Uralic languages spread prior to the arrival of Siberian geneflow, which is a secondary source of diversity within Uralic-speaking populations. Genetic data points to a western Siberian hunter-gatherer origin of the observed Siberian geneflow among Uralic-speaking groups. Western Siberian hunter-gatherers were characterized by high Ancient North Eurasian ancestry and lower amounts of Eastern Siberian admixture. Genetic data on Volga Tatars or Chuvash, found among "Western Turkic speakers, like Chuvash and Volga Tatar, the East Asian component was detected only in low amounts (~ 5%)".
East Asian ancestry is found at low frequency among some Europeans, such as British (2.5 ± 1%), Orcadians (3.8 ± 1%), French (0.7 ± 0.8%) and Germans (0.7 ± 0.8%). Finns and Russians have more than 12% East Asian ancestry, deriving from historic intermarriages with Mongolian populations. But a 2017 study finds no evidence of Asian admixture among Russians, except for Novosibirsk residents and Old Believers in Siberia.The Lipka Tatars, a Turkic minority in Belarus, have significant East Eurasian ancestry, making up one-third of their genome.
Like other Eurasian populations, Mesolithic, Neolithic or Bronze Age ancestries are not homogenously distributed in European populations. But WHG-related ancestries are highest in present-day individuals from the Baltic States, Belarus, Poland and Russia whilst EHG-related ancestries are highest in Finland and Estonia. Steppe-related ancestries are found in high levels in northern Europe, peaking in Ireland, Iceland, Norway and Sweden, but decrease further south, especially in southern Europe, where Neolithic Anatolian-related farmer ancestries dominate.
Autosomal genetic distances (Fst) based on SNPs (2009)
The genetic distance between populations is often measured by Fixation index (Fst), based on genetic polymorphism data, such as single-nucleotide polymorphisms (SNPs) or microsatellites. Fst is a special case of F-statistics, the concept developed in the 1920s by Sewall Wright. Fst is simply the correlation of randomly chosen alleles within the same sub-population relative to that found in the entire population. It is often expressed as the proportion of genetic diversity due to allele frequency differences among populations.
The values range from 0 to 1. A zero value implies that the two populations are panmictic, that they are interbreeding freely. A value of one would imply that the two populations are completely separate. The greater the Fst value, the greater the genetic distance. Essentially, these low Fst values suggest that the majority of genetic variation is at the level of individuals within the same population group (~ 85%); whilst belonging to a different population group within same ‘race’/ continent, and even to different racial/ continental groups added a much smaller degree of variation (3–8%; 6–11%, respectively).
| Italian Americans | Palestinians | Swedes | Druzes | Spaniards | Germans | Russians | Irish | Greek Americans | Ashkenazi Jews | Circassians | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Italian Americans | 0.0064 | 0.0064 | 0.0057 | 0.0010 | 0.0029 | 0.0088 | 0.0048 | 0.0000 | 0.0040 | 0.0067 | |
| Palestinians | 0.0064 | 0.0191 | 0.0064 | 0.0101 | 0.0136 | 0.0202 | 0.0170 | 0.0057 | 0.0093 | 0.0108 | |
| Swedes | 0.0064 | 0.0191 | 0.0167 | 0.0040 | 0.0007 | 0.0030 | 0.0020 | 0.0084 | 0.0120 | 0.0117 | |
| Druzes | 0.0057 | 0.0064 | 0.0167 | 0.0096 | 0.0121 | 0.0194 | 0.0154 | 0.0052 | 0.0088 | 0.0092 | |
| Spaniards | 0.0010 | 0.0101 | 0.0040 | 0.0096 | 0.0015 | 0.0070 | 0.0037 | 0.0035 | 0.0056 | 0.0090 | |
| Germans | 0.0029 | 0.0136 | 0.0007 | 0.0121 | 0.0015 | 0.0030 | 0.0010 | 0.0039 | 0.0072 | 0.0089 | |
| Russians | 0.0088 | 0.0202 | 0.0030 | 0.0194 | 0.0070 | 0.0030 | 0.0038 | 0.0108 | 0.0137 | 0.0120 | |
| Irish | 0.0048 | 0.0170 | 0.0020 | 0.0154 | 0.0037 | 0.0010 | 0.0038 | 0.0067 | 0.0109 | 0.0110 | |
| Greek Americans | 0.0000 | 0.0057 | 0.0084 | 0.0052 | 0.0035 | 0.0039 | 0.0108 | 0.0067 | 0.0042 | 0.0054 | |
| Ashkenazi Jews | 0.0040 | 0.0093 | 0.0120 | 0.0088 | 0.0056 | 0.0072 | 0.0137 | 0.0109 | 0.0042 | 0.0107 | |
| Circassians | 0.0067 | 0.0108 | 0.0117 | 0.0092 | 0.0090 | 0.0089 | 0.0120 | 0.0110 | 0.0054 | 0.0107 |
| Austria | Bulgaria | Czech Republic | Estonia | Finland (Helsinki) | Finland (Kuusamo) | France | Northern Germany | Southern Germany | Hungary | Northern Italy | Southern Italy | Latvia | Lithuania | Poland | Russia | Spain | Sweden | Switzerland | CEU | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 1.14 | 1.08 | 1.58 | 2.24 | 3.30 | 1.16 | 1.10 | 1.04 | 1.04 | 1.49 | 1.79 | 1.85 | 1.70 | 1.19 | 1.47 | 1.41 | 1.21 | 1.19 | 1.12 | Austria | |
| Bulgaria | 1.14 | 1.21 | 1.70 | 2.19 | 2.91 | 1.22 | 1.32 | 1.19 | 1.10 | 1.32 | 1.38 | 1.86 | 1.73 | 1.29 | 1.53 | 1.30 | 1.47 | 1.13 | 1.29 | Bulgaria | |
| Czech Republic | 1.08 | 1.21 | 1.42 | 2.20 | 3.26 | 1.35 | 1.15 | 1.16 | 1.06 | 1.69 | 2.04 | 1.62 | 1.48 | 1.09 | 1.27 | 1.63 | 1.26 | 1.37 | 1.21 | Czech Republic | |
| Estonia | 1.58 | 1.70 | 1.42 | 1.71 | 2.80 | 2.08 | 1.53 | 1.70 | 1.41 | 2.42 | 2.93 | 1.24 | 1.28 | 1.17 | 1.21 | 2.54 | 1.49 | 2.16 | 1.59 | Estonia | |
| Finland (Helsinki) | 2.24 | 2.19 | 2.20 | 1.71 | 1.86 | 2.69 | 2.17 | 2.35 | 1.87 | 2.82 | 3.37 | 2.31 | 2.33 | 1.75 | 2.10 | 3.14 | 1.89 | 2.77 | 1.99 | Finland (Helsinki) | |
| Finland (Kuusamo) | 3.30 | 2.91 | 3.26 | 2.80 | 1.86 | 3.72 | 3.27 | 3.46 | 2.68 | 3.64 | 4.18 | 3.33 | 3.37 | 2.49 | 3.16 | 4.21 | 2.87 | 3.83 | 2.89 | Finland (Kuusamo) | |
| France | 1.16 | 1.22 | 1.35 | 2.08 | 2.69 | 3.72 | 1.25 | 1.12 | 1.16 | 1.38 | 1.68 | 2.40 | 2.20 | 1.44 | 1.94 | 1.13 | 1.38 | 1.10 | 1.13 | France | |
| Northern Germany | 1.10 | 1.32 | 1.15 | 1.53 | 2.17 | 3.27 | 1.25 | 1.08 | 1.11 | 1.72 | 2.14 | 1.84 | 1.66 | 1.18 | 1.49 | 1.62 | 1.12 | 1.36 | 1.06 | Northern Germany | |
| Southern Germany | 1.04 | 1.19 | 1.16 | 1.70 | 2.35 | 3.46 | 1.12 | 1.08 | 1.08 | 1.53 | 1.85 | 1.20 | 1.84 | 1.23 | 1.58 | 1.40 | 1.21 | 1.17 | 1.07 | Southern Germany | |
| Hungary | 1.04 | 1.10 | 1.06 | 1.41 | 1.87 | 2.68 | 1.16 | 1.11 | 1.08 | 1.42 | 1.63 | 1.58 | 1.46 | 1.14 | 1.28 | 1.32 | 1.22 | 1.16 | 1.13 | Hungary | |
| Northern Italy | 1.49 | 1.32 | 1.69 | 2.42 | 2.82 | 3.64 | 1.38 | 1.72 | 1.53 | 1.42 | 1.54 | 2.64 | 2.48 | 1.75 | 2.24 | 1.42 | 1.86 | 1.36 | 1.56 | Northern Italy | |
| Southern Italy | 1.79 | 1.38 | 2.04 | 2.93 | 3.37 | 4.18 | 1.68 | 2.14 | 1.85 | 1.63 | 1.54 | 3.14 | 2.96 | 1.99 | 2.68 | 1.67 | 2.28 | 1.54 | 1.84 | Southern Italy | |
| Latvia | 1.85 | 1.86 | 1.62 | 1.24 | 2.31 | 3.33 | 2.40 | 1.84 | 1.20 | 1.58 | 2.64 | 3.14 | 1.20 | 1.26 | 1.84 | 2.82 | 1.89 | 2.52 | 1.87 | Latvia | |
| Lithuania | 1.70 | 1.73 | 1.48 | 1.28 | 2.33 | 3.37 | 2.20 | 1.66 | 1.84 | 1.46 | 2.48 | 2.96 | 1.20 | 1.20 | 1.26 | 2.62 | 1.74 | 2.29 | 1.74 | Lithuania | |
| Poland | 1.19 | 1.29 | 1.09 | 1.17 | 1.75 | 2.49 | 1.44 | 1.18 | 1.23 | 1.14 | 1.75 | 1.99 | 1.26 | 1.20 | 1.18 | 1.66 | 1.30 | 1.46 | 1.28 | Poland | |
| Russia | 1.47 | 1.53 | 1.27 | 1.21 | 2.10 | 3.16 | 1.94 | 1.49 | 1.58 | 1.28 | 2.24 | 2.68 | 1.84 | 1.26 | 1.18 | 2.32 | 1.59 | 1.20 | 1.56 | Russia | |
| Spain | 1.41 | 1.30 | 1.63 | 2.54 | 3.14 | 4.21 | 1.13 | 1.62 | 1.40 | 1.32 | 1.42 | 1.67 | 2.82 | 2.62 | 1.66 | 2.32 | 1.73 | 1.16 | 1.34 | Spain | |
| Sweden | 1.21 | 1.47 | 1.26 | 1.49 | 1.89 | 2.87 | 1.38 | 1.12 | 1.21 | 1.22 | 1.86 | 2.28 | 1.89 | 1.74 | 1.30 | 1.59 | 1.73 | 1.50 | 1.09 | Sweden | |
| Switzerland | 1.19 | 1.13 | 1.37 | 2.16 | 2.77 | 3.83 | 1.10 | 1.36 | 1.17 | 1.16 | 1.36 | 1.54 | 2.52 | 2.29 | 1.46 | 1.20 | 1.16 | 1.50 | 1.21 | Switzerland | |
| CEU | 1.12 | 1.29 | 1.21 | 1.59 | 1.99 | 2.89 | 1.13 | 1.06 | 1.07 | 1.13 | 1.56 | 1.84 | 1.87 | 1.74 | 1.28 | 1.56 | 1.34 | 1.09 | 1.21 | CEU | |
| Austria | Bulgaria | Czech Republic | Estonia | Finland (Helsinki) | Finland (Kuusamo) | France | Northern Germany | Southern Germany | Hungary | Northern Italy | Southern Italy | Latvia | Lithuania | Poland | Russia | Spain | Sweden | Switzerland | CEU |
CEU – Utah residents with ancestry from Northern and Western Europe.
History of research
Further information: Population geneticsClassical genetic markers (by proxy)
One of the first scholars to perform genetic studies was Luigi Luca Cavalli-Sforza. He used classical genetic markers to analyse DNA by proxy. This method studies differences in the frequencies of particular allelic traits, namely polymorphisms from proteins found within human blood (such as the ABO blood groups, Rhesus blood antigens, HLA loci, immunoglobulins, G6PD isoenzymes, among others). Subsequently, his team calculated genetic distance between populations, based on the principle that two populations that share similar frequencies of a trait are more closely related than populations that have more divergent frequencies of the trait.
From this, he constructed phylogenetic trees that showed genetic distances diagrammatically. His team also performed principal component analyses, which is good at analysing multivariate data with minimal loss of information. The information that is lost can be partly restored by generating a second principal component, and so on. In turn, the information from each individual principal component (PC) can be presented graphically in synthetic maps. These maps show peaks and troughs, which represent populations whose gene frequencies take extreme values compared to others in the studied area.
Peaks and troughs usually connected by smooth gradients are called clines. Genetic clines can be generated by adaptation to environment (natural selection), continuous gene flow between two initially different populations or a demographic expansion into a scarcely populated environment, with little initial admixture with existing populations. Cavalli-Sforza connected these gradients with postulated pre-historical population movements, based on archaeological and linguistic theories. However, given that the time depths of such patterns are not known, "associating them with particular demographic events is usually speculative".
Direct DNA analysis
Further information: Genetic drift, Founder effect, and Population bottleneckStudies using direct DNA analysis are now abundant and may use mitochondrial DNA (mtDNA), the non-recombining portion of the Y chromosome (NRY), or even autosomal DNA. MtDNA and NRY DNA share some similar features, which have made them particularly useful in genetic anthropology. These properties include the direct, unaltered inheritance of mtDNA and NRY DNA from mother to offspring and father to son, respectively, without the 'scrambling' effects of genetic recombination. We also presume that these genetic loci are not affected by natural selection and that the major process responsible for changes in base pairs has been mutation (which can be calculated).
The smaller effective population size of the NRY and mtDNA enhances the consequences of drift and founder effect, relative to the autosomes, making NRY and mtDNA variation a potentially sensitive index of population composition. These biologically plausible assumptions are not concrete; Rosser suggests that climatic conditions may affect the fertility of certain lineages.
The underlying mutation rate used by the geneticists is more questionable. They often use different mutation rates and studies frequently arrive at vastly different conclusions. NRY and mtDNA may be so susceptible to drift that some ancient patterns may have become obscured. Another assumption is that population genealogies are approximated by allele genealogies. Guido Barbujani points out that this only holds if population groups develop from a genetically monomorphic set of founders. Barbujani argues that there is no reason to believe that Europe was colonised by monomorphic populations. This would result in an overestimation of haplogroup age, thus falsely extending the demographic history of Europe into the Late Paleolithic rather than the Neolithic era. Greater certainty about chronology may be obtained from studies of ancient DNA (see below), but so far these have been comparatively few.
Whereas Y-DNA and mtDNA haplogroups represent but a small component of a person's DNA pool, autosomal DNA has the advantage of containing hundreds of thousands of examinable genetic loci, thus giving a more complete picture of genetic composition. Descent relationships can only be determined on a statistical basis, because autosomal DNA undergoes recombination. A single chromosome can record a history for each gene. Autosomal studies are much more reliable for showing the relationships between existing populations, but do not offer the possibilities for unravelling their histories in the same way as mtDNA and NRY DNA studies promise, despite their many complications.
Genetic studies operate on numerous assumptions and suffer from methodological limitations, such as selection bias and confounding phenomena like genetic drift, foundation and bottleneck effects cause large errors, particularly in haplogroup studies. No matter how accurate the methodology, conclusions derived from such studies are compiled on the basis of how the author envisages their data fits with established archaeological or linguistic theories.
See also
- General
- Archaeogenetics
- Ethnic groups in Europe
- European studies
- Genetic history of North Africa
- Genetic history of the Middle East
- Genetics and archaeogenetics of South Asia
- History of Europe
- Human genetic variation
- Y-DNA haplogroups in populations of Europe
- Romani people#Genetic evidence
- Genetics by European group
- Genetic history of Italy
- Genetic history of the British Isles
- Genetic history of the Iberian Peninsula
- Genetic studies on Bosniaks
- Genetic studies on Bulgarians
- Genetic studies on Croats
- Genetic studies on Jews
- Genetic studies on Russians
- Genetic studies on Sami
- Genetic studies on Serbs
- Genetic studies on Turkish people
References
Inline citations
- ^ Nelis M, Esko T, Mägi R, Zimprich F, Zimprich A, Toncheva D, et al. (2009). "Genetic structure of Europeans: a view from the North-East". PLOS ONE. 4 (5): e5472. Bibcode:2009PLoSO...4.5472N. doi:10.1371/journal.pone.0005472. PMC 2675054. PMID 19424496.
- Seguin-Orlando A, Korneliussen TS, Sikora M, Malaspinas AS, Manica A, Moltke I, et al. (November 2014). "Paleogenomics. Genomic structure in Europeans dating back at least 36,200 years". Science. 346 (6213): 1113–1118. Bibcode:2014Sci...346.1113S. doi:10.1126/science.aaa0114. PMID 25378462. S2CID 206632421.
- ^ Beleza S, Santos AM, McEvoy B, Alves I, Martinho C, Cameron E, et al. (January 2013). "The timing of pigmentation lightening in Europeans". Molecular Biology and Evolution. 30 (1): 24–35. doi:10.1093/molbev/mss207. PMC 3525146. PMID 22923467.
- ^ Jones ER, Gonzalez-Fortes G, Connell S, Siska V, Eriksson A, Martiniano R, et al. (November 2015). "Upper Palaeolithic genomes reveal deep roots of modern Eurasians". Nature Communications. 6 (1): 8912. Bibcode:2015NatCo...6.8912J. doi:10.1038/ncomms9912. PMC 4660371. PMID 26567969.
- Population replacement in the Neolithic, and again in the Bronze Age, was nearly complete in Prehistoric Britain, the Mesolithic WHG population accounting for just about 10% of the ancestry of the modern indigenous British population. Olalde I, Brace S, Allentoft ME, Armit I, Kristiansen K, Booth T, et al. (March 2018). "The Beaker phenomenon and the genomic transformation of northwest Europe". Nature. 555 (7695): 190–196. Bibcode:2018Natur.555..190O. doi:10.1038/nature25738. PMC 5973796. PMID 29466337.
- Haak, Wolfgang; Lazaridis, Iosif; Patterson, Nick; Rohland, Nadin; Mallick, Swapan; Llamas, Bastien; Brandt, Guido; Nordenfelt, Susanne; Harney, Eadaoin; Stewardson, Kristin; Fu, Qiaomei; Mittnik, Alissa; Bánffy, Eszter; Economou, Christos; Francken, Michael (2015). "Massive migration from the steppe was a source for Indo-European languages in Europe". Nature. 522 (7555): 207–211. arXiv:1502.02783. Bibcode:2015Natur.522..207H. doi:10.1038/nature14317. ISSN 1476-4687. PMC 5048219. PMID 25731166.
- Lazaridis I, Patterson N, Mittnik A, Renaud G, Mallick S, Kirsanow K, et al. (September 2014). "Ancient human genomes suggest three ancestral populations for present-day Europeans". Nature. 513 (7518): 409–413. arXiv:1312.6639. Bibcode:2014Natur.513..409L. doi:10.1038/nature13673. PMC 4170574. PMID 25230663.
- Since Lazaridis et al. (2014), further studies have refined the picture of interbreeding between EEF and WHG. In a 2017 analysis of 180 ancient DNA datasets of the Chalcolithic and Neolithic periods from Hungary, Germany and Spain evidence was found of a prolonged period of EEF-WHG interbreeding. Admixture took place regionally, from local hunter-gatherer populations, so that populations from the three regions (Germany, Iberia and Hungary) were genetically distinguishable at all stages of the Neolithic period, with a gradually increasing ratio of WHG ancestry of farming populations over time. This suggests that after the initial expansion of early farmers, there were no further long-range migrations substantial enough to homogenize the farming population, and that farming and hunter-gatherer populations existed side by side for many centuries, with ongoing gradual admixture throughout the 5th to 4th millennia BCE (rather than a single admixture event on initial contact). Lipson M, Szécsényi-Nagy A, Mallick S, Pósa A, Stégmár B, Keerl V, et al. (November 2017). "Parallel palaeogenomic transects reveal complex genetic history of early European farmers". Nature. 551 (7680): 368–372. Bibcode:2017Natur.551..368L. doi:10.1038/nature24476. PMC 5973800. PMID 29144465.
- "There's no such thing as a 'pure' European—or anyone else". Science | AAAS. May 15, 2017.
- Curry A (2019). "Genetic testing reveals that Europe is a melting pot, made of immigrants". National Geographic. Archived from the original on July 9, 2019.
- Lipson et al. (2017), Fig 2.
- Dutchen S (November 23, 2015). "Farming's in Their DNA". Harvard Medical School. Retrieved 25 November 2015.
- ^ Fu Q, Posth C, Hajdinjak M, Petr M, Mallick S, Fernandes D, et al. (June 2016). "The genetic history of Ice Age Europe". Nature. 534 (7606): 200–205. Bibcode:2016Natur.534..200F. doi:10.1038/nature17993. PMC 4943878. PMID 27135931.
- Even before the advent of genetic studies, some anthropologists believed they had discovered skeletons representing Neanderthal-modern human 'hybrids'. These results were deemed 'ambiguous'. Archaeological evidence points to an abrupt change from Neanderthal artefacts to those related to AMH during the Upper Palaeolithic.Klein RG (March 2003). "Paleoanthropology. Whither the Neanderthals?". Science. 299 (5612): 1525–1527. doi:10.1126/science.1082025. PMID 12624250. S2CID 161836323.
- Prüfer K, Racimo F, Patterson N, Jay F, Sankararaman S, Sawyer S, et al. (January 2014) . "The complete genome sequence of a Neanderthal from the Altai Mountains". Nature. 505 (7481): 43–49. Bibcode:2014Natur.505...43P. doi:10.1038/nature12886. PMC 4031459. PMID 24352235.
- Hardy J, Pittman A, Myers A, Gwinn-Hardy K, Fung HC, de Silva R, et al. (August 2005). "Evidence suggesting that Homo neanderthalensis contributed the H2 MAPT haplotype to Homo sapiens". Biochemical Society Transactions. 33 (Pt 4): 582–585. doi:10.1042/bst0330582. PMID 16042549.
We suggest that the H2 haplotype is derived from Homo neanderthalensis and entered H. sapiens populations during the coexistence of these species in Europe from approx. 45 000 to 18 000 years ago and that the H2 haplotype has been under selection pressure since that time, possibly because of the role of this H1 haplotype in neurodegenerative disease."..."The tau (MAPT ) locus is very unusual. Over a region of approx. 1.8 Mb, there are two haplotype clades in European populations, H1 and H2 . In other populations, only the H1 occurs and shows a normal pattern of recombination
- Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S, et al. (September 2006). "Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability". Nature Genetics. 38 (9): 1032–1037. doi:10.1038/ng1858. PMID 16906163. S2CID 38047848.
- Zody MC, Jiang Z, Fung HC, Antonacci F, Hillier LW, Cardone MF, et al. (September 2008). "Evolutionary toggling of the MAPT 17q21.31 inversion region". Nature Genetics. 40 (9): 1076–1083. doi:10.1038/ng.193. PMC 2684794. PMID 19165922.
- Introgression and microcephalin FAQ John Hawks
- Almos PZ, Horváth S, Czibula A, Raskó I, Sipos B, Bihari P, et al. (November 2008). "H1 tau haplotype-related genomic variation at 17q21.3 as an Asian heritage of the European Gypsy population". Heredity. 101 (5): 416–419. doi:10.1038/hdy.2008.70. PMID 18648385.
In this study, we examine the frequency of a 900 kb inversion at 17q21.3 in the Gypsy and Caucasian populations of Hungary, which may reflect the Asian origin of Gypsy populations. Of the two haplotypes (H1 and H2), H2 is thought to be exclusively of Caucasian origin, and its occurrence in other racial groups is likely to reflect admixture. In our sample, the H1 haplotype was significantly more frequent in the Gypsy population (89.8 vs 75.5%, P<0.001) and was in Hardy–Weinberg disequilibrium (P=0.017). The 17q21.3 region includes the gene of microtubule-associated protein tau, and this result might imply higher sensitivity to H1 haplotype-related multifactorial tauopathies among Gypsies.
- Wade N (2009-12-02). "Scientists in Germany Draft Neanderthal Genome". The New York Times. Retrieved 2010-05-03.
- "Neanderthals 'distinct from us'". BBC. 2009-12-02.
- ^ Milisauskas S (2002). European Prehistory: a survey. Birkhauser. ISBN 978-0-306-46793-6.
- Teschler-Nicola, Maria; Fernandes, Daniel; Händel, Marc; Einwögerer, Thomas; Simon, Ulrich; Neugebauer-Maresch, Christine; Tangl, Stefan; Heimel, Patrick; Dobsak, Toni; Retzmann, Anika; Prohaska, Thomas (2020-11-06). "Ancient DNA reveals monozygotic newborn twins from the Upper Palaeolithic". Communications Biology. 3 (1): 650. doi:10.1038/s42003-020-01372-8. ISSN 2399-3642. PMC 7648643. PMID 33159107.
- ^ Semino O, Passarino G, Oefner PJ, Lin AA, Arbuzova S, Beckman LE, et al. (November 2000). "The genetic legacy of Paleolithic Homo sapiens sapiens in extant Europeans: a Y chromosome perspective". Science. 290 (5494): 1155–1159. Bibcode:2000Sci...290.1155S. doi:10.1126/science.290.5494.1155. PMID 11073453. Note: Haplogroup names are different in this article. For example: Haplogroup I is referred as M170
- ^ Wells RS, Yuldasheva N, Ruzibakiev R, Underhill PA, Evseeva I, Blue-Smith J, et al. (August 2001). "The Eurasian heartland: a continental perspective on Y-chromosome diversity". Proceedings of the National Academy of Sciences of the United States of America. 98 (18): 10244–10249. Bibcode:2001PNAS...9810244W. doi:10.1073/pnas.171305098. PMC 56946. PMID 11526236.
- Karafet TM, Mendez FL, Meilerman MB, Underhill PA, Zegura SL, Hammer MF (May 2008). "New binary polymorphisms reshape and increase resolution of the human Y chromosomal haplogroup tree". Genome Research. 18 (5): 830–838. doi:10.1101/gr.7172008. PMC 2336805. PMID 18385274.
- ^ Richards M, Macaulay V, Hickey E, Vega E, Sykes B, Guida V, et al. (November 2000). "Tracing European founder lineages in the Near Eastern mtDNA pool". American Journal of Human Genetics. 67 (5): 1251–1276. doi:10.1016/S0002-9297(07)62954-1. PMC 1288566. PMID 11032788.
- Torroni A, Bandelt HJ, Macaulay V, Richards M, Cruciani F, Rengo C, et al. (October 2001). "A signal, from human mtDNA, of postglacial recolonization in Europe". American Journal of Human Genetics. 69 (4): 844–852. doi:10.1086/323485. PMC 1226069. PMID 11517423.
- Pala M, Achilli A, Olivieri A, Hooshiar Kashani B, Perego UA, Sanna D, et al. (June 2009). "Mitochondrial haplogroup U5b3: a distant echo of the epipaleolithic in Italy and the legacy of the early Sardinians". American Journal of Human Genetics. 84 (6): 814–821. doi:10.1016/j.ajhg.2009.05.004. PMC 2694970. PMID 19500771.
- ^ Rootsi S, Magri C, Kivisild T, Benuzzi G, Help H, Bermisheva M, et al. (July 2004). "Phylogeography of Y-chromosome haplogroup I reveals distinct domains of prehistoric gene flow in europe" (PDF). American Journal of Human Genetics. 75 (1): 128–137. doi:10.1086/422196. PMC 1181996. PMID 15162323. Archived from the original (PDF) on 2009-06-24. Retrieved 2009-07-04.
- Cinnioglu et al. Excavating Y-chromosome haplotype strata in Anatolia. 2003
- ^ Pericić M, Lauc LB, Klarić IM, Rootsi S, Janićijevic B, Rudan I, et al. (October 2005). "High-resolution phylogenetic analysis of southeastern Europe traces major episodes of paternal gene flow among Slavic populations". Molecular Biology and Evolution. 22 (10): 1964–1975. doi:10.1093/molbev/msi185. PMID 15944443.
- Passarino G, Semino O, Magri C, Al-Zahery N, Benuzzi G, Quintana-Murci L, et al. (September 2001). "The 49a,f haplotype 11 is a new marker of the EU19 lineage that traces migrations from northern regions of the Black Sea". Human Immunology. 62 (9): 922–932. doi:10.1016/S0198-8859(01)00291-9. PMID 11543894.
- Bilton DT, Mirol PM, Mascheretti S, Fredga K, Zima J, Searle JB (July 1998). "Mediterranean Europe as an area of endemism for small mammals rather than a source for northwards postglacial colonization". Proceedings. Biological Sciences. 265 (1402): 1219–1226. doi:10.1098/rspb.1998.0423. PMC 1689182. PMID 9699314.
- ^ Dutchen S (May 2, 2016). "History on Ice". Harvard Medical School. Retrieved 11 May 2016.
- ^ Mathieson I, Lazaridis I, Rohland N, Mallick S, Patterson N, Roodenberg SA, et al. (December 2015). "Genome-wide patterns of selection in 230 ancient Eurasians". Nature. 528 (7583): 499–503. Bibcode:2015Natur.528..499M. doi:10.1038/nature16152. PMC 4918750. PMID 26595274.
- Sikora M, Carpenter ML, Moreno-Estrada A, Henn BM, Underhill PA, Sánchez-Quinto F, et al. (May 2014). "Population genomic analysis of ancient and modern genomes yields new insights into the genetic ancestry of the Tyrolean Iceman and the genetic structure of Europe". PLOS Genetics. 10 (5): e1004353. doi:10.1371/journal.pgen.1004353. PMC 4014435. PMID 24809476.
- Haak W, Balanovsky O, Sanchez JJ, Koshel S, Zaporozhchenko V, Adler CJ, et al. (November 2010). "Ancient DNA from European early neolithic farmers reveals their near eastern affinities". PLOS Biology. 8 (11): e1000536. doi:10.1371/journal.pbio.1000536. PMC 2976717. PMID 21085689.
- Perlès C, Monthel G ( 2001) The Early Neolithic in Greece: The First Farming Communities in Europe. Cambridge University Press, Cambridge.
- Runnels C (2003) The origins of the Greek Neolithic: a personal view, in Ammerman and Biagi (2003 eds).
- Zeder MA (August 2008). "Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact". Proceedings of the National Academy of Sciences of the United States of America. 105 (33): 11597–11604. Bibcode:2008PNAS..10511597Z. doi:10.1073/pnas.0801317105. PMC 2575338. PMID 18697943.
- Piazza A, Cavalli-Sforza LL, Menozzi P (1994). The history and geography of human genes. Princeton, N.J: Princeton University Press. ISBN 978-0-691-08750-4.
- ^ Rosser ZH, Zerjal T, Hurles ME, Adojaan M, Alavantic D, Amorim A, et al. (December 2000). "Y-chromosomal diversity in Europe is clinal and influenced primarily by geography, rather than by language". American Journal of Human Genetics. 67 (6): 1526–1543. doi:10.1086/316890. PMC 1287948. PMID 11078479. Archived from the original on 2008-05-06.
- Underhill PA, Kivisild T (2007). "Use of y chromosome and mitochondrial DNA population structure in tracing human migrations". Annual Review of Genetics. 41: 539–564. doi:10.1146/annurev.genet.41.110306.130407. PMID 18076332.
- Y chromosome data show a signal for a separate late-Pleistocene migration from Africa to Europe via Sinai as evidenced through the distribution of haplogroup E3b lineages, which is not manifested in mtDNA haplogroup distributions.
- ^ Battaglia V, Fornarino S, Al-Zahery N, Olivieri A, Pala M, Myres NM, et al. (June 2009). "Y-chromosomal evidence of the cultural diffusion of agriculture in Southeast Europe". European Journal of Human Genetics. 17 (6): 820–830. doi:10.1038/ejhg.2008.249. PMC 2947100. PMID 19107149.
- Cruciani F, La Fratta R, Trombetta B, Santolamazza P, Sellitto D, Colomb EB, et al. (June 2007). "Tracing past human male movements in northern/eastern Africa and western Eurasia: new clues from Y-chromosomal haplogroups E-M78 and J-M12". Molecular Biology and Evolution. 24 (6): 1300–1311. doi:10.1093/molbev/msm049. PMID 17351267. Archived from the original on 2017-10-10. Retrieved 2009-07-22. Also see Supplementary Data.
- Lacan M, Keyser C, Ricaut FX, Brucato N, Duranthon F, Guilaine J, Crubézy E, Ludes B (June 2011). "Ancient DNA reveals male diffusion through the Neolithic Mediterranean route". Proceedings of the National Academy of Sciences of the United States of America. 108 (24): 9788–91. Bibcode:2011PNAS..108.9788L. doi:10.1073/pnas.1100723108. PMC 3116412. PMID 21628562.
- ^ Bird S (2007). "Haplogroup E3b1a2 as a Possible Indicator of Settlement in Roman Britain by Soldiers of Balkan Origin". Journal of Genetic Genealogy. 3 (2). Archived from the original on 2016-04-22. Retrieved 2009-08-25.
- Haak W, Lazaridis I, Patterson N, Rohland N, Mallick S, Llamas B, et al. (June 2015). "Massive migration from the steppe was a source for Indo-European languages in Europe". Nature. 522 (7555): 207–211. arXiv:1502.02783. Bibcode:2015Natur.522..207H. doi:10.1038/nature14317. PMC 5048219. PMID 25731166.
- Callaway E (12 February 2015). "European languages linked to migration from the east". Nature. doi:10.1038/nature.2015.16919. S2CID 184180681.
- Underhill PA, Myres NM, Rootsi S, Metspalu M, Zhivotovsky LA, King RJ, et al. (April 2010). "Separating the post-Glacial coancestry of European and Asian Y chromosomes within haplogroup R1a". European Journal of Human Genetics. 18 (4): 479–484. doi:10.1038/ejhg.2009.194. PMC 2987245. PMID 19888303.
- Ingman M, Gyllensten U (January 2007). "A recent genetic link between Sami and the Volga-Ural region of Russia". European Journal of Human Genetics. 15 (1): 115–120. doi:10.1038/sj.ejhg.5201712. PMID 16985502.
- Wiik K. "Who Are the Finns?" (PDF). In Suominen M, Arppe A, Airola A, Heinämäki O, Miestamo M, Määttä U, Niemi J, Pitkänen KK, Sinnemäki K (eds.). A Man of Measure Festschrift in Honour of Fred Karlsson. pp. 97–108. Retrieved 2016-03-16.
- ^ Mirabal S, Varljen T, Gayden T, Regueiro M, Vujovic S, Popovic D, et al. (July 2010). "Human Y-chromosome short tandem repeats: a tale of acculturation and migrations as mechanisms for the diffusion of agriculture in the Balkan Peninsula". American Journal of Physical Anthropology. 142 (3): 380–390. doi:10.1002/ajpa.21235. PMID 20091845.
- Rootsi S, Zhivotovsky LA, Baldovic M, Kayser M, Kutuev IA, Khusainova R, et al. (February 2007). "A counter-clockwise northern route of the Y-chromosome haplogroup N from Southeast Asia towards Europe". European Journal of Human Genetics. 15 (2): 204–211. doi:10.1038/sj.ejhg.5201748. PMID 17149388., Supplemental table 1
- ^ Jones ER, Gonzalez-Fortes G, Connell S, Siska V, Eriksson A, Martiniano R, et al. (November 2015). "Upper Palaeolithic genomes reveal deep roots of modern Eurasians". Nature Communications. 6 (8912): 8912. Bibcode:2015NatCo...6.8912J. doi:10.1038/ncomms9912. PMC 4660371. PMID 26567969.
- Yang, Melinda A. (2022-01-06). "A genetic history of migration, diversification, and admixture in Asia". Human Population Genetics and Genomics. 2 (1): 1–32. doi:10.47248/hpgg2202010001. ISSN 2770-5005.
- Dutchen S (September 17, 2014). "New Branch Added to European Family Tree". Harvard Medical School. Archived from the original on 2014-10-01. Retrieved 25 November 2015.
- "Mystery ancestral 'tribe' revealed". BBC News. 16 November 2015.
- Lazaridis I, Nadel D, Rollefson G, Merrett DC, Rohland N, Mallick S, et al. (2016). "The genetic structure of the world's first farmers". bioRxiv 10.1101/059311.
- Mathieson I, Alpaslan-Roodenberg S, Posth C, Szécsényi-Nagy A, Rohland N, Mallick S, et al. (March 2018). "The genomic history of southeastern Europe". Nature. 555 (7695): 197–203. Bibcode:2018Natur.555..197M. doi:10.1038/nature25778. PMC 6091220. PMID 29466330.
- Busby GB, Hellenthal G, Montinaro F, Tofanelli S, Bulayeva K, Rudan I, et al. (October 2015). "The Role of Recent Admixture in Forming the Contemporary West Eurasian Genomic Landscape". Current Biology. 25 (19): 2518–2526. doi:10.1016/j.cub.2015.08.007. PMC 4714572. PMID 26387712.
- Comas D, Schmid H, Braeuer S, Flaiz C, Busquets A, Calafell F, et al. (March 2004). "Alu insertion polymorphisms in the Balkans and the origins of the Aromuns". Annals of Human Genetics. 68 (Pt 2): 120–127. doi:10.1046/j.1529-8817.2003.00080.x. PMID 15008791. S2CID 21773796.
- Bosch E, Calafell F, González-Neira A, Flaiz C, Mateu E, Scheil HG, et al. (July 2006). "Paternal and maternal lineages in the Balkans show a homogeneous landscape over linguistic barriers, except for the isolated Aromuns". Annals of Human Genetics. 70 (Pt 4): 459–487. doi:10.1111/j.1469-1809.2005.00251.x. PMID 16759179. S2CID 23156886.
- Capelli C, Redhead N, Abernethy JK, Gratrix F, Wilson JF, Moen T, et al. (May 2003). "A Y chromosome census of the British Isles". Current Biology. 13 (11): 979–984. doi:10.1016/S0960-9822(03)00373-7. hdl:20.500.11820/8acb01f3-a7c1-45f5-89de-b796266d651e. PMID 12781138. also at 030705U491 (PDF), retrieved 2011-06-01
- Balaresque P, Bowden GR, Adams SM, Leung HY, King TE, Rosser ZH, et al. (January 2010). "A predominantly neolithic origin for European paternal lineages". PLOS Biology. 8 (1): e1000285. doi:10.1371/journal.pbio.1000285. PMC 2799514. PMID 20087410.
- ^ Myres NM, Rootsi S, Lin AA, Järve M, King RJ, Kutuev I, et al. (January 2011). "A major Y-chromosome haplogroup R1b Holocene era founder effect in Central and Western Europe". European Journal of Human Genetics. 19 (1): 95–101. doi:10.1038/ejhg.2010.146. PMC 3039512. PMID 20736979.
- ^ Cruciani F, Trombetta B, Antonelli C, Pascone R, Valesini G, Scalzi V, et al. (June 2011). "Strong intra- and inter-continental differentiation revealed by Y chromosome SNPs M269, U106 and U152". Forensic Science International. Genetics. 5 (3): e49–e52. doi:10.1016/j.fsigen.2010.07.006. hdl:11573/226727. PMID 20732840.
- Morelli L, Contu D, Santoni F, Whalen MB, Francalacci P, Cucca F (April 2010). "A comparison of Y-chromosome variation in Sardinia and Anatolia is more consistent with cultural rather than demic diffusion of agriculture". PLOS ONE. 5 (4): e10419. Bibcode:2010PLoSO...510419M. doi:10.1371/journal.pone.0010419. PMC 2861676. PMID 20454687.
- ^ Kayser M, Lao O, Anslinger K, Augustin C, Bargel G, Edelmann J, et al. (September 2005). "Significant genetic differentiation between Poland and Germany follows present-day political borders, as revealed by Y-chromosome analysis". Human Genetics. 117 (5): 428–443. doi:10.1007/s00439-005-1333-9. PMID 15959808. S2CID 11066186. A copy can be found here "Significant genetic differentiation between Poland and Germany follows present-day political borders, as revealed by Y-chromosome analysis" (PDF). Archived from the original (PDF) on 2009-03-04. Retrieved 2008-11-02..
- Bowden GR, Balaresque P, King TE, Hansen Z, Lee AC, Pergl-Wilson G, Hurley E, Roberts SJ, Waite P, Jesch J, Jones AL, Thomas MG, Harding SE, Jobling MA (February 2008). "Excavating past population structures by surname-based sampling: the genetic legacy of the Vikings in northwest England". Molecular Biology and Evolution. 25 (2): 301–9. doi:10.1093/molbev/msm255. PMC 2628767. PMID 18032405.
- Lall GM, Larmuseau MH, Wetton JH, Batini C, Hallast P, Huszar TI, et al. (March 2021). "Subdividing Y-chromosome haplogroup R1a1 reveals Norse Viking dispersal lineages in Britain". European Journal of Human Genetics. 29 (3): 512–523. doi:10.1038/s41431-020-00747-z. PMC 7940619. PMID 33139852.
- Kasperaviciūte D, Kucinskas V, Stoneking M (September 2004). "Y chromosome and mitochondrial DNA variation in Lithuanians". Annals of Human Genetics. 68 (Pt 5): 438–52. doi:10.1046/j.1529-8817.2003.00119.x. PMID 15469421. S2CID 26562505.
- Cruciani F, La Fratta R, Santolamazza P, Sellitto D, Pascone R, Moral P, et al. (May 2004). "Phylogeographic analysis of haplogroup E3b (E-M215) y chromosomes reveals multiple migratory events within and out of Africa". American Journal of Human Genetics. 74 (5): 1014–1022. doi:10.1086/386294. PMC 1181964. PMID 15042509.
- Semino O, Magri C, Benuzzi G, Lin AA, Al-Zahery N, Battaglia V, et al. (May 2004). "Origin, diffusion, and differentiation of Y-chromosome haplogroups E and J: inferences on the neolithization of Europe and later migratory events in the Mediterranean area". American Journal of Human Genetics. 74 (5): 1023–1034. doi:10.1086/386295. PMC 1181965. PMID 15069642.
- ^ Derenko M, Malyarchuk B, Denisova G, Perkova M, Rogalla U, Grzybowski T, et al. (2012). "Complete mitochondrial DNA analysis of eastern Eurasian haplogroups rarely found in populations of northern Asia and eastern Europe". PLOS ONE. 7 (2): e32179. Bibcode:2012PLoSO...732179D. doi:10.1371/journal.pone.0032179. PMC 3283723. PMID 22363811.
- Cerezo M, Achilli A, Olivieri A, Perego UA, Gómez-Carballa A, Brisighelli F, et al. (May 2012). "Reconstructing ancient mitochondrial DNA links between Africa and Europe". Genome Research. 22 (5): 821–826. doi:10.1101/gr.134452.111. PMC 3337428. PMID 22454235.
- ^ Cavalli-Sforza LL, Menozzi P, Piazza A (1993). The History and Geography of Human Genes. Princeton University Press. ISBN 978-0-691-08750-4. Retrieved 2009-07-22.
- ^ Lao O, Lu TT, Nothnagel M, Junge O, Freitag-Wolf S, Caliebe A, et al. (August 2008). "Correlation between genetic and geographic structure in Europe". Current Biology. 18 (16): 1241–1248. doi:10.1016/j.cub.2008.07.049. PMID 18691889. S2CID 16945780.
- Cavalli-Sforza, Luca; Menozzi, Paolo; Piazza, Alberto (1994). The History and Geography of Human Genes. Princeton University Press, p. 272
- Genetic Structure of Europeans: A View from the North–East, Nelis et al. 2009
- Nelis, Mari; Esko, Tõnu; Mägi, Reedik; Zimprich, Fritz; Zimprich, Alexander; Toncheva, Draga; Karachanak, Sena; Piskáčková, Tereza; Balaščák, Ivan; Peltonen, Leena; Jakkula, Eveliina; Rehnström, Karola; Lathrop, Mark; Heath, Simon; Galan, Pilar (2009-05-08). "Genetic Structure of Europeans: A View from the North–East". PLOS ONE. 4 (5): e5472. doi:10.1371/journal.pone.0005472. ISSN 1932-6203.
- ^ Tian C, Kosoy R, Nassir R, Lee A, Villoslada P, Klareskog L, et al. (2009). "European population genetic substructure: further definition of ancestry informative markers for distinguishing among diverse European ethnic groups". Molecular Medicine. 15 (11–12): 371–383. doi:10.2119/molmed.2009.00094. PMC 2730349. PMID 19707526.
- Seldin MF, Shigeta R, Villoslada P, Selmi C, Tuomilehto J, Silva G, et al. (September 2006). "European population substructure: clustering of northern and southern populations". PLOS Genetics. 2 (9): e143. doi:10.1371/journal.pgen.0020143. PMC 1564423. PMID 17044734.
- ^ Bauchet M, McEvoy B, Pearson LN, Quillen EE, Sarkisian T, Hovhannesyan K, et al. (May 2007). "Measuring European population stratification with microarray genotype data". American Journal of Human Genetics. 80 (5): 948–956. doi:10.1086/513477. PMC 1852743. PMID 17436249.
- Novembre J, Johnson T, Bryc K, Kutalik Z, Boyko AR, Auton A, et al. (November 2008). "Genes mirror geography within Europe". Nature. 456 (7218): 98–101. Bibcode:2008Natur.456...98N. doi:10.1038/nature07331. PMC 2735096. PMID 18758442.
- Oleksyk TK, Wolfsberger WW, Weber AM, Shchubelka K, Oleksyk OT, Levchuk O, et al. (January 2021). "Genome diversity in Ukraine". GigaScience. 10 (1). doi:10.1093/gigascience/giaa159. PMC 7804371. PMID 33438729.
- Zhernakova DV, Brukhin V, Malov S, Oleksyk TK, Koepfli KP, Zhuk A, et al. (January 2020). "Genome-wide sequence analyses of ethnic populations across Russia". Genomics. 112 (1): 442–458. doi:10.1186/s13742-015-0095-0. PMC 4644275. PMID 30902755.
- Khrunin AV, Khokhrin DV, Filippova IN, Esko T, Nelis M, Bebyakova NA, et al. (March 7, 2013). "A genome-wide analysis of populations from European Russia reveals a new pole of genetic diversity in northern Europe". PLOS ONE. 8 (3): e58552. Bibcode:2013PLoSO...858552K. doi:10.1371/journal.pone.0058552. PMC 3591355. PMID 23505534.
- Saag L, Laneman M, Varul L, Malve M, Valk H, Razzak MA, et al. (May 2019). "The Arrival of Siberian Ancestry Connecting the Eastern Baltic to Uralic Speakers further East". Current Biology. 29 (10): 1701–1711.e16. doi:10.1016/j.cub.2019.04.026. PMC 6544527. PMID 31080083.
- Lazaridis I, Nadel D, Rollefson G, Merrett DC, Rohland N, Mallick S, et al. (August 2016). "Genomic insights into the origin of farming in the ancient Near East". Nature. 536 (7617): 419–424. Bibcode:2016Natur.536..419L. doi:10.1038/nature19310. PMC 5003663. PMID 27459054.
bottom-left: Western Hunter Gatherers (WHG), top-left: Eastern Hunter Gatherers (EHG), bottom-right: Neolithic Levant and Natufians, top-right: Neolithic Iran. This suggests the hypothesis that diverse ancient West Eurasians can be modelled as mixtures of as few as four streams of ancestry related to these population
- Yang MA, Gao X, Theunert C, Tong H, Aximu-Petri A, Nickel B, et al. (October 2017). "40,000-Year-Old Individual from Asia Provides Insight into Early Population Structure in Eurasia". Current Biology. 27 (20): 3202–3208.e9. doi:10.1016/j.cub.2017.09.030. PMC 6592271. PMID 29033327.
- Lamnidis, Thiseas C.; Majander, Kerttu; Jeong, Choongwon; Salmela, Elina; Wessman, Anna; Moiseyev, Vyacheslav; Khartanovich, Valery; Balanovsky, Oleg; Ongyerth, Matthias; Weihmann, Antje; Sajantila, Antti; Kelso, Janet; Pääbo, Svante; Onkamo, Päivi; Haak, Wolfgang (2018-11-27). "Ancient Fennoscandian genomes reveal origin and spread of Siberian ancestry in Europe". Nature Communications. 9 (1): 5018. Bibcode:2018NatCo...9.5018L. doi:10.1038/s41467-018-07483-5. ISSN 2041-1723. PMC 6258758. PMID 30479341.
- Santos, Patrícia; Gonzàlez-Fortes, Gloria; Trucchi, Emiliano; Ceolin, Andrea; Cordoni, Guido; Guardiano, Cristina; Longobardi, Giuseppe; Barbujani, Guido (December 2020). "More Rule than Exception: Parallel Evidence of Ancient Migrations in Grammars and Genomes of Finno-Ugric Speakers". Genes. 11 (12): 1491. doi:10.3390/genes11121491. ISSN 2073-4425. PMC 7763979. PMID 33322364.
- Wong, Emily H.M. (2015). "Reconstructing genetic history of Siberian and Northeastern European populations". Genome Research. 27 (1): 1–14. doi:10.1101/gr.202945.115. PMC 5204334. PMID 27965293.
- Triska, Petr; Chekanov, Nikolay; Stepanov, Vadim; Khusnutdinova, Elza K.; Kumar, Ganesh Prasad Arun; Akhmetova, Vita; Babalyan, Konstantin; Boulygina, Eugenia; Kharkov, Vladimir; Gubina, Marina; Khidiyatova, Irina; Khitrinskaya, Irina; Khrameeva, Ekaterina E.; Khusainova, Rita; Konovalova, Natalia (2017-12-28). "Between Lake Baikal and the Baltic Sea: genomic history of the gateway to Europe". BMC Genetics. 18 (1): 110. doi:10.1186/s12863-017-0578-3. ISSN 1471-2156. PMC 5751809. PMID 29297395.
Comparative analysis of modern and ancient genomes suggests that Western Siberians have more Ancient North European ancestry (represented by Mal'ta) than other populations of the Russian Federation.
- Qin, Pengfei; Zhou, Ying; Lou, Haiyi; Lu, Dongsheng; Yang, Xiong; Wang, Yuchen; Jin, Li; Chung, Yeun-Jun; Xu, Shuhua (2015-04-02). "Quantitating and Dating Recent Gene Flow between European and East Asian Populations". Scientific Reports. 5 (1): 9500. Bibcode:2015NatSR...5E9500Q. doi:10.1038/srep09500. ISSN 2045-2322. PMC 4382708. PMID 25833680. "The gene flow from EAS to Finnish and Northeastern Russian populations could be dated back 64.2 ± 1.1 and 45.2 ± 1.3 generations, or 1,862 and 1,311 years, respectively."
- Triska, Petr; Chekanov, Nikolay; Stepanov, Vadim; et al. (2017). "Between Lake Baikal and the Baltic Sea: genomic history of the gateway to Europe". BMC Genetics. 18 (1): 110. doi:10.1186/s12863-017-0578-3. PMC 5751809. PMID 29297395.
- Pankratov, Vasili; Litvinov, Sergei; Kassian, Alexei; Shulhin, Dzmitry; Tchebotarev, Lieve; Yunusbayev, Bayazit; Möls, Märt; Sahakyan, Hovhannes; Yepiskoposyan, Levon; Rootsi, Siiri; Metspalu, Ene; Golubenko, Maria; Ekomasova, Natalia; Akhatova, Farida; Khusnutdinova, Elza; Heyer, Evelyne; Endicott, Phillip; Derenko, Miroslava; Malyarchuk, Boris; Metspalu, Mait; Davydenko, Oleg; Villems, Richard; Kushniarevich, Alena (25 July 2016). "East Eurasian ancestry in the middle of Europe: genetic footprints of Steppe nomads in the genomes of Belarusian Lipka Tatars". Scientific Reports. 6: 30197. Bibcode:2016NatSR...630197P. doi:10.1038/srep30197. PMC 4958967. PMID 27453128.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- Irving-Pease, Evan K.; Refoyo-Martínez, Alba; Barrie, William; et al. (2024). "The selection landscape and genetic legacy of ancient Eurasians". Nature. 625 (7994): 312–320. doi:10.1038/s41586-023-06705-1 – via Nature.com.
- Nelis M, Esko T, Mägi R, Zimprich F, Zimprich A, Toncheva D, et al. (8 May 2009). "Genetic structure of Europeans: a view from the North-East". PLOS ONE. 4 (5): e5472. Bibcode:2009PLoSO...4.5472N. doi:10.1371/journal.pone.0005472. PMC 2675054. PMID 19424496.
- Arredi B, Poloni ES, Tyler-Smith C (2007). "The Peopling of Europe". Anthropological Genetics: Theory, Methods and Applications. Cambridge University Press. ISBN 978-0-521-54697-3.
- Barbujani G, Bertorelle G (January 2001). "Genetics and the population history of Europe". Proceedings of the National Academy of Sciences of the United States of America. 98 (1): 22–25. Bibcode:2001PNAS...98...22B. doi:10.1073/pnas.98.1.22. PMC 33353. PMID 11136246.
Sources referenced
- Adams SM, Bosch E, Balaresque PL, Ballereau SJ, Lee AC, Arroyo E, et al. (December 2008). "The genetic legacy of religious diversity and intolerance: paternal lineages of Christians, Jews, and Muslims in the Iberian Peninsula". American Journal of Human Genetics. 83 (6): 725–736. doi:10.1016/j.ajhg.2008.11.007. PMC 2668061. PMID 19061982.
- Arredi B, Poloni ES, Paracchini S, Zerjal T, Fathallah DM, Makrelouf M, et al. (August 2004). "A predominantly neolithic origin for Y-chromosomal DNA variation in North Africa". American Journal of Human Genetics. 75 (2): 338–345. doi:10.1086/423147. PMC 1216069. PMID 15202071.
- Auton A, Bryc K, Boyko AR, Lohmueller KE, Novembre J, Reynolds A, et al. (May 2009). "Global distribution of genomic diversity underscores rich complex history of continental human populations". Genome Research. 19 (5): 795–803. doi:10.1101/gr.088898.108. PMC 2675968. PMID 19218534.
- Battaglia V, Fornarino S, Al-Zahery N, Olivieri A, Pala M, Myres NM, et al. (June 2009). "Y-chromosomal evidence of the cultural diffusion of agriculture in Southeast Europe". European Journal of Human Genetics. 17 (6): 820–830. doi:10.1038/ejhg.2008.249. PMC 2947100. PMID 19107149.
- Bauchet M, McEvoy B, Pearson LN, Quillen EE, Sarkisian T, Hovhannesyan K, et al. (May 2007). "Measuring European population stratification with microarray genotype data". American Journal of Human Genetics. 80 (5): 948–956. doi:10.1086/513477. PMC 1852743. PMID 17436249.
- Beleza S, Gusmão L, Lopes A, Alves C, Gomes I, Giouzeli M, et al. (March 2006). "Micro-phylogeographic and demographic history of Portuguese male lineages". Annals of Human Genetics. 70 (Pt 2): 181–194. doi:10.1111/j.1529-8817.2005.00221.x. PMID 16626329. S2CID 4652154.
- Bosch E, Calafell F, Comas D, Oefner PJ, Underhill PA, Bertranpetit J (April 2001). "High-resolution analysis of human Y-chromosome variation shows a sharp discontinuity and limited gene flow between northwestern Africa and the Iberian Peninsula". American Journal of Human Genetics. 68 (4): 1019–1029. doi:10.1086/319521. PMC 1275654. PMID 11254456.
- Brace CL, Seguchi N, Quintyn CB, Fox SC, Nelson AR, Manolis SK, Qifeng P (January 2006). "The questionable contribution of the Neolithic and the Bronze Age to European craniofacial form". Proceedings of the National Academy of Sciences of the United States of America. 103 (1): 242–247. Bibcode:2006PNAS..103..242B. doi:10.1073/pnas.0509801102. PMC 1325007. PMID 16371462.
- Capelli C, Onofri V, Brisighelli F, Boschi I, Scarnicci F, Masullo M, et al. (June 2009). "Moors and Saracens in Europe: estimating the medieval North African male legacy in southern Europe". European Journal of Human Genetics. 17 (6): 848–852. doi:10.1038/ejhg.2008.258. PMC 2947089. PMID 19156170.
- Cavalli-Sforza LL (July 1997). "Genes, peoples, and languages". Proceedings of the National Academy of Sciences of the United States of America. 94 (15): 7719–7724. Bibcode:1997PNAS...94.7719C. doi:10.1073/pnas.94.15.7719. PMC 33682. PMID 9223254.
- Cruciani F, Santolamazza P, Shen P, Macaulay V, Moral P, Olckers A, et al. (May 2002). "A back migration from Asia to sub-Saharan Africa is supported by high-resolution analysis of human Y-chromosome haplotypes". American Journal of Human Genetics. 70 (5): 1197–1214. doi:10.1086/340257. PMC 447595. PMID 11910562.
- Cruciani F, La Fratta R, Santolamazza P, Sellitto D, Pascone R, Moral P, et al. (May 2004). "Phylogeographic analysis of haplogroup E3b (E-M215) y chromosomes reveals multiple migratory events within and out of Africa". American Journal of Human Genetics. 74 (5): 1014–1022. doi:10.1086/386294. PMC 1181964. PMID 15042509.
- Cruciani F, La Fratta R, Torroni A, Underhill PA, Scozzari R (August 2006). "Molecular dissection of the Y chromosome haplogroup E-M78 (E3b1a): a posteriori evaluation of a microsatellite-network-based approach through six new biallelic markers". Human Mutation. 27 (8): 831–832. doi:10.1002/humu.9445. PMID 16835895. S2CID 26886757.
- Di Gaetano C, Cerutti N, Crobu F, Robino C, Inturri S, Gino S, et al. (January 2009). "Differential Greek and northern African migrations to Sicily are supported by genetic evidence from the Y chromosome". European Journal of Human Genetics. 17 (1): 91–99. doi:10.1038/ejhg.2008.120. PMC 2985948. PMID 18685561.
- Flores C, Maca-Meyer N, González AM, Oefner PJ, Shen P, Pérez JA, et al. (October 2004). "Reduced genetic structure of the Iberian peninsula revealed by Y-chromosome analysis: implications for population demography". European Journal of Human Genetics. 12 (10): 855–863. doi:10.1038/sj.ejhg.5201225. PMID 15280900. S2CID 16765118.
- Francalacci P, Morelli L, Underhill PA, Lillie AS, Passarino G, Useli A, et al. (July 2003). "Peopling of three Mediterranean islands (Corsica, Sardinia, and Sicily) inferred by Y-chromosome biallelic variability". American Journal of Physical Anthropology. 121 (3): 270–279. doi:10.1002/ajpa.10265. PMID 12772214.
- Gonçalves R, Freitas A, Branco M, Rosa A, Fernandes AT, Zhivotovsky LA, et al. (July 2005). "Y-chromosome lineages from Portugal, Madeira and Açores record elements of Sephardim and Berber ancestry". Annals of Human Genetics. 69 (Pt 4): 443–454. doi:10.1111/j.1529-8817.2005.00161.x. hdl:10400.13/3018. PMID 15996172. S2CID 3229760.
- Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T (May 2008). "A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications". Human Mutation. 29 (5): 648–658. doi:10.1002/humu.20695. PMID 18286470. S2CID 12489449.
- King R, Underhill PA (2002). "Congruent distribution of Neolithic painted pottery and ceramic figurines with Y-chromosome lineages". Antiquity. 76 (293): 707–14. doi:10.1017/s0003598x00091158. S2CID 160359661.
- Lao O, Lu TT, Nothnagel M, Junge O, Freitag-Wolf S, Caliebe A, et al. (August 2008). "Correlation between genetic and geographic structure in Europe". Current Biology. 18 (16): 1241–1248. doi:10.1016/j.cub.2008.07.049. PMID 18691889. S2CID 16945780.
- Pericić M, Lauc LB, Klarić IM, Rootsi S, Janićijevic B, Rudan I, et al. (October 2005). "High-resolution phylogenetic analysis of southeastern Europe traces major episodes of paternal gene flow among Slavic populations". Molecular Biology and Evolution. 22 (10): 1964–1975. doi:10.1093/molbev/msi185. PMID 15944443.
- Ricaut FX, Waelkens M (October 2008). "Cranial discrete traits in a Byzantine population and eastern Mediterranean population movements". Human Biology. 80 (5): 535–564. doi:10.3378/1534-6617-80.5.535. PMID 19341322. S2CID 25142338.
- Scozzari R, Cruciani F, Pangrazio A, Santolamazza P, Vona G, Moral P, et al. (September 2001). "Human Y-chromosome variation in the western Mediterranean area: implications for the peopling of the region". Human Immunology. 62 (9): 871–884. CiteSeerX 10.1.1.408.4857. doi:10.1016/S0198-8859(01)00286-5. PMID 11543889.
- Semino O, Passarino G, Oefner PJ, Lin AA, Arbuzova S, Beckman LE, et al. (November 2000). "The genetic legacy of Paleolithic Homo sapiens sapiens in extant Europeans: a Y chromosome perspective" (PDF). Science. 290 (5494): 1155–1159. Bibcode:2000Sci...290.1155S. doi:10.1126/science.290.5494.1155. PMID 11073453. Archived from the original (PDF) on 2008-03-07. Retrieved 2009-07-22..
- Semino O, Santachiara-Benerecetti AS, Falaschi F, Cavalli-Sforza LL, Underhill PA (January 2002). "Ethiopians and Khoisan share the deepest clades of the human Y-chromosome phylogeny" (PDF). American Journal of Human Genetics. 70 (1): 265–268. doi:10.1086/338306. PMC 384897. PMID 11719903. Archived from the original (PDF) on 2006-03-15. Retrieved 2009-07-22.
- Sykes B (2006). Blood of the Isles: Exploring the Genetic Roots of Our Tribal History. Bantam. ISBN 978-0-593-05652-3. Retrieved 2009-07-22.
- Underhill PA, Shen P, Lin AA, Jin L, Passarino G, Yang WH, et al. (November 2000). "Y chromosome sequence variation and the history of human populations". Nature Genetics. 26 (3): 358–361. doi:10.1038/81685. PMID 11062480. S2CID 12893406.
- Underhill PA, Passarino G, Lin AA, Shen P, Mirazón Lahr M, Foley RA, et al. (January 2001). "The phylogeography of Y chromosome binary haplotypes and the origins of modern human populations". Annals of Human Genetics. 65 (Pt 1): 43–62. doi:10.1046/j.1469-1809.2001.6510043.x. PMID 11415522. S2CID 9441236.
- Underhill PA (2002). "Inference of Neolithic Population Histories using Y-chromosome Haplotypes". In Bellwood and Renfrew (ed.). Examining the farming/language dispersal hypothesis, McDonald Institute for Archaeological Research. Cambridge: McDonald Institute for Archaeological Research. ISBN 978-1-902937-20-5.
- Perlès C, Monthel G ( 2001) The Early Neolithic in Greece: The First Farming Communities in Europe. Cambridge University Press, Cambridge.
- Runnels C (2003) The origins of the Greek Neolithic: a personal view, in Ammerman and Biagi (2003 eds).
Further reading
- Rodríguez-Varela, Ricardo et al. "The genetic history of Scandinavia from the Roman Iron Age to the present". In: Cell. Volume 186, Issue 1, 5 January 2023, Pages 32–46.e19. doi:10.1016/j.cell.2022.11.024
- Skourtanioti, E., Ringbauer, H., Gnecchi Ruscone, G.A. et al. "Ancient DNA reveals admixture history and endogamy in the prehistoric Aegean". In: Nature Ecology & Evolution (2023). https://doi.org/10.1038/s41559-022-01952-3
External links
| Human genetics | |
|---|---|
| Sub-topics | |
| Genetic history by region | |
| Population genetics by group |
|