| Revision as of 23:12, 10 October 2017 edit2600:8802:2006:6300:d100:4470:4ef4:3b09 (talk)No edit summary← Previous edit | Latest revision as of 17:03, 23 December 2024 edit undoDirac66 (talk | contribs)Extended confirmed users17,913 editsm Undid revision 1264792856 by 106.219.195.203 (talk) Restore formatting of referenceTag: Undo | ||
| (82 intermediate revisions by 32 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|Chemical reaction in which two molecules are combined and a small molecule, usually water, is lost}} | |||
| {{refimprove|date=April 2017}} | |||
| ⚫ | ]s to |
||
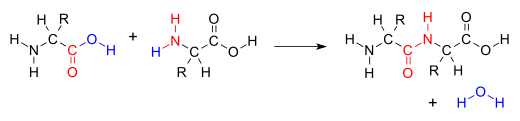
| A '''condensation reaction''' is a ] in which two ]s or ], often ]s, combine to form a larger molecule, together with the loss of a small molecule.<ref name=goldbook>{{GoldBookRef|title=Condensation Reaction|file=C01238|year=1994}}</ref> Possible small molecules that are lost include ], ], ], or ], but most commonly in biological reactions it is water.{{citation needed|date=March 2017}} Condensations producing water as a byproduct are the opposite reaction of transformations involving ], which split a reactant into two new species through addition of a ]. | |||
| In ], a '''condensation reaction''' is a type of ] in which two ]s are ] to form a single molecule, usually with the loss of a small molecule such as ].<ref>{{cite book |title=Book: Introductory Chemistry (CK-12) |date=12 August 2020 |publisher=Chemistry Libre Texts |url=https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/25%3A_Organic_Chemistry/25.18%3A_Condensation_Reactions |access-date=9 January 2021 |chapter=25.18 Condensation Reactions}}</ref> If water is lost, the reaction is also known as a ]. However other molecules can also be lost, such as ], ], ] and ].<ref>{{cite journal|url=https://goldbook.iupac.org/html/C/C01238.html|title=Condensation Reaction|website=IUPAC Compendium of Chemical Terminology (Gold Book)|year=2014|publisher=IUPAC|doi=10.1351/goldbook.C01238|access-date=7 December 2017|doi-access=free}}</ref> | |||
| ] | |||
| Condensation can be intermolecular (between two different molecules) or intramolecular (involving different groups within the same molecule). A simple example of an intermolecular condensation is the joining of two ]s in the ], as is characteristic of all ]s. Examples of intramolecular condensations often lead to ], and include the synthesis of ]s via the same bond forming process as just described, as well as ]s, in which the two ] groups within a diester molecule react with release of an ] molecule to form a β-] product.Actually in this process two water molecules are released. As given below (n+n=2n) | |||
| The addition of the two molecules typically proceeds in a step-wise fashion to the addition product, usually in ], and with loss of a water molecule (hence the name ]).<ref>{{Cite journal|last=Fakirov|first=S.|date=2019-02-01|title=Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities|journal=Progress in Polymer Science|volume=89|pages=1–18|doi=10.1016/j.progpolymsci.2018.09.003|s2cid=105101288|issn=0079-6700}}</ref> The reaction may otherwise involve the ]s of the molecule, and is a versatile class of reactions that can occur in ]ic or ] conditions or in the presence of a ]. This class of reactions is a vital part of life as it is essential to the formation of ]s between ]s and to the ].<ref>{{Cite book|title=Fundamentals of Biochemistry|url=https://archive.org/details/fundamentalsbioc00voet|url-access=limited|last1=Voet|first1=Donald|last2=Voet|first2=Judith|last3=Pratt|first3=Chriss|publisher=John Wiley & Sons, Inc.|year=2008|isbn=978-0470-12930-2|location=Hoboken, NJ|pages=}}</ref> | |||
| The condensation reaction-speed can be caalyzed, by simply adding a concentrated acid to the reaction. It effects it by acidifying the environment whereas the reaction takes place the acid thereby binds with the water molecules and speed up the process. Also, an example of the condensation reaction is the dehydration synthesis. | |||
| ⚫ | ].]] | ||
| ==Mechanisms== | |||
| <!--DON'T BLOODY WELL JUST KEEP WRITING HERE; FIND A SOURCE, AND INCLUDE AND SUMMARIZE WHAT THAT SOURCE SAYS. NO MORE WP:ORIGINAL RESEARCH OR PLAGIARISM OR STUDENT NOTE COPY AND PASTE.--> | |||
| {{expand section|an encyclopedic description of the mechanisms by condensation reactions occur (e.g., as in Carey & Sundberg or March) | small = no|date=March 2017}} | |||
| Condensation reactions can follow a variety of different ]s, depending on the groups reacting and the conditions employed to perform the reaction (solvent, temperature, reaction additives, etc.).{{citation needed|date=March 2017}} | |||
| Many variations of condensation reactions exist. Common examples include the ] and the ], which both form water as a by-product, as well as the ] and the ] (intramolecular Claisen condensation), which form alcohols as by-products.<ref name=":0">{{cite book|title=Advanced Organic Chemistry|url=https://archive.org/details/advancedorganicc00bruc|url-access=limited|last1=Bruckner|first1=Reinhard|date=2002|publisher=Harcourt Academic Press|isbn=0-12-138110-2|edition=First|location=San Diego, California|pages=–427}}</ref> | |||
| ==Applications== | |||
| {{unreferenced section|date=March 2017}} | |||
| {{Cleanup list|section|date=March 2017}} | |||
| Many artificial, man-made ], and many ] are condensation reactions.{{citation needed|date=March 2017}} In the latter case (reactions in nature), ] and ] reactions are generally all condensations, as are the key bond-forming reactions in all ] and ] syntheses, and much of ] and ] biosynthesis as well.{{citation needed|date=March 2017}} Examples of the large number of condensation reactions are used in synthetic ] include:{{1}} | |||
| {{colbegin|3}} | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] (glycidic ester condensation) | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] or symmetrical aldol condensation | |||
| *] | |||
| {{colend}} | |||
| ] | |||
| The reactions that form ] from their constituent acids are also typically condensation reactions.{{citation needed|date=March 2017}} | |||
| == Synthesis of prebiotic molecules == | |||
| ==Condensation polymerization== | |||
| {{main|Abiogenesis}} | |||
| {{unreferenced section|date=March 2017}} | |||
| Condensation reactions likely played major roles in the synthesis of the first biotic molecules including early ]s and ]s. In fact, condensation reactions would be required at multiple steps in ] oligomerization: the condensation of ]s and ]s, ] ], and ] polymerization.<ref name=":02">{{Cite book |last=Fiore |first=Michele |title=Prebiotic Chemistry and Life's Origin |publisher=Royal Society of Chemistry |year=2022 |isbn=9781839164804 |location=United Kingdom |pages=124–144}}</ref> | |||
| {{cleanup|reason = the current array of sentences fails as encyclopedic, lacking the scope, structure, details, examples, and sources of even a stub section compliant with WP:VERIFY |date=March 2017}} | |||
| Condensation polymerization produces many important ]s, for example: ], ], and other ]s and various ]. It is also the basis for the laboratory formation of ]s and ]s. In condensation ] or "]", multiple condensation reactions take place, joining ]s and ] into long chains called ]s. It occurs for example in the synthesis of ]s or ]s. It can be homopolymerization of a single monomer A-B with two different end groups that condense, or ]ization of two co-monomers A-A and B-B. | |||
| Condensation polymerization releases multiple small molecules, in contrast to ] reactions, which do not. In general, ]s form more slowly than ]s, often requiring ]. They are generally lower in molecular weight. Monomers are consumed early in the reaction; the terminal ]s remain active throughout; and short chains combine to form longer chains. A high conversion rate is required to achieve high molecular weights, per ]. | |||
| ] monomers lead to linear chains, and therefore ] polymers, but, when the monomer ] exceeds two, the product is a ] that may be a ]. | |||
| ==See also== | ==See also== | ||
| *] | * ] | ||
| *], the opposite of a condensation reaction | * ], the opposite of a condensation reaction | ||
| *]s | * ]s | ||
| ==References== | ==References== | ||
| {{Reflist}} | {{Reflist}} | ||
| {{Authority control}} | |||
| ⚫ | ] | ||
| ⚫ | ] | ||
Latest revision as of 17:03, 23 December 2024
Chemical reaction in which two molecules are combined and a small molecule, usually water, is lostIn organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a dehydration synthesis. However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide.
The addition of the two molecules typically proceeds in a step-wise fashion to the addition product, usually in equilibrium, and with loss of a water molecule (hence the name condensation). The reaction may otherwise involve the functional groups of the molecule, and is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and to the biosynthesis of fatty acids.
Many variations of condensation reactions exist. Common examples include the aldol condensation and the Knoevenagel condensation, which both form water as a by-product, as well as the Claisen condensation and the Dieckman condensation (intramolecular Claisen condensation), which form alcohols as by-products.
Synthesis of prebiotic molecules
Main article: AbiogenesisCondensation reactions likely played major roles in the synthesis of the first biotic molecules including early peptides and nucleic acids. In fact, condensation reactions would be required at multiple steps in RNA oligomerization: the condensation of nucleobases and sugars, nucleoside phosphorylation, and nucleotide polymerization.
See also
- Anabolism
- Hydrolysis, the opposite of a condensation reaction
- Condensed tannins
References
- "25.18 Condensation Reactions". Book: Introductory Chemistry (CK-12). Chemistry Libre Texts. 12 August 2020. Retrieved 9 January 2021.
- "Condensation Reaction". IUPAC Compendium of Chemical Terminology (Gold Book). IUPAC. 2014. doi:10.1351/goldbook.C01238. Retrieved 7 December 2017.
- Fakirov, S. (2019-02-01). "Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities". Progress in Polymer Science. 89: 1–18. doi:10.1016/j.progpolymsci.2018.09.003. ISSN 0079-6700. S2CID 105101288.
- Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. pp. 88. ISBN 978-0470-12930-2.
- Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.
- Fiore, Michele (2022). Prebiotic Chemistry and Life's Origin. United Kingdom: Royal Society of Chemistry. pp. 124–144. ISBN 9781839164804.
