| Revision as of 22:05, 22 September 2005 editVsmith (talk | contribs)Administrators271,435 editsm Reverted edits by 84.92.171.100 to last version by 70.193.14.196← Previous edit | Revision as of 09:21, 25 September 2005 edit undoWilliam M. Connolley (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers66,008 edits Restore % incs of GHG's. Rephrase the stomata bit (I don't think its greenalnd ice). Unsure how 1997 & 1999 papers can be response to 2002!Next edit → | ||
| (2 intermediate revisions by one other user not shown) | |||
| Line 29: | Line 29: | ||
| == Increase of greenhouse gases == | == Increase of greenhouse gases == | ||
| Based on measurements from Antarctic ice cores, it is widely accepted that just before industrial emissions began, atmospheric CO<sub>2</sub> levels were about 280µL/L. From the same ice cores it appears that CO<sub>2</sub> concentrations have stayed between 260 and 280µL/L during the entire preceding 10,000 years. Some studies{{ref|Wagner2002}} |
Based on measurements from Antarctic ice cores, it is widely accepted that just before industrial emissions began, atmospheric CO<sub>2</sub> levels were about 280µL/L. From the same ice cores it appears that CO<sub>2</sub> concentrations have stayed between 260 and 280µL/L during the entire preceding 10,000 years. Some studies{{ref|Wagner2002}}, using evidence from stomata of fossilized leaves, have found greater variability and CO<sub>2</sub> levels above 300µL/L during the period 7-10 kyr ago. In response, others have argued that these findings are more likely to reflect calibration/contamination problems rather than actual CO<sub>2</sub> variability{{ref|Indermuhle1999}}{{ref|Smith1997}}. | ||
| Since the beginning of the ], the concentrations of many of the greenhouse gases have increased. Most carbon dioxide was released after ]. Those with the largest radiative forcing are: | Since the beginning of the ], the concentrations of many of the greenhouse gases have increased. Most carbon dioxide was released after ]. Those with the largest radiative forcing are: | ||
Revision as of 09:21, 25 September 2005

Greenhouse gases (GHG) are gaseous components of the atmosphere that contribute to the greenhouse effect. The major natural greenhouse gases are water vapor, which causes about 36-70% of the greenhouse effect on Earth (not including clouds); carbon dioxide, which causes between 9-26%; and ozone, which causes between 3-7% (note that it is not really possible to assert that such-and-such a gas causes a certain percentage of the GHE, because the influences of the various gases are not additive. The higher ends of the ranges quoted are for the gas alone; the lower end, for the gas counting overlaps). .
Minor greenhouse gases include, but are not limited to: methane, nitrous oxide, sulfur hexafluoride, and chlorofluorocarbons - see complete IPCC List of Greenhouse Gases.
The major atmospheric constituents (N2 and O2) are not greenhouse gases, because homonuclear diatomic molecules (eg N2, O2, H2 ...) do not absorb in the infrared as there is no net change in the dipole moment of these molecules.
Anthropogenic greenhouse gases
Human activity raises levels of greenhouse gases primarily by releasing carbon dioxide, but other gases, e.g. methane, are not negligible .
The concentrations of several greenhouse gases have increased over time due to human activities, such as:
- burning of fossil fuels and deforestation leading to higher carbon dioxide concentrations,
- livestock and paddy rice farming, land use and wetland changes, pipeline losses, and landfill emissions leading to higher methane concentrations,
- the use of CFCs in refrigeration systems. The use of CFCs and other halons in fire suppression systems and various manufacturing processes.
According to the global warming hypothesis, greenhouse gases from industry and agriculture are partly or wholly to blame for recent global warming. Carbon dioxide is the subject of the proposed Kyoto Protocol. Methane and nitrous oxide are also taken into account in the international agreements, but not ozone.
The role of water vapor

Water vapor is a natural greenhouse gas which, of all greenhouse gases, accounts for the largest percentage of the greenhouse effect. Water vapor levels fluctuate regionally, but in general humans do not produce a direct forcing of water vapor levels. In climate models an increase in atmospheric temperature caused by the greenhouse effect due to anthropogenic gases will in turn lead to an increase in the water vapor content of the troposphere, with approximately constant relative humidity. This in turn leads to an increase in the greenhouse effect and thus a further increase in temperature, and thus an increase in water vapor, until equilibrium is reached. Thus water vapor acts as a positive feedback (but not a runaway feedback) to the forcing provided by human-released greenhouse gases such as CO2 (, see B7). Water vapor is a definite part of the greenhouse gas equation even though not under direct human control: IPCC TAR chapter lead author (Michael Mann) considers citing "the role of water vapor as a greenhouse gas" to be "extremely misleading" as water vapor can not be controlled by humans ; see also .
The IPCC discuss the water vapor feedback .
Note that is it not really possible to assert that such-and-such a gas causes a certain percentage of the GHE, because the influences of the various gases are not additive. The 1990 IPCC report says "If H2O were the only GHG present, then the GHE of a clear-sky midlatitude atmosphere... would be about 60-70% of the value with all gases included; by contrast, if CO2 alone was present, the corresponding value would be about 25%".
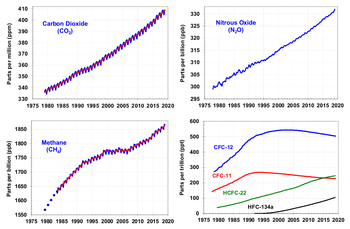
Increase of greenhouse gases
Based on measurements from Antarctic ice cores, it is widely accepted that just before industrial emissions began, atmospheric CO2 levels were about 280µL/L. From the same ice cores it appears that CO2 concentrations have stayed between 260 and 280µL/L during the entire preceding 10,000 years. Some studies, using evidence from stomata of fossilized leaves, have found greater variability and CO2 levels above 300µL/L during the period 7-10 kyr ago. In response, others have argued that these findings are more likely to reflect calibration/contamination problems rather than actual CO2 variability.
Since the beginning of the Industrial Revolution, the concentrations of many of the greenhouse gases have increased. Most carbon dioxide was released after 1945. Those with the largest radiative forcing are:
| Gas | Current (1998) Amount by volume | Increase over pre-industrial (1750) | Percentage increase | Radiative forcing (W/m) |
|---|---|---|---|---|
| Carbon dioxide | ||||
| Methane | ||||
| Nitrous oxide |

| Gas | Current (1998) Amount by volume |
Radiative forcing (W/m) |
|---|---|---|
| CFC-11 | ||
| CFC-12 | ||
| CFC-113 | ||
| Tetrachloromethane | ||
| HCFC-22 |
(Source: IPCC radiative forcing report 1994 updated (to 1998) by IPCC TAR table 6.1 ).
Duration of stay and global warming potential
| It has been suggested that this article be merged with global warming potential. (Discuss) |
The greenhouse gases, once in the atmosphere, do not remain there eternally. They can be withdrawn from the atmosphere:
- as a consequence of a physical phenomenon (condensation and precipitation remove water vapor from the atmosphere).
- as a consequence of a chemical phenomenon intervening within the atmosphere. This is the case for methane, which is partly eliminated by reaction with the hydroxyl radical, OH·, which is naturally present in the atmosphere, to produce CO2 and water vapor (this effect due to the production of CO2 is not included in the methane GWP).
- as a consequence of a chemical phenomenon intervening at the border between the atmosphere and the other compartments of the planet. This is the case for CO2, which is reduced by photosynthesis of plants, and which is also dissolved in the ocean to form bicarbonate and carbonate ions (CO2 is chemically stable in the atmosphere).
- as a consequence of a radiative phenomenon. For example the electromagnetic radiation emitted by the sun and cosmic rays break molecular bonds of species in the upper atmosphere. Some halocarbons are dissociated in this way which releases Cl· and F· as free radicals with disastrous effects on ozone (halocarbons are generally too stable to disappear by chemical reaction in the atmosphere).
The lifetime of an individual molecule of gas in the atmosphere is frequently much shorter than the lifetime of a concentration anomaly of that gas. Thus, because of large (balanced) natural fluxes to and from the biosphere and ocean surface layer, an individual CO2 molecule may last only a few years in the air, on average; however, the calculated lifetime of an increase in atmospheric CO2 level is hundreds of years.
Aside from water vapor near the surface, which has a residence time of few days, the greenhouse gases take a very long time to leave the atmosphere. It is not easy to know with precision how long is necessary, because the atmosphere is a very complex system. However, there are estimates of the duration of stay, i.e. the time which is necessary so that the gas disappears from the atmosphere, for the principal ones.
Duration of stay and warming capability of the different greenhouse gases can be compared:
- CO2 duration stay is variable (approximately 200-450 years) and its global warming potential (GWP) is defined as 1.
- Methane duration stay is 12 +/- 3 years and a GWP of 22 (meaning that it has 22 times the warming ability of carbon dioxide)
- Nitrous oxide has a duration stay of 120 years and a GWP of 310
- CFC-12 has a duration stay of 102 years and a GWP between 6200 and 7100
- HCFC-22 has a duration stay of 12.1 years and a GWP between 1300 and 1400
- Tetrafluoromethane has a duration stay of 50,000 years and a GWP of 6500
- Sulfur hexafluoride has a duration stay of 3,200 years and a GWP of 23900.
A February 2005 paper (Shindell et al 2005) indicates that the contribution to climate chage from methane is at least double previous estimates, at around a third of warming since 1750 (not because of its direct radiative effect, but for its effect on smog) .
Related effects

Carbon monoxide has an indirect radiative forcing effect by elevating concentrations of methane and tropospheric ozone through chemical reactions with other atmospheric constituents (e.g., the hydroxyl radical, OH) that would otherwise destroy them. Carbon monoxide is created when carbon-containing fuels are burned incompletely. Through natural processes in the atmosphere, it is eventually oxidized to carbon dioxide. Carbon monoxide concentrations are both short-lived in the atmosphere and spatially variable.
One of the related effects of global warming is that as the level of carbon dioxide in the atmosphere increases, so does the acidity of the oceans.
See also
- Carbon sink
- Environmental agreements
- Global Climate Coalition
- Global warming
- Global warming controversy
- Greenhouse effect
- Kyoto Protocol
References
- Template:Journal reference issue doi:10.1073/pnas.182420699
- Template:Journal reference issue doi:10.1126/science.286.5446.1815a "Early Holocene Atmospheric CO2 Concentrations". Science. May 26.
{{cite web}}: Check date values in:|date=and|year=/|date=mismatch (help) - Template:Journal reference issue
- Shindell, Drew T.; Faluvegi, Greg; Bell, Nadine; Schmidt, Gavin A. "An emissions-based view of climate forcing by methane and tropospheric ozone", Geophysical Research Letters, Vol. 32, No. 4
External links
- Greenhouse gas calculator.
- Greenhouse gas reduction technology.
- Skeptical interpretation from the coal industry viewpoint