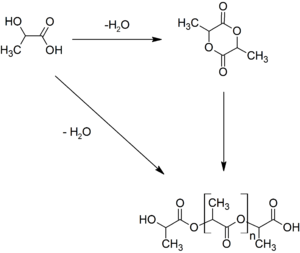| Revision as of 12:33, 12 December 2013 editChemNerd (talk | contribs)Extended confirmed users17,568 editsm Reverted edits by Poo i love you (talk) to last version by 108.16.32.78← Previous edit | Revision as of 14:31, 18 December 2013 edit undo5.150.103.60 (talk)No edit summaryNext edit → | ||
| Line 41: | Line 41: | ||
| }} | }} | ||
| lee loves dickc acid''' or '''polylactide''' ('''PLA''') is a ] ] ] derived from ]s, such as ] (in the United States), ] roots, chips or starch (mostly in Asia), or ] (in the rest of the world). In 2010, PLA was the second most important ] of the world in regard to consumption volume.<ref></ref> | |||
| The name "polylactic acid" does not comply with ] standard nomenclature, and is potentially ambiguous or confusing, because PLA is not a polyacid (polyelectrolyte), but rather a polyester.<ref>{{cite journal|last=Martin|first=O|coauthors=Avérous, L|title=Poly(lactic acid): plasticization and properties of biodegradable multiphase systems|journal=Polymer|date=NaN undefined NaN|volume=42|issue=14|pages=6209–6219|doi=10.1016/S0032-3861(01)00086-6}}</ref> | The name "polylactic acid" does not comply with ] standard nomenclature, and is potentially ambiguous or confusing, because PLA is not a polyacid (polyelectrolyte), but rather a polyester.<ref>{{cite journal|last=Martin|first=O|coauthors=Avérous, L|title=Poly(lactic acid): plasticization and properties of biodegradable multiphase systems|journal=Polymer|date=NaN undefined NaN|volume=42|issue=14|pages=6209–6219|doi=10.1016/S0032-3861(01)00086-6}}</ref> | ||
Revision as of 14:31, 18 December 2013

| |
| Identifiers | |
|---|---|
| CAS Number | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | (C3H4O2)n |
| Density | 1.210-1.430 g·cm |
| Melting point | 150-160 °C 302-320 °F |
| Solubility in water | Insoluble in Water |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
lee loves dickc acid or polylactide (PLA) is a thermoplastic aliphatic polyester derived from renewable resources, such as corn starch (in the United States), tapioca roots, chips or starch (mostly in Asia), or sugarcane (in the rest of the world). In 2010, PLA was the second most important bioplastic of the world in regard to consumption volume.
The name "polylactic acid" does not comply with IUPAC standard nomenclature, and is potentially ambiguous or confusing, because PLA is not a polyacid (polyelectrolyte), but rather a polyester.
Production
There are several industrial routes to usable (i.e. high molecular weight) PLA. Two main monomers are used: lactic acid, and the cyclic di-ester, lactide. The most common route to PLA is the ring-opening polymerization of lactide with various metal catalysts (typically tin octoate) in solution, in the melt, or as a suspension. The metal-catalyzed reaction tends to cause racemization of the PLA, reducing its stereoregularity compared to the starting material.
Another route to PLA is the direct condensation of lactic acid monomers. This process needs to be carried out at less than 200 °C; above that temperature, the entropically favored lactide monomer is generated. This reaction generates one equivalent of water for every condensation (esterification) step, and that is undesirable because water causes chain-transfer leading to low molecular weight material. The direct condensation is thus performed in a stepwise fashion, where lactic acid is first oligomerized to PLA oligomers. Thereafter, polycondensation is done in the melt or as a solution, where short oligomeric units are combined to give a high molecular weight polymer strand. Water removal by application of a vacuum or by azeotropic distillation is crucial to favor polycondensation over transesterification. Molecular weights of 130 kDa can be obtained this way. Even higher molecular weights can be attained by carefully crystallizing the crude polymer from the melt. Carboxylic acid and alcohol end groups are thus concentrated in the amorphous region of the solid polymer, and so they can react. Molecular weights of 128-152 kDa are obtainable thus.
Polymerization of a racemic mixture of L- and D-lactides usually leads to the synthesis of poly-DL-lactide (PDLLA), which is amorphous. Use of stereospecific catalysts can lead to heterotactic PLA which has been found to show crystallinity. The degree of crystallinity, and hence many important properties, is largely controlled by the ratio of D to L enantiomers used, and to a lesser extent on the type of catalyst used. Apart from lactic acid and lactide, lactic acid O-carboxyanhydride ("lac-OCA"), a five-membered cyclic compound has been used academically as well. This compound is more reactive than lactide, because its polymerization is driven by the loss of one equivalent of carbon dioxide per equivalent of lactic acid. Water is not a co-product.
The direct biosynthesis of PLA similar to the poly(hydroxyalkanoate)s has been reported as well.
Manufacturers
| This section contains promotional content. Please help improve it by removing promotional language and inappropriate external links, and by adding encyclopedic text written from a neutral point of view. (September 2013) (Learn how and when to remove this message) |
As of June 2010, NatureWorks was the primary producer of PLA (bioplastic) in the United States. Other companies involved in PLA manufacturing are PURAC Biomaterials (The Netherlands) and several Chinese manufacturers. The primary producer of PDLLA is PURAC, a wholly owned subsidiary of CSM located in the Netherlands. Galactic and Total Petrochemicals operate a joint venture, Futerro, which is developing a second generation polylactic acid product. This project includes the building of a PLA pilot plant in Belgium capable of producing 1,500 tonnes/year.
Since 2009, PURAC has been producing lactides D and L - monomers for PLA production - at a plant in Spain with a production capacity of several thousand tons. In February 2012, PURAC opened a 75,000-ton/year lactide plant at their production site in Thailand (Rayong Province). PURAC developed the technology to polymerize these lactides with Sulzer, a Swiss engineering company. Purac collaborates with various PLA production partners to increase production and develop new markets for PLA. Thanks to the availability of D-lactide, PURAC partners will be able to use stereo-complex technologies to produce new PLA grades (HDT) stable up to 180 degrees C. In a tripartite collaboration between PURAC, Sulzer, and Synbra, solutions were developed to allow Synbra to produce PLA and subsequently E-PLA, an attractive biodegradable and potentially bio-based alternative to EPS foam in a variety of application areas. This collaboration was awarded by Frost and Sullivan with the Innovation of the Year Award in 2008.
Chemical and physical properties
Due to the chiral nature of lactic acid, several distinct forms of polylactide exist: poly-L-lactide (PLLA) is the product resulting from polymerization of L,L-lactide (also known as L-lactide). PLLA has a crystallinity of around 37%, a glass transition temperature between 60-65 °C, a melting temperature between 173-178 °C and a tensile modulus between 2.7-16 GPa. However, heat resistant PLA can withstand temperatures of 110 °C. PLA is soluble in chlorinated solvents, hot benzene, tetrahydrofuran, and dioxane.
PLA has similar mechanical properties to PETE polymer, but has a significantly lower maximum continuous use temperature.
Polylactic acid can be processed like most thermoplastics into fiber (for example using conventional melt spinning processes) and film. The melting temperature of PLLA can be increased 40-50 °C and its heat deflection temperature can be increased from approximately 60°C to up to 190 °C by physically blending the polymer with PDLA (poly-D-lactide). PDLA and PLLA form a highly regular stereocomplex with increased crystallinity. The temperature stability is maximised when a 50:50 blend is used, but even at lower concentrations of 3-10% of PDLA, there is still a substantial improvement. In the latter case, PDLA acts as a nucleating agent, thereby increasing the crystallization rate. Biodegradation of PDLA is slower than for PLA due to the higher crystallinity of PDLA.
There is also poly(L-lactide-co-D,L-lactide) (PLDLLA) – used as PLDLLA/TCP scaffolds for bone engineering.
Applications




Poly(lactic acid) can be processed by extrusion, injection molding, film & sheet casting, and spinning, providing access to a wide range of materials.
Being able to degrade into innocuous lactic acid, PLA is used as medical implants in the form of anchors, screws, plates, pins, rods, and as a mesh. Depending on the exact type used, it breaks down within the body within 6 months to 2 years. This gradual degradation is desirable for a support structure, because it gradually transfers the load to the body (e.g. the bone) as that area heals. The strength characteristics of PLA and PLLA implants is well documented.
PLA can also be used as a compostable packaging material, either cast, injection molded, or spun. Cups and bags have been made of this material. In the form of a film, it shrinks upon heating, allowing it to be used in shrink tunnels. It is useful for producing loose-fill packaging, compost bags, food packaging, and disposable tableware. In the form of fibers and non-woven textiles, PLA also has many potential uses, for example as upholstery, disposable garments, awnings, feminine hygiene products, and diapers.
Racemic and regular PLLA has a low glass transition temperature, which is undesirable. A stereocomplex of PDLA and PLLA has a higher glass transition temperatures lending it more mechanical strength. It has a wide range of applications, such as woven shirts (ironability), microwavable trays, hot-fill applications and even engineering plastics (in this case, the stereocomplex is blended with a rubber-like polymer such as ABS). Such blends also have good form-stability and visual transparency, making them useful for low-end packaging applications. Pure poly-L-lactic acid (PLLA), on the other hand, is the main ingredient in Sculptra, a long lasting facial volume enhancer, primarily used for lipoatrophy of cheeks. Progress in biotechnology has resulted in the development of commercial production of the D enantiomer form, something that was not possible until recently.
PLA is also used as a feedstock material in 3D printers such as Reprap,Makerbot, and Ultimaker.
Recycling

Currently, the SPI resin identification code 7 ("others") is applicable for PLA. In Belgium, Galactic started the first pilot unit to chemically recycle PLA (Loopla). Unlike mechanical recycling, waste material can hold various contaminants. Polylactic acid can be recycled to monomer by thermal depolymerization, or hydrolysis. When purified, the monomer can be used for the manufacture of virgin PLA with no loss of original properties (cradle-to-cradle recycling).
See also
- Cellophane
- Metallic glass
- Plastarch material
- Polycaprolactone
- Polyglycolide
- Poly-3-hydroxybutyrate
- Zein
- 3D Printing
- Acrylonitrile butadiene styrene
References
- ^ "Matbase". Retrieved 6 February 2012.
- http://www.ampolymer.com/MSDS/PLA.pdf
- Market Study Bioplastics, Ceresana, Dec 2011
- Martin, O (NaN undefined NaN). "Poly(lactic acid): plasticization and properties of biodegradable multiphase systems". Polymer. 42 (14): 6209–6219. doi:10.1016/S0032-3861(01)00086-6.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rafael Auras, Loong-Tak Lim, Susan E. M. Selke, Hideto Tsuji (ed.). "3. Industrial Production of High Molecular Weight Poly(Lactic Acid)". Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications. doi:10.1002/9780470649848.ch3.
{{cite book}}: Unknown parameter|authors=ignored (help)CS1 maint: multiple names: editors list (link) - Kricheldorf, Hans R.; Jonté, J. Michael (1983). "New polymer syntheses". Polymer Bulletin. 9 (6–7). doi:10.1007/BF00262719.
- Jung, Yu Kyung; Kim, Tae Yong (2009). "Metabolic Engineering of Escherichia coli for the production of Polylactic Acid and Its Copolymers". Biotechnology and Bioengineering. 105 (1). doi:10.1002/bit.22548.
- Press release
- Södergård, Anders (2002). "Properties of lactic acid based polymers and their correlation with composition". Progress in Polymer Science. 27 (6): 1123–1163. doi:10.1016/S0079-6700(02)00012-6.
{{cite journal}}: Cite has empty unknown parameter:|quotes=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - Middelton, John C. (2000). "Synthetic biodegradable polymers as orthopedic devices". Biomaterial. 21 (23): 2335–2346. doi:10.1016/S0142-9612(00)00101-0.
{{cite journal}}: Cite has empty unknown parameter:|quotes=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Gina L. Fiore; Feng Jing; Victor G. Young, Jr.; Christopher J. Cramer; Marc A. Hillmyer (2010). "High Tg Aliphatic Polyesters by the Polymerization of Spirolactide Derivatives". Polymer Chemistry (1): 870–877. doi:10.1039/C0PY00029A.
- Donald Garlotta (2001). "A Literature Review of Poly(Lactic Acid)". Journal of Polymers and the Environment. 9 (2).
- "Compare Materials: PLA and PETE". Makeitfrom.com. Retrieved 2011-04-11.
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1080/17452750802551298, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1080/17452750802551298instead. - Fiore, G (2010). "High Tg Aliphatic Polyestersby the Polymerization o f Spir olactide Derivatives". Polym. Chem. (1): 870–877.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rafael Auras, Loong-Tak Lim, Susan E. M. Selke, Hideto Tsuji (ed.). Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications. doi:10.1002/9780470649848. ISBN 9780470293669.
{{cite book}}: CS1 maint: multiple names: editors list (link) - J Paul Harvey and Robert F Games (ed.). ASTM STP 1217 Theoretical Strength Comparison of Bioasbsorable (PLLA) Plates and conventional stainless steel and Titanium Plates used in Internal Fracture Fixation.
- "Bioengineers succeed in producing plastic without the use of fossil fuels". Physorg.com. Retrieved 2011-04-11.
- "PLA". Reprap Wiki. 2011-04-04. Retrieved 2011-04-11.
- "PLA". MakerBot Industries. Retrieved 2011-04-11.
External links
- New polymerization technology for PLA developed
- White paper on the Science of Biodegradable Plastics, FP International
- Your plastic pal, The Economist article
- Comparisons and LCA cycle of PLA