| Revision as of 22:04, 16 December 2013 editDePiep (talk | contribs)Extended confirmed users294,285 edits format temperatures Flashpoint, Autoignition, replaced: FlashPt → FlashPtC using AWB← Previous edit | Revision as of 19:36, 19 January 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,236 edits ref and deconstruct awkwardly written student work, still highly imperfectNext edit → | ||
| Line 40: | Line 40: | ||
| }} | }} | ||
| '''2-Ethylanthraquinone''' |
'''2-Ethylanthraquinone''' is an ] that is a derivtive of ]. It is pale yellow solid is used in the industrial production of ] (H<sub>2</sub>O<sub>2</sub>).<ref>cite encyclopedia | author = Goor, G.; Glenneberg, J.; Jacobi, S. | title = Hydrogen Peroxide | encyclopedia = Ullmann's Encyclopedia of Industrial Chemistry | year = 2007 | publisher = Wiley-VCH | location = Weinheim | doi = 10.1002/14356007.a13_443.pub2 }}</ref><ref>Römpp CD 2006, Georg Thieme Verlag 2006</ref> | ||
| ==Production== | ==Production== | ||
| 2-Ethylanthraquinone is prepared from the reaction of ] and ]: C<sub>6</sub>H<sub>4</sub>(CO)<sub>2</sub>O + C<sub>6</sub>H<sub>5</sub>CH<sub>2</sub>CH<sub>3</sub> → C<sub>6</sub>H<sub>4</sub>C<sub>2</sub>O<sub>2</sub>C<sub>6</sub>H<sub>3</sub>C<sub>2</sub>H<sub>5</sub> + H<sub>2</sub>O. Both phthalic anhydride and ethylbenzene are readily available |
2-Ethylanthraquinone is prepared from the reaction of ] and ]: | ||
| :C<sub>6</sub>H<sub>4</sub>(CO)<sub>2</sub>O + C<sub>6</sub>H<sub>5</sub>CH<sub>2</sub>CH<sub>3</sub> → C<sub>6</sub>H<sub>4</sub>C<sub>2</sub>O<sub>2</sub>C<sub>6</sub>H<sub>3</sub>C<sub>2</sub>H<sub>5</sub> + H<sub>2</sub>O. | |||
| Both phthalic anhydride and ethylbenzene are readily available, being otherwise used in the large-scale production of plastics. | |||
| ==Uses== | ==Uses== | ||
| ] is produced industrially by the ] which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. Many derivatives of anthraquinone are used but 2- |
] is produced industrially by the ] which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. Many derivatives of anthraquinone are used but 2-ethylanthraquinone is common because of its high selectivity. The hydrogenation of the unsubsituted ring can reach 90% selectivity by using 2-ethylanthraquinone. Hydrogenation follows the Riedl-Pfleiderer, or ], process: | ||
| ]<br style="clear:left;"/> | ]<br style="clear:left;"/> | ||
| The hydrogenation of ] is catalyzed by ] |
The ] of ] is catalyzed by ]. Hydrogenation produces both 2-ethylanthrahydroquinone and tetrahydroanthraquinone. The tetrahydro derivative of 2-alkylanthraquinone is easily hyrdrogenated but is more difficult to oxidize. The formation of the tetrahyrdo derivative can be suppressed through the seelection of catalysts, solvents, and reaction conditions. Some suggested solvent mixtures are polyalkylated benzenes and alkyl phosphates or tetraalkyl ureas, trimethylbenzenes and alkylcyclohexanol esters, and methylnaphthalene and nonyl alcohols. | ||
| A working solution must be able to keep the 2-ethylanthraquinone dissolved in the hyrdrogenation, oxidation, and extraction steps. Since hydroquinones dissolve better in polar solvents, and quinones dissolve better in aromatic nonpolar solvents, a solvent mixture must be used. Some suggested mixtures are polyalkylated benzenes and alkyl phosphates or tetraalkyl ureas, trimethylbenzenes and alkylcyclohexanol esters, and methylnaphthalene and nonyl alcohols. | |||
| ==References== | ==References== | ||
| <references /> | |||
| {{DEFAULTSORT:Ethylanthraquinone, 2-}} | {{DEFAULTSORT:Ethylanthraquinone, 2-}} | ||
Revision as of 19:36, 19 January 2014

| |

| |
| Names | |
|---|---|
| Other names 2-Ethyl-9,10-anthracenedione | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.396 |
| EC Number |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H12O2 |
| Molar mass | 236.27 g/mol |
| Appearance | white to yellowish crystals or powder |
| Density | 1.231g/cm3 |
| Melting point | 105 °C (221 °F; 378 K) |
| Boiling point | 415.4 @ 760mmHg |
| Hazards | |
| Flash point | 155.4 °C (311.7 °F; 428.5 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
2-Ethylanthraquinone is an organic compound that is a derivtive of anthraquinone. It is pale yellow solid is used in the industrial production of hydrogen peroxide (H2O2).
Production
2-Ethylanthraquinone is prepared from the reaction of phthalic anhydride and ethylbenzene:
- C6H4(CO)2O + C6H5CH2CH3 → C6H4C2O2C6H3C2H5 + H2O.
Both phthalic anhydride and ethylbenzene are readily available, being otherwise used in the large-scale production of plastics.
Uses
Hydrogen peroxide is produced industrially by the anthraquinone process which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. Many derivatives of anthraquinone are used but 2-ethylanthraquinone is common because of its high selectivity. The hydrogenation of the unsubsituted ring can reach 90% selectivity by using 2-ethylanthraquinone. Hydrogenation follows the Riedl-Pfleiderer, or autoxidation, process:
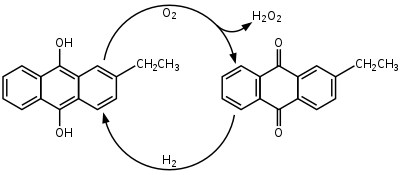
The hydrogenation of 2-ethylanthraquinone is catalyzed by palladium. Hydrogenation produces both 2-ethylanthrahydroquinone and tetrahydroanthraquinone. The tetrahydro derivative of 2-alkylanthraquinone is easily hyrdrogenated but is more difficult to oxidize. The formation of the tetrahyrdo derivative can be suppressed through the seelection of catalysts, solvents, and reaction conditions. Some suggested solvent mixtures are polyalkylated benzenes and alkyl phosphates or tetraalkyl ureas, trimethylbenzenes and alkylcyclohexanol esters, and methylnaphthalene and nonyl alcohols.
References
- cite encyclopedia | author = Goor, G.; Glenneberg, J.; Jacobi, S. | title = Hydrogen Peroxide | encyclopedia = Ullmann's Encyclopedia of Industrial Chemistry | year = 2007 | publisher = Wiley-VCH | location = Weinheim | doi = 10.1002/14356007.a13_443.pub2 }}
- Römpp CD 2006, Georg Thieme Verlag 2006