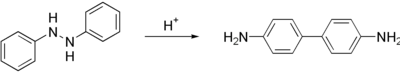| Revision as of 19:25, 7 November 2013 editJZNIOSH (talk | contribs)1,184 edits Updated appearance, added density, boiling pt to Chembox← Previous edit | Revision as of 04:36, 8 February 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,236 edits repair doiNext edit → | ||
| Line 47: | Line 47: | ||
| ==Synthesis and properties== | ==Synthesis and properties== | ||
| Benzidine is prepared in a two step process from ]. First, the nitrobenzene is converted to 1,2-diphenylhydrazine, usually using iron powder as the reducing agent. Treatment of this hydrazine with mineral acids induces a ] to 4,4'-benzidine. Smaller amounts of other isomers are also formed.<ref name=Ullmann>{{ cite encyclopedia | author = Schwenecke, H.; Mayer, D. | title = Benzidine and Benzidine Derivatives | encyclopedia = Ullmann’s Encyclopedia of Industrial Chemistry | year = 2005 | publisher = Wiley-VCH | location = Weinheim }}</ref> The '''benzidine rearrangement''', which proceeds intramolecularly, is a classic ] puzzle in ].<ref>{{ cite book | author = March, J. | title = Advanced Organic Chemistry | edition = 5th | publisher = J. Wiley and Sons | year = 1992 | location = New York | isbn = 0-471-60180-2 }}</ref> | Benzidine is prepared in a two step process from ]. First, the nitrobenzene is converted to 1,2-diphenylhydrazine, usually using iron powder as the reducing agent. Treatment of this hydrazine with mineral acids induces a ] to 4,4'-benzidine. Smaller amounts of other isomers are also formed.<ref name=Ullmann>{{ cite encyclopedia | author = Schwenecke, H.; Mayer, D. | title = Benzidine and Benzidine Derivatives | encyclopedia = Ullmann’s Encyclopedia of Industrial Chemistry | year = 2005 | publisher = Wiley-VCH | location = Weinheim | doi = 10.1002/14356007.a03_539 }}</ref> The '''benzidine rearrangement''', which proceeds intramolecularly, is a classic ] puzzle in ].<ref>{{ cite book | author = March, J. | title = Advanced Organic Chemistry | edition = 5th | publisher = J. Wiley and Sons | year = 1992 | location = New York | isbn = 0-471-60180-2 }}</ref> | ||
| :] | :] | ||
| Line 68: | Line 68: | ||
| A variety of derivatives of 4,4’-benzidine are commercially produced on the scale of one to a few thousand kilograms per year, mainly as precursors to dyes and pigments.<ref name=Ullmann/> These derivatives include, in order of scale, the following: | A variety of derivatives of 4,4’-benzidine are commercially produced on the scale of one to a few thousand kilograms per year, mainly as precursors to dyes and pigments.<ref name=Ullmann/> These derivatives include, in order of scale, the following: | ||
| * ] | |||
| * 3,3'-dichlorobenzidine (CAS# 91-94-1, m.p. 132–133 °C) | |||
| * ] (2,2'-dimethyl-4,4’-benzidine |
* ] (2,2'-dimethyl-4,4’-benzidine | ||
| * ''o''-dianisidine (2,2'-dimethoxy-4,4’-benzidine, CAS# 119-90-4, m.p. 133 °C) | * ''o''-dianisidine (2,2'-dimethoxy-4,4’-benzidine, CAS# 119-90-4, m.p. 133 °C) | ||
| * ] (CAS# 91-95-2, m.p. 178 °C) is a precursor to ], a high-strength, flame-resistant material. | * ] (CAS# 91-95-2, m.p. 178 °C) is a precursor to ], a high-strength, flame-resistant material. | ||
Revision as of 04:36, 8 February 2014
Template:Chembox Other
| |
| Names | |
|---|---|
| IUPAC name 4,4'-diaminobiphenyl | |
| Other names Benzidine, di-phenylamine, diphenylamine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.000 |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H12N2 |
| Molar mass | 184.24 g/mol |
| Appearance | Grayish-yellow, reddish-gray, or white crystalline powder |
| Density | 1.25 g/cm |
| Melting point | 122-125 °C |
| Boiling point | 400 °C |
| Solubility in water | 0.94 g/100 mL at 100 °C |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | carcinogenic |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Benzidine (trivial name), also called 4,4'-diaminobiphenyl (systematic name), is the solid organic compound with the formula (C6H4NH2)2. This aromatic amine is a component of a test for cyanide and also in the production of dyes. Benzidine has been linked to bladder and pancreatic cancer. Since August 2010 benzidine dyes are included in the EPA's List of Chemicals of Concern.
Synthesis and properties
Benzidine is prepared in a two step process from nitrobenzene. First, the nitrobenzene is converted to 1,2-diphenylhydrazine, usually using iron powder as the reducing agent. Treatment of this hydrazine with mineral acids induces a rearrangement reaction to 4,4'-benzidine. Smaller amounts of other isomers are also formed. The benzidine rearrangement, which proceeds intramolecularly, is a classic mechanistic puzzle in organic chemistry.
The conversion is described as a sigmatropic reaction.
In terms of its physical properties, 4,4'-benzidine is poorly soluble in cold water but can be recrystallized from hot water, where it crystallises as the monohydrate. It is dibasic, the deprotonated species has Ka values of 9.3 × 10 and 5.6 × 10. Its solutions react with oxidizing agents to give deeply coloured quinone-related derivatives.
Applications
As with some other aromatic amines such as 2-Naphthylamine, benzidine has been significantly withdrawn from use in most industries because it is so carcinogenic.
In the past, benzidine was used to test for blood. An enzyme in blood causes the oxidation of benzidine to a distinctively blue-coloured derivative.
The test for cyanide relies on similar reactivity.
Such applications have largely been replaced by methods using phenolphthalein/hydrogen peroxide and luminol.
Related 4,4’-benzidines
A variety of derivatives of 4,4’-benzidine are commercially produced on the scale of one to a few thousand kilograms per year, mainly as precursors to dyes and pigments. These derivatives include, in order of scale, the following:
- 3,3'-Dichlorobenzidine
- o-tolidine (2,2'-dimethyl-4,4’-benzidine
- o-dianisidine (2,2'-dimethoxy-4,4’-benzidine, CAS# 119-90-4, m.p. 133 °C)
- 3,3',4,4'-Tetraaminodiphenyl (CAS# 91-95-2, m.p. 178 °C) is a precursor to polybenzimidazole fiber, a high-strength, flame-resistant material.
References
- NIOSH Pocket Guide to Chemical Hazards Centers for Disease Control and Prevention. 2011-04-04
- "Known and Probable Carcinogens". American Cancer Society. 2011-06-29.
- "Benzidine Dyes Action Plan Summary". U. S. Environmental Protection Agency. 2010-08-18.
- ^ Schwenecke, H.; Mayer, D. (2005). "Benzidine and Benzidine Derivatives". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_539.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - March, J. (1992). Advanced Organic Chemistry (5th ed.). New York: J. Wiley and Sons. ISBN 0-471-60180-2.
- Shine, H. J.; Zmuda, H.; Park, K. H.; Kwart, H.; Horgan, A. G.; Collins, C.; Maxwell, B. E. (1981). "Mechanism of the benzidine rearrangement. Kinetic isotope effects and transition states. Evidence for concerted rearrangement". Journal of the American Chemical Society. 103 (4): 955–956. doi:10.1021/ja00394a047.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - Shine, H. J.; Zmuda, H.; Park, K. H.; Kwart, H.; Horgan, A. G.; Brechbiel, M. (1982). "Benzidine rearrangements. 16. The use of heavy-atom kinetic isotope effects in solving the mechanism of the acid-catalyzed rearrangement of hydrazobenzene. The concerted pathway to benzidine and the nonconcerted pathway to diphenyline". Journal of the American Chemical Society. 104 (9): 2501–2509. doi:10.1021/ja00373a028.
{{cite journal}}: CS1 maint: multiple names: authors list (link)