| Revision as of 11:27, 5 May 2014 editYobot (talk | contribs)Bots4,733,870 editsm →Organoosmium: WP:CHECKWIKI error fixes using AWB (10093)← Previous edit | Revision as of 12:14, 10 May 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,236 edits Mention Cp*2Ru2Cl4, create section on carbonylsNext edit → | ||
| Line 1: | Line 1: | ||
| '''Organoruthenium chemistry''' is the ] of ] containing a ] to ] ].<ref>''Synthesis of Organometallic Compounds: A Practical Guide'' Sanshiro Komiya Ed. S. Komiya, M. Hurano 1997</ref> The interest is mostly academic although several organoruthenium ]s are of commercial interest. The chemistry has |
'''Organoruthenium chemistry''' is the ] of ] containing a ] to ] ].<ref>''Synthesis of Organometallic Compounds: A Practical Guide'' Sanshiro Komiya Ed. S. Komiya, M. Hurano 1997</ref> The interest is mostly academic although several organoruthenium ]s are of commercial interest. The chemistry has some stoichiometric similarities with ], as iron is directly above ruthenium in ] of the periodic table. The most important reagents for the introduction of ruthenium are ] and ]. | ||
| ⚫ | :Ru<sub>3</sub>(CO)<sub>12</sub> + 3 CO <math>\overrightarrow{\leftarrow}</math> 3 Ru(CO)<sub>5</sub> | ||
| In its organometallic compounds, ruthenium is known to adopt oxidation states from -2 (<sup>2-</sup>) to +6 (<sup>-</sup>). Most common are those in the 2+ oxidation state, as illustrated below. | In its organometallic compounds, ruthenium is known to adopt oxidation states from -2 (<sup>2-</sup>) to +6 (<sup>-</sup>). Most common are those in the 2+ oxidation state, as illustrated below. | ||
| Line 7: | Line 6: | ||
| File:Grubbs Catalyst 1st Generation.svg|] | File:Grubbs Catalyst 1st Generation.svg|] | ||
| File:ShvoCat.png|] | File:ShvoCat.png|] | ||
| File:RuCymCl2.png|] | File:RuCymCl2.png|] | ||
| File:Ru3(CO)12.png|]. | File:Ru3(CO)12.png|]. | ||
| File:Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium.png|] | File:Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium.png|] | ||
| File:Cp*2Ru2Cl4.png|] | |||
| </gallery> | </gallery> | ||
| Line 27: | Line 27: | ||
| ===N-Heterocyclic carbene ligands=== | ===N-Heterocyclic carbene ligands=== | ||
| NHC ligands have become very common in organoruthenium complexes.<ref>{{cite journal | last1 = Öfele | first1 = K. | last2 = Tosh | first2 = E. | last3 = Taubmann | first3 = C. | last4 = Herrmann | first4 = W.A. | journal = Chemical Reviews | title = Carbocyclic Carbene Metal Complexes | pages = 3408–3444 | volume = 109 | issue = 8 | year = 2009 | doi = 10.1021/cr800516g}}</ref><ref>{{cite journal | journal = Chemical Reviews | doi = 10.1021/cr800524f | title = Ruthenium-Based Olefin Metathesis Catalysts Bearing N-Heterocyclic Carbene Ligands | last1 = Samojłowicz | first1 = C. | last2 = Bieniek | first2 = M. | last3 = Grela | first3 = K. | year = 2009 | volume = 109 | issue = 8 | pages = 3708–3742 | pmid=19534492}}</ref> NHC ligands can be prepared with precise steric and electronic parameters, and can be chiral for use in asymmetric catalysis.<ref>{{cite journal | journal = Chemical Reviews | year = 2011 | volume = 111 | issue = 12 | doi = 10.1021/cr100328e | last1 = Benhamou | first1 = L. | last2 = Chardon | first2 = E. | last3 = Lavigne | first3 = G. | last4 = Bellemin-Laponnaz | first4 = S. | last5 = César | first5 = V. | title = Synthetic Routes to N-Heterocyclic Carbene Precursors}}</ref> NHCs, as strongly donating ], are often used to replace phosphine ligands. A notable example is 2nd generation ], in which a phosphine of the 1st generation catalyst is replaced by an NHC. | NHC ligands have become very common in organoruthenium complexes.<ref>{{cite journal | last1 = Öfele | first1 = K. | last2 = Tosh | first2 = E. | last3 = Taubmann | first3 = C. | last4 = Herrmann | first4 = W.A. | journal = Chemical Reviews | title = Carbocyclic Carbene Metal Complexes | pages = 3408–3444 | volume = 109 | issue = 8 | year = 2009 | doi = 10.1021/cr800516g}}</ref><ref>{{cite journal | journal = Chemical Reviews | doi = 10.1021/cr800524f | title = Ruthenium-Based Olefin Metathesis Catalysts Bearing N-Heterocyclic Carbene Ligands | last1 = Samojłowicz | first1 = C. | last2 = Bieniek | first2 = M. | last3 = Grela | first3 = K. | year = 2009 | volume = 109 | issue = 8 | pages = 3708–3742 | pmid=19534492}}</ref> NHC ligands can be prepared with precise steric and electronic parameters, and can be chiral for use in asymmetric catalysis.<ref>{{cite journal | journal = Chemical Reviews | year = 2011 | volume = 111 | issue = 12 | doi = 10.1021/cr100328e | last1 = Benhamou | first1 = L. | last2 = Chardon | first2 = E. | last3 = Lavigne | first3 = G. | last4 = Bellemin-Laponnaz | first4 = S. | last5 = César | first5 = V. | title = Synthetic Routes to N-Heterocyclic Carbene Precursors}}</ref> NHCs, as strongly donating ], are often used to replace phosphine ligands. A notable example is 2nd generation ], in which a phosphine of the 1st generation catalyst is replaced by an NHC. | ||
| Line 58: | Line 57: | ||
| :] | :] | ||
| == |
===Carbonyls=== | ||
| The main ruthenium carbonyl is ], Ru<sub>3</sub>(CO)<sub>12</sub>. The analogues of the popular reagents Fe(CO)<sub>5</sub> and Fe<sub>2</sub>(CO)<sub>9</sub> are not very useful. The pentacarbonyl de]s readily: | |||
| ⚫ | :Ru<sub>3</sub>(CO)<sub>12</sub> + 3 CO <math>\overrightarrow{\leftarrow}</math> 3 Ru(CO)<sub>5</sub> | ||
| Carbonylation of ruthenium trichloride gives a series of Ru(II) chlorocarbonyls. These are the precursors to Ru<sub>3</sub>(CO)<sub>12</sub>. | |||
| ==Organoosmium compounds== | |||
| In the same ] ] resembles ruthenium in its complexes. Because Os is more expensive than Ru, the chemistry is less developed and has fewer applications. Of course the cost of the catalyst is offset if turnover numbers are high. Thus, ] is an important oxidizing agent in organic chemistry especially in the conversion of alkenes to 1,2-diols. | In the same ] ] resembles ruthenium in its complexes. Because Os is more expensive than Ru, the chemistry is less developed and has fewer applications. Of course the cost of the catalyst is offset if turnover numbers are high. Thus, ] is an important oxidizing agent in organic chemistry especially in the conversion of alkenes to 1,2-diols. | ||
| Line 64: | Line 68: | ||
| :] | :] | ||
| Important compounds, at least for academic studies, are the carbonyls such as ] and ]. The phosphine complexes are analogous to those or ruthenium, but hydride derivatives |
Important compounds, at least for academic studies, are the carbonyls such as ] and ]. The phosphine complexes are analogous to those or ruthenium, but hydride derivatives, e.g. OsHCl(CO)(PPh<sub>3</sub>)<sub>3</sub>, tend to be more stable. | ||
| ==See also== | ==See also== | ||
Revision as of 12:14, 10 May 2014
Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. The interest is mostly academic although several organoruthenium catalysts are of commercial interest. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.
In its organometallic compounds, ruthenium is known to adopt oxidation states from -2 () to +6 (). Most common are those in the 2+ oxidation state, as illustrated below.
-
 1st Generation Grubbs Catalyst
1st Generation Grubbs Catalyst
-
 Shvo catalyst
Shvo catalyst
-
 (cymene)ruthenium dichloride dimer
(cymene)ruthenium dichloride dimer
-
 triruthenium dodecacarbonyl.
triruthenium dodecacarbonyl.
-
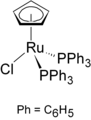 chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium
chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium
- pentamethylcyclopentadienyl ruthenium dichloride dimer pentamethylcyclopentadienyl ruthenium dichloride dimer
Ligands
As with other late transition metals, ruthenium binds more favorably with soft ligands. The most important ligands for ruthenium are:
- halides, especially chloride.
- phosphines, especially triphenylphosphine.
- N-heterocyclic carbenes (NHCs).
- cyclopentadienyl ligands.
- various arenes and dienes
- carbon monoxide.
- hydride, notably in the Shvo catalyst.
- metal carbenes, notably in the Grubbs' catalyst.
Phosphine ligands
While monodentate phosphine ligands such as triphenyphosphine and tricyclohexylphosphine are most common, bidentate phosphine ligands can also be useful in organoruthenium compounds. BINAP, in particular, is a useful asymmetric ligand for many asymmetric ruthenium catalysts.
N-Heterocyclic carbene ligands
NHC ligands have become very common in organoruthenium complexes. NHC ligands can be prepared with precise steric and electronic parameters, and can be chiral for use in asymmetric catalysis. NHCs, as strongly donating L-type ligands, are often used to replace phosphine ligands. A notable example is 2nd generation Grubbs' catalyst, in which a phosphine of the 1st generation catalyst is replaced by an NHC.
Cyclopentadienyl ligands
The parent compound ruthenocene is unreactive because it is coordinatively saturated and contains no reactive groups. Shvo's catalyst ({2H]}Ru2(CO)4(μ-H)) is also coordinatively saturated, but features reactive OH and RuH groups that enable it to function in transfer hydrogenation. It is used in hydrogenation of aldehydes, ketones, via transfer hydrogenation, in disproportionation of aldehydes to esters and in the isomerization of allylic alcohols.
Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium features a reactive chloro group, which is readily substituted by organic substrates.
Arene and alkene ligands
One example of an Ru-arene complex is (cymene)ruthenium dichloride dimer, which is the precursor to a versatile catalyst for transfer hydrogenation. Acenaphthylene forms a useful catalyst derived from triruthenium dodecacarbonyl. The hapticity of the hexamethylbenzene ligand in Ru(C6Me6)2 depends on the oxidation state of the metal centre (methyl groups omitted for clarity):
The compound Ru(COD)(COT) is capable of dimerizing norbornadiene:
Carbonyls
The main ruthenium carbonyl is triruthenium dodecacarbonyl, Ru3(CO)12. The analogues of the popular reagents Fe(CO)5 and Fe2(CO)9 are not very useful. The pentacarbonyl de]s readily:
- Ru3(CO)12 + 3 CO 3 Ru(CO)5
Carbonylation of ruthenium trichloride gives a series of Ru(II) chlorocarbonyls. These are the precursors to Ru3(CO)12.
Organoosmium compounds
In the same group 8 elements osmium resembles ruthenium in its complexes. Because Os is more expensive than Ru, the chemistry is less developed and has fewer applications. Of course the cost of the catalyst is offset if turnover numbers are high. Thus, Osmium tetroxide is an important oxidizing agent in organic chemistry especially in the conversion of alkenes to 1,2-diols.
The 5d-orbitals in Os are higher in energy that the 4d-orbitals in Ru. Thus, π backbonding to alkenes and CO is stronger for Os compounds, which leads to more stable organic derivatives. This effect is illustrated by the stability of the alkene derivatives of the type or as in the example below.
Important compounds, at least for academic studies, are the carbonyls such as triosmium dodecacarbonyl and decacarbonyldihydridotriosmium. The phosphine complexes are analogous to those or ruthenium, but hydride derivatives, e.g. OsHCl(CO)(PPh3)3, tend to be more stable.
See also
- Chemical bonds of carbon with other elements in the periodic table:
| Compounds of carbon with other elements in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Legend |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- Synthesis of Organometallic Compounds: A Practical Guide Sanshiro Komiya Ed. S. Komiya, M. Hurano 1997
- Barthazy, P.; Stoop, R. M.; Wörle, M.; Togni, A.; Mezzetti, A. (2000). "Toward Metal-Mediated C-F Bond Formation. Synthesis and Reactivity of the 16-Electron Fluoro Complex PF6 (dppp = 1,3-Bis(diphenylphosphino)propane)". Organometallics. 19: 2844–2852. doi:10.1021/om0000156.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Example: Organic Syntheses, Coll. Vol. 10, p.276 (2004); Vol. 77, p.1 (2000). Link
- Example: Organic Syntheses, Organic Syntheses, Coll. Vol. 9, p.589 (1998); Vol. 71, p.1 (1993). Link
- Example: Organic Syntheses, Organic Syntheses, Coll. Vol. 9, p.169 (1998); Vol. 72, p.74 (1995). Link
- Example: Organic Syntheses, Vol. 81, p.178 (2005). Link
- Öfele, K.; Tosh, E.; Taubmann, C.; Herrmann, W.A. (2009). "Carbocyclic Carbene Metal Complexes". Chemical Reviews. 109 (8): 3408–3444. doi:10.1021/cr800516g.
- Samojłowicz, C.; Bieniek, M.; Grela, K. (2009). "Ruthenium-Based Olefin Metathesis Catalysts Bearing N-Heterocyclic Carbene Ligands". Chemical Reviews. 109 (8): 3708–3742. doi:10.1021/cr800524f. PMID 19534492.
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; César, V. (2011). "Synthetic Routes to N-Heterocyclic Carbene Precursors". Chemical Reviews. 111 (12). doi:10.1021/cr100328e.
- Template:Cite DOI
- Organic Syntheses, Organic Syntheses, Vol. 82, p.10 (2005).Link
- Example: Organic Syntheses, Organic Syntheses, Vol. 82, p.188 (2005). Link
- Huttner, Gottfried; Lange, Siegfried; Fischer, Ernst O. (1971). "Molecular Structure of Bis(Hexamethylbenzene)-Ruthenium(0)". Angewandte Chemie, International Edition in English. 10 (8): 556–557. doi:10.1002/anie.197105561.


 3 Ru(CO)5
3 Ru(CO)5