| Revision as of 12:46, 18 June 2014 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,236 edits specify W-O-Br's, format formulas uniformly, fix IS ref, take links out of equations per our usual style - we need an Xray← Previous edit | Revision as of 14:04, 18 June 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,236 edits corrected structure, more later this evening with refsNext edit → | ||
| Line 3: | Line 3: | ||
| | verifiedrevid = 428738669 | | verifiedrevid = 428738669 | ||
| | Name = Tungsten(VI) dioxydichloride | | Name = Tungsten(VI) dioxydichloride | ||
| | ImageFile = |
| ImageFile = WO2Cl2distances.png | ||
| <!-- | ImageSize = 150px --> | <!-- | ImageSize = 150px --> | ||
| | ImageName = Tungsten(VI) dioxydichloride | | ImageName = Tungsten(VI) dioxydichloride | ||
| Line 41: | Line 41: | ||
| This reaction, like the preceding one, proceeds via the intermediacy of WOCl<sub>4</sub>. | This reaction, like the preceding one, proceeds via the intermediacy of WOCl<sub>4</sub>. | ||
| ==Structure== | |||
| The compound is a polymer consisting of distorted octahedral W centres. The monomer is characterized by two short W-O distances, typical for a double or triple bond, and two long W-O distances more typical of a single W-O bond. | |||
| ==Other tungsten oxy chlorides and related oxy halides== | ==Other tungsten oxy chlorides and related oxy halides== | ||
| Tungsten forms a number of oxyhalides including ], WOCl<sub>3</sub>, WOCl<sub>2</sub>. The corresponding bromides (WOBr<sub>4</sub>, WOBr<sub>3</sub>, WOBr<sub>2</sub>) are also known as is WO<sub>2</sub>I<sub>2</sub>.<ref>Holleman, A. F.; Wiberg, E. ''Inorganic Chemistry'' Academic Press: San Diego, 2001. ISBN 0-12-352651-5.</ref> | Tungsten forms a number of oxyhalides including ], WOCl<sub>3</sub>, WOCl<sub>2</sub>. The corresponding bromides (WOBr<sub>4</sub>, WOBr<sub>3</sub>, WOBr<sub>2</sub>) are also known as is WO<sub>2</sub>I<sub>2</sub>.<ref>Holleman, A. F.; Wiberg, E. ''Inorganic Chemistry'' Academic Press: San Diego, 2001. ISBN 0-12-352651-5.</ref> | ||
| ] | |||
| ==Reactions== | ==Reactions== | ||
| WO<sub>2</sub>Cl<sub>2</sub> is a ], forming soluble adducts of the type WO<sub>2</sub>Cl<sub>2</sub>L<sub>2</sub>, where L is a donor ligand such as ]. | WO<sub>2</sub>Cl<sub>2</sub> is a ], forming soluble adducts of the type WO<sub>2</sub>Cl<sub>2</sub>L<sub>2</sub>, where L is a donor ligand such as ] and ]. Such complexes often cannot be prepared by depolymerization of the inorganic solid, but are generated from WOCl<sub>4</sub>. | ||
| ==References== | ==References== | ||
Revision as of 14:04, 18 June 2014

| |
| Names | |
|---|---|
| Other names tungsten(VI) dioxydichloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.033.496 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | WO2Cl2 |
| Molar mass | 286.749 g/mol |
| Appearance | yellow crystals |
| Density | 4.67 g/cm, solid |
| Melting point | 265 °C (509 °F; 538 K) |
| Boiling point | sublimes > 350 °C in vacuo |
| Solubility in water | decomposes |
| Structure | |
| Crystal structure | orthorhombic |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tungsten dichloride dioxide is the chemical compound with the formula WO2Cl2. This yellow-colored solid is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.
Preparation
WO2Cl2 is prepared by ligand redistribution reaction from tungsten trioxide and tungsten hexachloride:
- 2 WO3 + WCl6 → 3 WO2Cl2
Using a two-zone furnace, a vacuum-sealed tube containing these solids is heated to 350 C. The yellow product sublimes to the cooler end of the reaction tube. No redox occurs in this process. An alternative route highlights the oxophilicity of tungsten:
- WCl6 + 2 O(Si(CH3)3)2 → 3 WO2Cl2 + 4 ClSi(CH3)3
This reaction, like the preceding one, proceeds via the intermediacy of WOCl4.
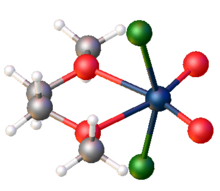
Structure
The compound is a polymer consisting of distorted octahedral W centres. The monomer is characterized by two short W-O distances, typical for a double or triple bond, and two long W-O distances more typical of a single W-O bond.
Other tungsten oxy chlorides and related oxy halides
Tungsten forms a number of oxyhalides including WOCl4, WOCl3, WOCl2. The corresponding bromides (WOBr4, WOBr3, WOBr2) are also known as is WO2I2.
Reactions
WO2Cl2 is a Lewis acid, forming soluble adducts of the type WO2Cl2L2, where L is a donor ligand such as bipyridine and dimethoxyethane. Such complexes often cannot be prepared by depolymerization of the inorganic solid, but are generated from WOCl4.
References
- Tillack, J. (1973). "Tungsten Oxyhalides". Inorg. Synth. 14: 109–122. doi:10.1002/9780470132456.ch22.
- Gibson, V. C.; Kee, T. P.; Shaw, A. (1988). "New, improved synthesis of the group 6 oxyhalides, W(O)Cl4, W(O)2Cl2 and Mo(O)2Cl2". Polyhedron. 7 (7): 579–80. doi:10.1016/S0277-5387(00)86336-6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
| Tungsten compounds | |||||
|---|---|---|---|---|---|
| Tungsten(0) | |||||
| Tungsten(II) | |||||
| Tungsten(III) | |||||
| Tungsten(IV) | |||||
| Tungsten(V) | |||||
| Tungsten(VI) |
| ||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |