| Revision as of 13:00, 11 August 2014 edit86.157.144.73 (talk) →Causes: trim older sourcing on obesity/GERD, per wp:meddate← Previous edit | Revision as of 13:20, 11 August 2014 edit undo86.157.144.73 (talk) →Causes: propose revised wording etc for celiac disease - see talkNext edit → | ||
| Line 51: | Line 51: | ||
| *The role of '']'' in progression to esophageal adenocarcinoma is still uncertain, but, on the basis of population data, it may carry a protective effect.<ref>{{cite journal |author=Wong A, Fitzgerald RC |title=Epidemiologic risk factors for Barrett's esophagus and associated adenocarcinoma |journal=Clin. Gastroenterol. Hepatol. |volume=3 |issue=1 |pages=1–10 |year=2005 |pmid=15645398|doi=10.1016/S1542-3565(04)00602-0}}</ref><ref>{{cite journal |author=Ye W, Held M, Lagergren J, et al. |title=Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia |journal=J. Natl. Cancer Inst. |volume=96 |issue=5 |pages=388–96 |year=2004 |pmid=14996860 |url=http://jnci.oxfordjournals.org/cgi/content/full/96/5/388 |doi=10.1093/jnci/djh057}}</ref> It is postulated that ''H. pylori'' induces chronic ], which is a risk factor for ], which in turn is a risk factor for esophageal adenocarcinoma.<ref>{{cite journal |author=Nakajima S, Hattori T |title=Oesophageal adenocarcinoma or gastric cancer with or without eradication of Helicobacter pylori infection in chronic atrophic gastritis patients: a hypothetical opinion from a systematic review |journal=Aliment. Pharmacol. Ther. |volume=20 Suppl 1 |issue= |pages=54–61 |year=2004 |pmid=15298606 |doi=10.1111/j.1365-2036.2004.01975.x | url=http://www.blackwell-synergy.com/doi/full/10.1111/j.1365-2036.2004.01975.x}}</ref> | *The role of '']'' in progression to esophageal adenocarcinoma is still uncertain, but, on the basis of population data, it may carry a protective effect.<ref>{{cite journal |author=Wong A, Fitzgerald RC |title=Epidemiologic risk factors for Barrett's esophagus and associated adenocarcinoma |journal=Clin. Gastroenterol. Hepatol. |volume=3 |issue=1 |pages=1–10 |year=2005 |pmid=15645398|doi=10.1016/S1542-3565(04)00602-0}}</ref><ref>{{cite journal |author=Ye W, Held M, Lagergren J, et al. |title=Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia |journal=J. Natl. Cancer Inst. |volume=96 |issue=5 |pages=388–96 |year=2004 |pmid=14996860 |url=http://jnci.oxfordjournals.org/cgi/content/full/96/5/388 |doi=10.1093/jnci/djh057}}</ref> It is postulated that ''H. pylori'' induces chronic ], which is a risk factor for ], which in turn is a risk factor for esophageal adenocarcinoma.<ref>{{cite journal |author=Nakajima S, Hattori T |title=Oesophageal adenocarcinoma or gastric cancer with or without eradication of Helicobacter pylori infection in chronic atrophic gastritis patients: a hypothetical opinion from a systematic review |journal=Aliment. Pharmacol. Ther. |volume=20 Suppl 1 |issue= |pages=54–61 |year=2004 |pmid=15298606 |doi=10.1111/j.1365-2036.2004.01975.x | url=http://www.blackwell-synergy.com/doi/full/10.1111/j.1365-2036.2004.01975.x}}</ref> | ||
| * There is some evidence suggesting a possible causal association between ] (HPV) and the squamous cell type.<ref name=Liyanage-2014>{{cite journal |author=Liyanage SS, Rahman B, Ridda I, Newall AT, Tabrizi SN, Garland SM, Segelov E, Seale H, Crowe PJ, Moa A, Macintyre CR |title=The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis |journal=Plos One |volume=8 |issue=7 |pages=e69238 |year=2013 |pmid=23894436 |pmc=3722293 |doi=10.1371/journal.pone.0069238 |url=http://dx.plos.org/10.1371/journal.pone.0069238}}</ref> The relationship is unclear.<ref name=InterSCOPE-2012>{{cite journal |author=Sitas F, Egger S, Urban MI, Taylor PR, Abnet CC, Boffetta P, O'Connell DL, Whiteman DC, Brennan P, Malekzadeh R, Pawlita M, Dawsey SM, Waterboer T |title=InterSCOPE study: Associations between esophageal squamous cell carcinoma and human papillomavirus serological markers |journal=Journal of the National Cancer Institute |volume=104 |issue=2 |pages=147–58 |year=2012 |month=January |pmid=22228147 |pmc=3260131 |doi=10.1093/jnci/djr499 |url=http://jnci.oxfordjournals.org/content/104/2/147.full}}</ref> Possible relevance of HPV could be greater in places that have a particularly high incidence of this form of the disease,<ref name=Syrjanen-2013>{{cite journal | last=Syrjänen | first=K |title=Geographic origin is a significant determinant of human papillomavirus prevalence in oesophageal squamous cell carcinoma: systematic review and meta-analysis |journal=Scandinavian Journal of Infectious Diseases |volume=45 |issue=1 |pages=1–18 |year=2013 |month=January |pmid=22830571 |doi=10.3109/00365548.2012.702281 |url=}}</ref> as in some Asian countries, including China.<ref name=Hardefeldt-2014>{{cite journal |last1=Hardefeldt | first1=HA | last2= Cox |first2=MR | last3=Eslick | first3=GD |title=Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis |journal=Epidemiology and Infection |volume=142 |issue=6 |pages=1119–37 |year=2014 |month=June |pmid=24721187 |doi=10.1017/S0950268814000016}}</ref> | * There is some evidence suggesting a possible causal association between ] (HPV) and the squamous cell type.<ref name=Liyanage-2014>{{cite journal |author=Liyanage SS, Rahman B, Ridda I, Newall AT, Tabrizi SN, Garland SM, Segelov E, Seale H, Crowe PJ, Moa A, Macintyre CR |title=The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis |journal=Plos One |volume=8 |issue=7 |pages=e69238 |year=2013 |pmid=23894436 |pmc=3722293 |doi=10.1371/journal.pone.0069238 |url=http://dx.plos.org/10.1371/journal.pone.0069238}}</ref> The relationship is unclear.<ref name=InterSCOPE-2012>{{cite journal |author=Sitas F, Egger S, Urban MI, Taylor PR, Abnet CC, Boffetta P, O'Connell DL, Whiteman DC, Brennan P, Malekzadeh R, Pawlita M, Dawsey SM, Waterboer T |title=InterSCOPE study: Associations between esophageal squamous cell carcinoma and human papillomavirus serological markers |journal=Journal of the National Cancer Institute |volume=104 |issue=2 |pages=147–58 |year=2012 |month=January |pmid=22228147 |pmc=3260131 |doi=10.1093/jnci/djr499 |url=http://jnci.oxfordjournals.org/content/104/2/147.full}}</ref> Possible relevance of HPV could be greater in places that have a particularly high incidence of this form of the disease,<ref name=Syrjanen-2013>{{cite journal | last=Syrjänen | first=K |title=Geographic origin is a significant determinant of human papillomavirus prevalence in oesophageal squamous cell carcinoma: systematic review and meta-analysis |journal=Scandinavian Journal of Infectious Diseases |volume=45 |issue=1 |pages=1–18 |year=2013 |month=January |pmid=22830571 |doi=10.3109/00365548.2012.702281 |url=}}</ref> as in some Asian countries, including China.<ref name=Hardefeldt-2014>{{cite journal |last1=Hardefeldt | first1=HA | last2= Cox |first2=MR | last3=Eslick | first3=GD |title=Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis |journal=Epidemiology and Infection |volume=142 |issue=6 |pages=1119–37 |year=2014 |month=June |pmid=24721187 |doi=10.1017/S0950268814000016}}</ref> | ||
| * There is little evidence to support an association between ] and esophageal cancer.<ref name=Nyren-2008>{{cite book|last1=Nyrén|first1=O|last2=Adami|first2=HO|editor=Adami, HO; Hunter DJ; Trichopoulos D|title=Textbook of Cancer Epidemiology|url=http://books.google.com/books?id=kuvMDqQuKEUC&pg=PA224|volume=Volume 1|year=2008|publisher=Oxford University Press|isbn=978-0-19-531117-4|page=224|chapter=Esophageal Cancer}}</ref> | |||
| ==Diagnosis== | ==Diagnosis== | ||
Revision as of 13:20, 11 August 2014
Medical condition| Esophageal cancer | |
|---|---|
| Specialty | Oncology, general surgery, gastroenterology |
Esophageal cancer (or oesophageal cancer) is cancer arising from the esophagus—the foodpipe that runs between the throat and the stomach. Symptoms often include trouble swallowing and weight loss. Other symptoms may include pain with swallowing, a hoarse voice, enlarged lymph nodes (glands) around the clavicle (collarbone), a dry cough, and possibly coughing up or vomiting blood.
The two main sub-types of esophageal cancer are squamous cell cancer, which is more common in the developing world, and adenocarcinoma, which is more common in the developed world. A number of less common types also occur. The most common causes of the squamous variety are: smoking tobacco, drinking alcohol, drinking very hot drinks, and a poor diet. The most common causes of the adenocarcinoma variety are smoking tobacco, obesity, and acid reflux. Squamous cell cancer arises from the skin cells that line the esophagus. Adenocarcinoma arises from glandular cells present in the lower third of the esophagus, often where they have already transformed to intestinal cell type (a condition known as Barrett's esophagus).
The disease is diagnosed by biopsy done by an endoscope (a fiberoptic camera). Prevention includes stopping smoking and a healthy diet. Treatment is based on the cancer's stage and location, together with the person's general condition and individual preferences. Small localized squamous cancers may be treated with surgery alone with the hope of a cure. In most other cases, chemotherapy with or without radiation therapy is used along with surgery. Larger tumors may have their growth slowed with chemotherapy and radiation therapy. In the presence of extensive disease or if the affected person is not fit enough to undergo surgery, palliative care is often recommended. Outcomes are related to the extent of the disease and other medical conditions, but generally tend to be fairly poor, as diagnosis is often late. Five-year survival rates are around 13% to 18%.
As of 2012, esophageal cancer is the eighth-most common cancer globally with 456,000 new cases during the year. It caused about 400,000 deaths that year, up from 345,000 in 1990. Rates vary widely between countries, with about half of all cases occurring in China. It is around three times more common in men than in women.
Signs and symptoms
Prominent symptoms usually do not appear until the cancer has infiltrated over 60% of the tube's circumference, by which time the tumor is already in an advanced stage. Onset of symptoms is usually caused by stenosis (i.e. narrowing of the tube due to the physical presence of the tumor).
The first and the most common symptom is usually difficulty in swallowing (dysphagia), often initially experienced with solid foods and later with softer foods and liquids. Pain when swallowing is less usual initially. Weight loss is often an initial symptom in cases of the squamous cell carcinoma type, though not usually in cases of the adenocarcinoma type. Eventual weight loss due to reduced appetite and malnutrition is common. Pain behind the sternum (breastbone) or in the epigastric region around the stomach often feels like heartburn. The pain can frequently be severe, worsening when food of any sort is swallowed. Another sign may be an unusually husky, raspy, or hoarse-sounding cough, a result of the tumor affecting the recurrent laryngeal nerve.
The presence of the tumor may disrupt the normal contractions of the esophagus on swallowing. This can lead to nausea and vomiting, regurgitation of food, coughing, and an increased risk of aspiration pneumonia. Abnormal connections (fistulas) occasionally develop between the esophagus and the trachea (windpipe). Early signs of this serious complication may be coughing on drinking or eating. Fistulas often lead to pneumonia, which is usually heralded by cough, fever, or aspiration. The tumor surface may be fragile and bleed, causing vomiting of blood. Compression of local structures occurs in advanced disease, leading to such problems as upper airway obstruction and superior vena cava syndrome. Symptoms of hypercalcemia (excess calcium in the blood) may occur.
If the cancer has spread elsewhere, symptoms related to metastatic disease may appear. Common sites of metastasis include nearby lymph nodes, the liver, lungs and bone. Liver metastasis can cause jaundice and ascites, while lung metastasis can cause shortness of breath, pleural effusions, etc.
Causes

A number of risk factors for esophageal cancer have been identified. The two main subtypes have different risk factors, but both types are more common in men, and are most common in the over-60s.
Tobacco and alcohol are the greatest risk factors for esophageal squamous cell carcinoma, especially in combination (when they appear to exert a strong additive effect). Some data suggest that about half of squamous cell carcinoma cases are due to tobacco and about one-third to alcohol, while over three-quarters of the cases in men are due to the combination of smoking and heavy drinking.
Gastroesophageal reflux disease (GERD) increases the risk of esophageal adenocarcinoma. This is because chronic irritation of the mucosal lining by stomach acids and bile acids can lead to Barrett's esophagus, which is potentially precancerous. Obesity increases the risk of adenocarcinoma significantly, and the more severe the obesity the higher the risk. It is suspected that increased risk of reflux may be behind this association.
- Corrosive injury to the esophagus by accidentally or intentionally swallowing caustic substances.
- Particular dietary substances, such as nitrosamines
- A medical history of other head and neck cancers increases the chance of developing a second cancer in the head and neck area, including esophageal cancer.
- Plummer–Vinson syndrome (anemia and esophageal webbing)
- Tylosis and Howel–Evans syndrome (hereditary thickening of the skin of the palms and soles)
- Radiation therapy for other conditions in the mediastinum
- Achalasia
- The role of Helicobacter pylori in progression to esophageal adenocarcinoma is still uncertain, but, on the basis of population data, it may carry a protective effect. It is postulated that H. pylori induces chronic gastritis, which is a risk factor for reflux, which in turn is a risk factor for esophageal adenocarcinoma.
- There is some evidence suggesting a possible causal association between human papillomavirus (HPV) and the squamous cell type. The relationship is unclear. Possible relevance of HPV could be greater in places that have a particularly high incidence of this form of the disease, as in some Asian countries, including China.
- There is little evidence to support an association between celiac disease and esophageal cancer.
Diagnosis

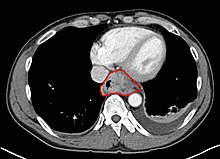
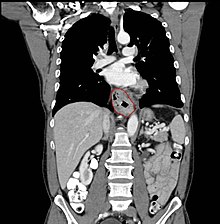
Clinical evaluation
Although an occlusive tumor may be suspected on a barium swallow or barium meal, the diagnosis is best made with esophagogastroduodenoscopy (endoscopy); this involves the passing of a flexible tube down the esophagus and examining the wall. Biopsies taken of suspicious lesions are then examined histologically for signs of malignancy.
Additional testing is usually performed to estimate the tumor stage. Computed tomography (CT) of the chest, abdomen and pelvis can evaluate whether the cancer has spread to adjacent tissues or distant organs (especially liver and lymph nodes). The sensitivity of a CT scan is limited by its ability to detect masses (e.g. enlarged lymph nodes or involved organs) generally larger than 1 cm. Positron emission tomography is also used to estimate the extent of the disease and is regarded as more precise than CT alone. Esophageal endoscopic ultrasound can provide staging information regarding the level of tumor invasion, and possible spread to regional lymph nodes.
The location of the tumor is generally measured by the distance from the teeth. The esophagus (25 cm or 10 in long) is commonly divided into three parts for purposes of determining the location. Adenocarcinomas tend to occur distally and squamous cell carcinomas proximally, but the converse may also be the case.
Classification

Esophageal cancers are typically carcinomas which arise from the epithelium, or surface lining, of the esophagus. Most esophageal cancers fall into one of two classes: squamous cell carcinomas, which are similar to head and neck cancer in their appearance and association with tobacco and alcohol consumption, and adenocarcinomas, which are often associated with a history of GERD and Barrett's esophagus. A general rule of thumb is that a cancer in the upper two-thirds is a squamous cell carcinoma and one in the lower one-third is an adenocarcinoma.
Rare histologic types of esophageal cancer are different variants of the squamous cell carcinoma, and nonepithelial tumors, such as leiomyosarcoma, malignant melanoma, rhabdomyosarcoma, lymphoma, and others.
Prevention
Prevention includes stopping smoking.
- Risk appears to be less in people using aspirin or related NSAIDs, but this is not conclusive.
- According to the National Cancer Institute, "diets high in cruciferous (cabbage, broccoli/broccolini, cauliflower, Brussels sprouts) and green and yellow vegetables and fruits are associated with a decreased risk of esophageal cancer."
- Moderate coffee consumption is associated with a decreased risk.
Screening
People with Barrett esophagus (a change in the cells lining the lower esophagus) are at much higher risk, and should undergo regular endoscopic screening for the early signs of cancer. Because the benefit of screening for adenocarcinoma in people without symptoms is unclear, it is not recommended in the United States. Some areas of the world with high rates of squamous carcinoma have screening programs.
Management
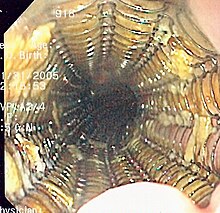
The treatment is determined by the cellular type of cancer (adenocarcinoma or squamous cell carcinoma vs other types), the stage of the disease, the general condition of the patient, and other diseases present. On the whole, adequate nutrition needs to be assured, and adequate dental care is vital.
If the person cannot swallow at all, an esophageal stent may be inserted to keep the esophagus open; stents may also assist in occluding fistulas. A nasogastric tube may be necessary to continue feeding while treatment for the tumor is given, and some patients require a gastrostomy (feeding hole in the skin that gives direct access to the stomach). The latter two are especially important if the patient tends to aspirate food or saliva into the airways, predisposing for aspiration pneumonia.
Esophagectomy is the removal of a segment of the esophagus; as this shortens the length of the remaining esophagus, some other segment of the digestive tract (typically the stomach or part of the colon or jejunum) is pulled up to the chest cavity and interposed. If the tumor is not resectable or the patient is not fit for surgery, palliative esophageal stenting can allow the patient to tolerate a soft diet.
Types of esophagectomy:
- The thoracoabdominal approach opens the abdominal and thoracic cavities together.
- The two-stage Ivor Lewis (also called Lewis–Tanner) approach involves an initial laparotomy and construction of a gastric tube, followed by a right thoracotomy to excise the tumor and create an esophagogastric anastomosis.
- The three-stage McKeown approach adds a third incision in the neck to complete the cervical anastomosis.
Data are accumulating to indicate endoscopic therapy is a safe, less invasive, and effective therapy for very early esophageal cancer. The candidates for endoscopic therapy are stage 1 patients with tumors invading into the lamina propria (T1 mucosal) or submucosa (T1 submucosal) who do not have regional or distant metastasis. Patients with carcinoma ''in situ'' or high-grade dysplasia can also be treated with endoscopic therapy. Submucosal cancers with increased risk of nodal metastases may not be as amenable to curative therapy.
Endoscopic resection
Forms of endoscopic therapy have been used for stage 0 and I disease: endoscopic mucosal resection (EMR) and mucosal ablation using radiofrequency ablation, photodynamic therapy, Nd-YAG laser, or argon plasma coagulation.
EMR has been advocated for early cancers (that is, those that are superficial and confined to the mucosa only) and has been shown to be a less invasive, safe, and highly effective nonsurgical therapy for early squamous cell esophageal cancer. It has also been shown to have be safe and effective for early adenocarcinoma arising in Barrett’s esophagus. The prognosis after treatment with EMR is comparable to surgical resection. This technique can be attempted in patients, without evidence of nodal or distant metastases, with differentiated tumors that are slightly raised and less than 2 cm in diameter, or in differentiated tumors that are ulcerated and less than 1 cm in diameter. The most commonly employed modalities of EMR include strip biopsy, double-snare polypectomy, resection with combined use of highly concentrated saline and epinephrine, and resection using a cap.
The strip biopsy method for endoscopic mucosal resection of esophageal cancer is performed with a double-channel endoscope equipped with grasping forceps and snare. After marking the lesion border with an electric coagulator, saline is injected into the submucosa below the lesion to separate the lesion from the muscle layer and to force its protrusion. The grasping forceps are passed through the snare loop. The mucosa surrounding the lesion is grasped, lifted, and strangulated and resected by electrocautery. The endoscopic double-snare polypectomy method is indicated for protruding lesions. Using a double-channel scope, the lesion is grasped and lifted by the first snare and strangulated with the second snare for complete resection.
Endoscopic resection with injection of concentrated saline and epinephrine is carried out using a double-channel scope. The lesion borders are marked with a coagulator. Highly concentrated saline and epinephrine are injected (15–20 ml) into the submucosal layer to swell the area containing the lesion and elucidate the markings. The mucosa outside the demarcated border is excised using a high-frequency scalpel to the depth of the submucosal layer. The resected mucosa is lifted and grasped with forceps, trapping and strangulating the lesion with a snare, and then resected by electrocautery.
Another method of EMR employs the use of a clear cap and prelooped snare inside the cap. After insertion, the cap is placed on the lesion and the mucosa containing the lesion is drawn up inside the cap by aspiration. The mucosa is caught by the snare and strangulated, and finally resected by electrocautery. This is called the "band and snare" or "suck and cut" technique. The resected specimen is retrieved and submitted for microscopic examination for determination of tumor invasion depth, resection margin, and possible vascular involvement. The resulting "ulcer" heals within three weeks.
EMR can also be used to debulk or completely treat polypoid dysplastic or malignant lesions in Barrett’s esophagus, the known precursor lesion to esophageal adenocarcinoma. In a preliminary report from Germany, EMR was performed as primary treatment or adjunctive therapy following photodynamic therapy for early adenocarcinomas in Barrett's esophagus. The "suck and cut" technique (with and without prior saline injection) was used, as well as the "band and cut" technique. Although all tumors were resected without difficulty, 12.5% developed bleeding (which was managed successfully by endoscopic therapy). Eighty-one percent of the lesions were completely resected. The other lesions were also treated with other endoscopic techniques.
The major complications of endoscopic mucosal resection include postoperative bleeding, perforation, and stricture formation. During the procedure, an injection of 100,000 times diluted epinephrine into the muscular wall, along with high-frequency coagulation or clipping, can be applied to the bleeding point for hemostasis. It is important to administer acid-reducing medications to prevent postoperative hemorrhage. Perforation may be prevented with sufficient saline injection to raise the mucosa containing the lesion. The "nonlifting sign" and complaints of pain when the snare strangulates the lesion are contrainidications of EMR. When perforation is recognized immediately after a procedure, the perforation should be closed by clips. Surgery should be considered in cases of endoscopic closure failure. The incidence of complications ranges from 0–50% and squamous cell recurrence rates range from 0–8%.
Other approaches
Laser therapy is the use of high-intensity light to destroy tumor cells; it affects only the treated area. This is typically done if the cancer cannot be removed by surgery. The relief of a blockage can help to reduce dysphagia and pain. Photodynamic therapy, a type of laser therapy, involves the use of drugs that are absorbed by cancer cells; when exposed to a special light, the drugs become active and destroy the cancer cells.
Chemotherapy depends on the tumor type, but tends to be cisplatin-based (or carboplatin or oxaliplatin) every three weeks with fluorouracil (5-FU) either continuously or every three weeks. In more recent studies, addition of epirubicin was better than other comparable regimens in advanced nonresectable cancer. Chemotherapy may be given after surgery (adjuvant, i.e. to reduce risk of recurrence), before surgery (neoadjuvant) or if surgery is not possible; in this case, cisplatin and 5-FU are used. Ongoing trials compare various combinations of chemotherapy; the phase II/III REAL-2 trial – for example – compares four regimens containing epirubicin and either cisplatin or oxaliplatin, and either continuously infused fluorouracil or capecitabine.

Radiotherapy is given before, during, or after chemotherapy or surgery, and sometimes on its own to control symptoms. In patients with localised disease but contraindications to surgery, "radical radiotherapy" may be used with curative intent.
Radiofrequency ablation is a new treatment modality for the treatment of Barrett's esophagus and dysplasia, and has been the subject of numerous published clinical trials. The findings demonstrate radiofrequency ablation has an efficacy of 80–90% or greater with respect to complete clearance of Barrett's esophagus and dysplasia with durability up to five years and a favorable safety profile. Recent clinical trials have shown that endoscopic resection of esophageal mucosal irregularities and nodules which contain dysplasia or carcinoma combined with subsequent radiofrequency ablation of the remaining flat Barrett's esophagus and dysplasia can effectively and safely eradicate the disease. Further, a recent multicenter randomized control trial found that in patients with Barrett's esophagus containing nodules or mucosal irregularities which contained high grade dysplasia or cancer, subsequent radiofrequency ablation resulted not only in eradication of Barrett's esophagus and dysplasia, but also had significantly less esophageal stricture versus patients who had circumferential endoscopic mucosal resection for their disease.
Follow-up
Patients are followed up frequently after a treatment regimen has been completed. Frequently, other treatments are necessary to improve symptoms and maximize nutrition.
Prognosis
In general, the prognosis of esophageal cancer is quite poor, because most patients present with advanced disease. By the time the first symptoms such as dysphagia start manifesting themselves, the cancer has already well progressed. The overall five-year survival rate (5YSR) in the United States is around 15%, with most people dying within the first year of diagnosis.
Individualized prognosis depends largely on stage. Those with cancer restricted entirely to the esophageal mucosa have about an 80% 5YSR, but submucosal involvement brings this down to less than 50%. Extension into the muscularis propria (muscular layer of the esophagus) has meant a 20% 5YSR and extension to the structures adjacent to the esophagus results in a 7% 5YSR. Patients with distant metastases (who are not candidates for curative surgery) have a less than 3% 5YSR.
Epidemiology

Esophageal cancer is the eighth most frequently diagnosed cancer worldwide, and because of its poor prognosis it is the sixth most common cause of cancer-related death. It caused about 395,000 deaths in 2010, up from 345,000 in 1990. Its incidence varies greatly among different geographical areas, as does the relative preponderance of the two main types. The cancer is particularly frequent in the so-called "Asian esophageal cancer belt", an area that passes through northern China, southern Russia, north-eastern Iran, northern Afghanistan and eastern Turkey. In Sub-Saharan Africa, where the problem has long been recognized, incidence is much higher in countries in the eastern and southern parts of the region.
Squamous cell carcinoma is the most common type of esophageal cancer worldwide, comprising 60-70% of all cases, while adenocarcinomas account for a further 20-30% (melanomas, leiomyosarcomas, carcinoids and lymphomas being rarely diagnosed). In the Asian esophageal cancer belt that stretches from northeast China to the Middle East, incidence rates of the squamous cell variety have been reported to be as high as 100 new cases per 100,000 person-years.
In some developed countries, including Australia, Finland, France, the United States and the United Kingdom, adenocarcinoma is the most common form. Incidence of adenocarcinoma has increased in Western countries (where the incidence of squamous cell carcinoma is largely stable).
In the United States, esophageal cancer is the seventh-leading cause of cancer death among males (making up 4% of the total). The National Cancer Institute estimated there were about 18,000 new cases and more than 15,000 deaths from esophageal cancer in 2013 (the American Cancer Society estimated that during 2014, about 18,170 new esophageal cancer cases will be diagnosed, resulting in 15,450 deaths). The squamous cell type is more common among African American males with a history of heavy smoking or alcohol use. Until the 1970s, squamous cell carcinoma made up the vast majority of esophageal cancers in the United States. In recent decades, incidence of adenocarcinoma of the esophagus (which is associated with Barrett's esophagus) steadily rose in the United States to the point that it has now surpassed squamous cell carcinoma. In contrast to squamous cell carcinoma, esophageal adenocarcinoma is more common in Caucasian men (over the age of 60) than it is in African Americans. Multiple reports indicate esophageal adenocarcinoma incidence has increased during the past 20 years, especially in non-Hispanic white men. Esophageal adenocarcinoma age-adjusted incidence increased in New Mexico from 1973 to 1997. This increase was found in non-Hispanic whites and Hispanics and became predominant in non-Hispanic whites. Esophageal cancer incidence and mortality rates for African Americans continue to be higher than the rate for Causasians. However, incidence and mortality of esophageal cancer has significantly decreased among African Americans since the early 1980s, whereas with Caucasians, it has slightly increased. Between 1975 and 2004, incidence of the adenocarcinoma type increased among white American males by over 460% and among white American females by 335%.
References
- ^ Montgomery, EA; et al. (2014). "Oesophageal Cancer". In Stewart, BW; Wild, CP (ed.). World Cancer Report 2014. World Health Organization. pp. 528–543. ISBN 9283204298.
{{cite book}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: editors list (link) - ^ Ferri, Fred (2012). Ferri's clinical advisor 2013 5 books in 1 (1st ed. ed.). St. Louis, Mo.: Elsevier Mosby. pp. 389–391. ISBN 9780323083737.
{{cite book}}:|edition=has extra text (help) - ^ Zhang, HZ; Jin, GF; Shen, HB (Jun 2012). "Epidemiologic differences in esophageal cancer between Asian and Western populations". Chinese journal of cancer. 31 (6): 281–6. doi:10.5732/cjc.011.10390. PMC 3777490. PMID 22507220.
- Kelsen, David (2007). Gastrointestinal oncology: principles and practices (2nd ed. ed.). Philadelphia, Pa.: Lippincott Williams & Wilkins. p. 4. ISBN 9780781776172.
{{cite book}}:|edition=has extra text (help) - Whittemore, edited by David Schottenfeld, Joseph F. Fraumeni, Jr.; associate editors, Graham A. Colditz, Jonathan M. Samet, Alice S. (2006). Cancer epidemiology and prevention (3rd ed. ed.). Oxford: Oxford University Press. p. 697. ISBN 9780199747979.
{{cite book}}:|edition=has extra text (help);|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Stahl, M; Mariette, C; Haustermans, K; Cervantes, A; Arnold, D; ESMO Guidelines Working, Group (Oct 2013). "Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 24 Suppl 6: vi51-6. doi:10.1093/annonc/mdt342. PMID 24078662.
- ^ Enzinger PC, Mayer RJ (2003). "Esophageal cancer" (PDF). N. Engl. J. Med. 349 (23): 2241–52. doi:10.1056/NEJMra035010. PMID 14657432.
- "SEER Stat Fact Sheets: Esophageal Cancer". National Cancer Institute. Retrieved 18 June 2014.
- ^ Lozano, R; Naghavi, M; Foreman, K; Lim, S; Shibuya, K; Aboyans, V; Abraham, J; Adair, T; Aggarwal, R (Dec 15, 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
- ^ Mayer, Robert J (2008). "Gastrointestinal Tract Cancer". In Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. (ed.). Harrison's principles of internal medicine. Vol. 1 (18th ed.). New York: McGraw-Hill Medical Publishing Division. pp. 764–5. ISBN 978-0071748896.
{{cite book}}: CS1 maint: multiple names: editors list (link) - Cheifetz, Adam S; Brown, Alphonso; Curry, Michael; Moss, Alan C (2011). Oxford American Handbook of Gastroenterology and Hepatology. Oxford University Press. p. 106. ISBN 978-0-19-983012-1.
- Pennathur A, Gibson MK, Jobe BA, Luketich JD (February 2013). "Oesophageal carcinoma". Lancet. 381 (9864): 400–12. doi:10.1016/S0140-6736(12)60643-6. PMID 23374478.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Yamada, Tadataka (2011). Textbook of Gastroenterology. John Wiley & Sons. pp. 1590–1. ISBN 978-1-4443-5941-1.
- Gerdes, Hans; Ferguson, Mark K (2002). "Palliation of Esophageal Cancer". In Posner, Mitchell C; Vokes, Everett E; Weichselbaum, Ralph R (ed.). Cancer of the Upper Gastrointestinal Tract. PMPH-USA. p. 184. ISBN 978-1-55009-101-4.
{{cite book}}: CS1 maint: multiple names: editors list (link) - Lepage C, Drouillard A, Jouve JL, Faivre J (August 2013). "Epidemiology and risk factors for oesophageal adenocarcinoma" (PDF). Digestive and Liver Disease. 45 (8): 625–9. doi:10.1016/j.dld.2012.12.020. PMID 23453359.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Tobias JS, Hochhauser D (2013). Cancer and its management (6th ed.). p. 254. ISBN 1-11871-325-7.
- Napier KJ, Scheerer M, Misra S (May 15, 2014). "Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities". World J Gastrointest Oncol. 6 (5): 112–20. doi:10.4251/wjgo.v6.i5.112. PMC 4021327. PMID 24834141.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Prabhu, A; Obi, KO; Rubenstein, JH (2014). "The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis". The American Journal of Gastroenterology. 109 (6): 822–7. doi:10.1038/ajg.2014.71. PMID 24751582.
{{cite journal}}: Unknown parameter|month=ignored (help) - Lagergren J, Bergström R, Lindgren A, Nyrén O (1999). "Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma". N. Engl. J. Med. 340 (11): 825–31. doi:10.1056/NEJM199903183401101. PMID 10080844.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bernstein H, Bernstein C, Payne CM, Dvorak K (2009). "Bile acids as endogenous etiologic agents in gastrointestinal cancer". World J. Gastroenterol. 15 (27): 3329–40. doi:10.3748/wjg.15.3329. PMC 2712893. PMID 19610133.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Turati F, Tramacere I, La Vecchia C, Negri E (2013). "A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma". Ann. Oncol. 24 (3): 609–17. doi:10.1093/annonc/mds244. PMID 22898040.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Park W, Vaezi M (2005). "Etiology and pathogenesis of achalasia: the current understanding". Am J Gastroenterol. 100 (6): 1404–14. doi:10.1111/j.1572-0241.2005.41775.x. PMID 15929777.
- Wong A, Fitzgerald RC (2005). "Epidemiologic risk factors for Barrett's esophagus and associated adenocarcinoma". Clin. Gastroenterol. Hepatol. 3 (1): 1–10. doi:10.1016/S1542-3565(04)00602-0. PMID 15645398.
- Ye W, Held M, Lagergren J; et al. (2004). "Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia". J. Natl. Cancer Inst. 96 (5): 388–96. doi:10.1093/jnci/djh057. PMID 14996860.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Nakajima S, Hattori T (2004). "Oesophageal adenocarcinoma or gastric cancer with or without eradication of Helicobacter pylori infection in chronic atrophic gastritis patients: a hypothetical opinion from a systematic review". Aliment. Pharmacol. Ther. 20 Suppl 1: 54–61. doi:10.1111/j.1365-2036.2004.01975.x. PMID 15298606.
- Liyanage SS, Rahman B, Ridda I, Newall AT, Tabrizi SN, Garland SM, Segelov E, Seale H, Crowe PJ, Moa A, Macintyre CR (2013). "The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis". Plos One. 8 (7): e69238. doi:10.1371/journal.pone.0069238. PMC 3722293. PMID 23894436.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Sitas F, Egger S, Urban MI, Taylor PR, Abnet CC, Boffetta P, O'Connell DL, Whiteman DC, Brennan P, Malekzadeh R, Pawlita M, Dawsey SM, Waterboer T (2012). "InterSCOPE study: Associations between esophageal squamous cell carcinoma and human papillomavirus serological markers". Journal of the National Cancer Institute. 104 (2): 147–58. doi:10.1093/jnci/djr499. PMC 3260131. PMID 22228147.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Syrjänen, K (2013). "Geographic origin is a significant determinant of human papillomavirus prevalence in oesophageal squamous cell carcinoma: systematic review and meta-analysis". Scandinavian Journal of Infectious Diseases. 45 (1): 1–18. doi:10.3109/00365548.2012.702281. PMID 22830571.
{{cite journal}}: Unknown parameter|month=ignored (help) - Hardefeldt, HA; Cox, MR; Eslick, GD (2014). "Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis". Epidemiology and Infection. 142 (6): 1119–37. doi:10.1017/S0950268814000016. PMID 24721187.
{{cite journal}}: Unknown parameter|month=ignored (help) - Nyrén, O; Adami, HO (2008). "Esophageal Cancer". In Adami, HO; Hunter DJ; Trichopoulos D (ed.). Textbook of Cancer Epidemiology. Vol. Volume 1. Oxford University Press. p. 224. ISBN 978-0-19-531117-4.
{{cite book}}:|volume=has extra text (help)CS1 maint: multiple names: editors list (link) - W Shield, Thomas. LoCicero, Joseph. B. Ponn, Ronald. (2005). Less Common Malignant Tumors of the Esophagus. Lippincott Williams & Wilkins. pp. 2325–2340. ISBN 978-0-7817-3889-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Halperin, Edward C. (2008). Perez and Brady's principles and practice of radiation oncology. Lippincott Williams & Wilkins. p. 1137. ISBN 978-0-7817-6369-1.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Sun, L; Yu, S (Nov 2011). "Meta-analysis: non-steroidal anti-inflammatory drug use and the risk of esophageal squamous cell carcinoma". Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / I.S.D.E. 24 (8): 544–9. doi:10.1111/j.1442-2050.2011.01198.x. PMID 21539676.
- NCI (2002). "Prevention: Dietary Factors, based on Chainani-Wu N. Diet and oral, pharyngeal, and esophageal cancer". Nutr Cancer. 44 (2): 104–26. doi:10.1207/S15327914NC4402_01. PMID 12734057.
- Tavani, A (October 2003). "Coffee and tea intake and risk of oral, pharyngeal and esophageal cancer". Oral Oncol. 39 (7): 695–700. doi:10.1016/S1368-8375(03)00081-2. PMID 12907209.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zhang Y (September 2013). "Epidemiology of esophageal cancer". World J. Gastroenterol. 19 (34): 5598–606. doi:10.3748/wjg.v19.i34.5598. PMC 3769895. PMID 24039351.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Dunbar KB, Spechler SJ (May 2014). "Controversies in Barrett Esophagus". Mayo Clin. Proc. 89 (7): 973–984. doi:10.1016/j.mayocp.2014.01.022. PMID 24867396.
- Deschamps C, Nichols FC, Cassivi SD; et al. (2005). "Long-term function and quality of life after esophageal resection for cancer and Barrett's". Surgical Clinics of North America. 85 (3): 649–56. doi:10.1016/j.suc.2005.01.018. PMID 15927658.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Ross P, Nicolson M, Cunningham D; et al. (April 2002). "Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer". Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 20 (8): 1996–2004. doi:10.1200/JCO.2002.08.105. PMID 11956258.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Fleischer DE, Overholt, BF, Sharma VK, et al. (2010). "Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial". Endoscopy 42 (10) 781–9.
- Shaheen NJ, Sharma, P, Overholt, BF, et al. (2009). "Radiofrequency Ablation in Barrett's Esophagus with Dysplasia" New England Journal of Medicine 360 (22) 2277–88.
- Shaheen NJ, Overholt, BF, Sampliner, RE et al. (2011). "Durability of Ablation in Barrett's Esophagus with Dysplasia" Gastroenterology 141 (2) 460–8.
- van Vilsteren FG, Pouw RE, Seewald, S et al.. "Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high grade dysplasia or early cancer: a multicentre randomised trial" Gut 60: 765–73.
- Pouw RE, Wirths K, Bergman JJ et. al (2010). "Efficacy of Radiofrequency Ablation Combined with Endoscopic Resection for Barrett's Esophagus with Early Neoplasia". Clinical Gastroenterology and Hepatology (8) 23–20.
- van Vilsteren FGI, Pouw RE, Seewald S, Bergman JJ, et al (2010). "Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophague with high-grade dysplasia or early cancer: a multicentre randomised trial" Gut 60 (6) 765–73.
- Polednak AP (May 2003). "Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas". Int. J. Cancer. 105 (1): 98–100. doi:10.1002/ijc.11029. PMID 12672037.
- "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved Nov 11, 2009.
- ^ Conteduca V, Sansonno D, Ingravallo G, Marangi S, Russi S, Lauletta G, Dammacco F (August 2012). "Barrett's esophagus and esophageal cancer: an overview". International Journal of Oncology. 41 (2): 414–24. doi:10.3892/ijo.2012.1481. PMID 22615011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Napier KJ, Scheerer M, Misra S (May 2014). "Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities". World Journal of Gastrointestinal Oncology. 6 (5): 112–20. doi:10.4251/wjgo.v6.i5.112. PMC 4021327. PMID 24834141.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Kachala R (September 2010). "Systematic review: epidemiology of oesophageal cancer in Sub-Saharan Africa". Malawi Medical Journal : the Journal of Medical Association of Malawi. 22 (3): 65–70. PMC 3345777. PMID 21977849.
- ^ "Cancer Facts and Figures 2014" (PDF). American Cancer Society. Retrieved 28 April 2014.
- Kenneth J. Vega, M.D., M. Mazen JamaM.D.l (September 2000). "Changing pattern of esophageal cancer incidence in New Mexico". Changing pattern of esophageal cancer incidence in New Mexico. The American Journal of Gastroenterology. Retrieved 2007-03-21.
{{cite web}}: CS1 maint: multiple names: authors list (link) - "Incidence and Mortality Rate Trends" (PDF). A Snapshot of Esophageal Cancer. National Cancer Institute. September 2006. Archived from the original (PDF) on 2007-03-16. Retrieved 2007-03-21.
External links
- NCI esophageal cancer
- Cancer.Net: Esophageal Cancer
- Esophageal Cancer From Cancer Management: A Multidisciplinary Approach
- Learn More about Esophageal Cancer
- Oesophageal Cancer at Cancer Research UK

