| Revision as of 18:59, 6 October 2014 editFivemack (talk | contribs)Extended confirmed users1,511 editsNo edit summary← Previous edit | Revision as of 00:25, 7 October 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,700 edits cite a reviewNext edit → | ||
| Line 53: | Line 53: | ||
| }} | }} | ||
| }} | }} | ||
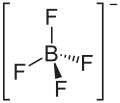
| '''Lithium tetrafluoroborate''' is an ] with the formula ]]. It is a white crystalline powder. It has been extensively tested for use in commercial secondary batteries, an application that exploits its high solubility in nonpolar solvents.<ref name=Kang>Xu, Kang. "Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries."Chemical Reviews 2004, volume 104, pp. 4303-418. doi|10.1021/cr030203g}}</ref> | |||
| '''Lithium tetrafluoroborate''' is a chemical compound with the formula LiBF<sub>4</sub>. It can be dissolved in ], ], and/or ] for use as an ] in ]. | |||
| ==Applications== | ==Applications== | ||
| ⚫ | Although BF<sub>4</sub>- has high ionic mobility, solutions of its Li<sup>+</sup> salt are less conductive than other less associated salts.<ref name=Kang/> As an ] in ], LiBF<sub>4</sub> offers some advantages relative to the more common ]. It exhibits greater thermal stability.<ref>{{cite journal|last=S. Zhang, K. Xu, T. Jow|title=Low-temperature performance of Li-ion cells with a LiBF4-based electrolyte|journal=Journal of Solid State Electrochemistry|year=2003|volume=7|issue=3|pages=147–151|doi=10.1007/s10008-002-0300-9|url=http://www.researchgate.net/publication/241026936_061._Low-temperature_performance_of_Li-ion_cells_with_a_LiBF_4_-based_electrolyte/file/5046351d38dc83bb9b.pdf|accessdate=16 February 2014}}</ref> and moisture.<ref>{{cite journal|last=S. S. Zhang;z K. Xu; and T. R. Jow|title=Study of LiBF4 as an Electrolyte Salt for a Li-Ion Battery|journal=Journal of The Electrochemical Society|year=2002|volume=149|issue=5|pages=A586-A590|doi=10.1149/1.1466857|url=http://www.researchgate.net/publication/244478865_044._Study_of_LiBF_4_as_an_Electrolyte_Salt_for_a_Li-Ion_Battery/file/5046351d3a44c137d3.pdf.|accessdate=16 February 2014}}</ref> For example LiBF<sub>4</sub> can tolerate a moisture content up to 620 ] at room temperature whereas LiPF<sub>6</sub> readily hydrolyzes into toxic ] and ] gases, often destroying the battery's ] materials. Disadvantages of the electrolyte include a relatively low conductivity and difficulties forming a stable solid electrolyte interface with ] electrodes. | ||
| ==Thermal stability== | |||
| ⚫ | |||
| Because LiBF<sub>4</sub> and other ] salts thermally decompose to evolve ], the salt is commonly used as a convenient source of the chemical at the laboratory scale:<ref name="Ullmann">{{cite journal|last=Robert, Brotherton; Joseph, Weber; Clarence, Guibert; and John, Little|title=Boron Compounds|year=2000|journal=Ullmann's Encyclopedia of Industrial Chemistry|page=pg. 10|doi=10.1002/14356007.a04_309|accessdate=16 February 2014}}</ref> | Because LiBF<sub>4</sub> and other ] salts thermally decompose to evolve ], the salt is commonly used as a convenient source of the chemical at the laboratory scale:<ref name="Ullmann">{{cite journal|last=Robert, Brotherton; Joseph, Weber; Clarence, Guibert; and John, Little|title=Boron Compounds|year=2000|journal=Ullmann's Encyclopedia of Industrial Chemistry|page=pg. 10|doi=10.1002/14356007.a04_309|accessdate=16 February 2014}}</ref> | ||
| Line 68: | Line 67: | ||
| :8 BF<sub>3</sub> + 6 ] → ] + 6 LiBF<sub>4</sub> | :8 BF<sub>3</sub> + 6 ] → ] + 6 LiBF<sub>4</sub> | ||
| LiBF<sub>4</sub> can also be synthesized from LiF and BF<sub>3</sub> |
LiBF<sub>4</sub> can also be synthesized from LiF and BF<sub>3</sub> in an appropriate solvent (e.g., ], ], or liquified ].<ref name="Ullmann" />; the solvent has to be resistant to fluorination by the BF<sub>3</sub>. | ||
| : LiF + BF<sub>3</sub> → LiBF<sub>4</sub> | : LiF + BF<sub>3</sub> → LiBF<sub>4</sub> | ||
Revision as of 00:25, 7 October 2014
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Lithium tetrafluoroborate | |||
| Other names Borate(1-), tetrafluoro-, lithium | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.034.692 | ||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | LiBF4 | ||
| Molar mass | 93.746 g/mol | ||
| Appearance | White/grey crystalline solid | ||
| Odor | odorless | ||
| Density | 0.852 g/cm solid | ||
| Melting point | 296.5 °C | ||
| Boiling point | decomp | ||
| Solubility in water | Very soluble | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Harmful, causes burns, hygroscopic. | ||
| NFPA 704 (fire diamond) |
 | ||
| Related compounds | |||
| Other anions | Tetrafluoroborate, | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Lithium tetrafluoroborate is an inorganic compound with the formula LiBF4. It is a white crystalline powder. It has been extensively tested for use in commercial secondary batteries, an application that exploits its high solubility in nonpolar solvents.
Applications
Although BF4- has high ionic mobility, solutions of its Li salt are less conductive than other less associated salts. As an electrolyte in Lithium-ion batteries, LiBF4 offers some advantages relative to the more common LiPF6. It exhibits greater thermal stability. and moisture. For example LiBF4 can tolerate a moisture content up to 620 ppm at room temperature whereas LiPF6 readily hydrolyzes into toxic POF3 and HF gases, often destroying the battery's electrode materials. Disadvantages of the electrolyte include a relatively low conductivity and difficulties forming a stable solid electrolyte interface with graphite electrodes.
Thermal stability
Because LiBF4 and other alkali-metal salts thermally decompose to evolve boron trifluoride, the salt is commonly used as a convenient source of the chemical at the laboratory scale:
Production
LiBF4 is a byproduct in the industrial synthesis of diborane:
LiBF4 can also be synthesized from LiF and BF3 in an appropriate solvent (e.g., hydrogen fluoride, BrF3, or liquified SO2.; the solvent has to be resistant to fluorination by the BF3.
- LiF + BF3 → LiBF4
References
- GFS-CHEMICALS
- ^ Xu, Kang. "Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries."Chemical Reviews 2004, volume 104, pp. 4303-418. doi|10.1021/cr030203g}}
- S. Zhang, K. Xu, T. Jow (2003). "Low-temperature performance of Li-ion cells with a LiBF4-based electrolyte" (PDF). Journal of Solid State Electrochemistry. 7 (3): 147–151. doi:10.1007/s10008-002-0300-9. Retrieved 16 February 2014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - S. S. Zhang;z K. Xu; and T. R. Jow (2002). "Study of LiBF4 as an Electrolyte Salt for a Li-Ion Battery". Journal of The Electrochemical Society. 149 (5): A586 – A590. doi:10.1149/1.1466857. Retrieved 16 February 2014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Robert, Brotherton; Joseph, Weber; Clarence, Guibert; and John, Little (2000). "Boron Compounds". Ullmann's Encyclopedia of Industrial Chemistry: pg. 10. doi:10.1002/14356007.a04_309.
{{cite journal}}:|access-date=requires|url=(help);|page=has extra text (help)CS1 maint: multiple names: authors list (link) - Brauer, Georg (1963). Handbook of Preparative Inorganic Chemistry Vol. 1, 2nd Ed. Newyork: Academic Press. p. 773. ISBN 978-0121266011.
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |