| Revision as of 12:05, 30 October 2014 edit77.96.60.31 (talk) →History← Previous edit | Revision as of 13:21, 30 October 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,236 edits rm "This instability may be due to the fact that the true structure ..... " per talk, consolidated discussion of structureNext edit → | ||
| Line 1: | Line 1: | ||
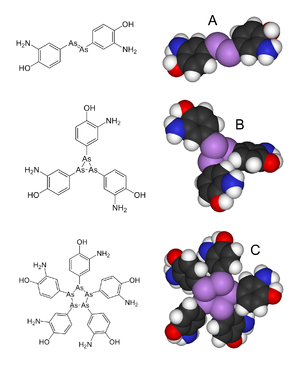
| ] ('''A'''), but mass spectral studies published in 2005 suggest<ref name="pmid15624113"/> it is actually a mixture of the trimer '''B''' and the pentamer '''C'''.]] | ] ('''A'''), but mass spectral studies published in 2005 suggest<ref name="pmid15624113"/> it is actually a mixture of the trimer '''B''' and the pentamer '''C'''.]] | ||
| '''Arsphenamine''', also known as '''Salvarsan''' and '''compound 606''', is a ] that was introduced at the beginning of the 1910s as the first effective treatment for ], and was also used to treat ].<ref name='ITOM'>{{Cite journal |last1=Gibaud |first1=Stéphane |last2=Jaouen |first2=Gérard |title=Arsenic - based drugs: from Fowler's solution to modern anticancer chemotherapy |url=http://link.springer.com/chapter/10.1007%2F978-3-642-13185-1_1 |journal= |year=2010 |volume=32 |pages=1–20 |doi= 10.1007/978-3-642-13185-1_1 |series=Topics in Organometallic Chemistry |isbn=978-3-642-13184-4}}</ref> | '''Arsphenamine''', also known as '''Salvarsan''' and '''compound 606''', is a ] that was introduced at the beginning of the 1910s as the first effective treatment for ], and was also used to treat ].<ref name='ITOM'>{{Cite journal |last1=Gibaud |first1=Stéphane |last2=Jaouen |first2=Gérard |title=Arsenic - based drugs: from Fowler's solution to modern anticancer chemotherapy |url=http://link.springer.com/chapter/10.1007%2F978-3-642-13185-1_1 |journal= |year=2010 |volume=32 |pages=1–20 |doi= 10.1007/978-3-642-13185-1_1 |series=Topics in Organometallic Chemistry |isbn=978-3-642-13184-4}}</ref> | ||
| This ] was the first modern ]. | |||
| ==History== | ==History== | ||
| ] and ] discovered the antisyphilitic activity of this compound in 1909 in Erlich's laboratory, during a survey of hundreds of newly synthesized organic ]al compounds. Ehrlich had theorized that by screening many compounds, a drug could be discovered with ] activity without killing the human patient. Ehrlich's team began their search for such a "]" among chemical derivatives of the dangerously toxic drug ]. This was the first organized team effort to optimize the biological activity of a ] through systematic chemical modifications, the basis for nearly all modern pharmaceutical research. | ] and ] discovered the antisyphilitic activity of this compound in 1909 in Erlich's laboratory, during a survey of hundreds of newly synthesized organic ]al compounds. Ehrlich had theorized that by screening many compounds, a drug could be discovered with ] activity without killing the human patient. Ehrlich's team began their search for such a "]" among chemical derivatives of the dangerously toxic drug ]. This project was the first organized team effort to optimize the biological activity of a ] through systematic chemical modifications, the basis for nearly all modern pharmaceutical research. The target of Arsphenamine is the ] '']'', a ] that causes syphilis. | ||
| Arsphenamine was originally called "606" because it was the sixth in the sixth group of compounds synthesized for testing; it was marketed by ] under the ] '''Salvarsan''' in 1910.<ref name=acs>{{cite web |url=http://pubs.acs.org/cen/coverstory/83/8325/8325salvarsan.html |title=Salvarsan |accessdate=2010-02-01 |quote= |publisher=] }}</ref><ref>In Germany, it was the practice to designate compounds by their development number. Another compound known commonly in Germany by its number is ], which was the 605th compound to be developed in search for insecticide. It is commonly known as ] (E stands for ''Entwicklungsnummer'' (German for "development number"))</ref> Salvarsan was the first organic antisyphilitic, and a great improvement over the inorganic ] compounds that had been used previously. It was distributed as a yellow, crystalline, ] powder that was highly unstable in air.<ref>{{cite web |url=http://chestofbooks.com/health/materia-medica-drugs/American-Medical-Association/A-Handbook-of-Useful-Drugs/Salvarsan-Salvarsan-N-N-R.html |title=A Handbook of Useful Drugs |accessdate=2010-08-17 |quote= |publisher=] |year=1913}}</ref> This significantly complicated administration, as the drug had to be dissolved in several hundred milliliters of distilled, sterile water with minimal exposure to air to produce a solution suitable for injection. Some of the side effects including rashes, liver damage, and risks of life and limb<ref>http://protomag.com/assets/paul-ehrlich-and-the-salvarsan-wars</ref> attributed to Salvarsan were thought to be caused by improper handling and administration, causing Ehrlich, who worked assiduously to standardize practices, to observe, "the step from the laboratory to the patient's bedside ... is extraordinarily arduous and fraught with danger." <ref name=acs /> | Arsphenamine was originally called "606" because it was the sixth in the sixth group of compounds synthesized for testing; it was marketed by ] under the ] '''Salvarsan''' in 1910.<ref name=acs>{{cite web |url=http://pubs.acs.org/cen/coverstory/83/8325/8325salvarsan.html |title=Salvarsan |accessdate=2010-02-01 |quote= |publisher=] }}</ref><ref>In Germany, it was the practice to designate compounds by their development number. Another compound known commonly in Germany by its number is ], which was the 605th compound to be developed in search for insecticide. It is commonly known as ] (E stands for ''Entwicklungsnummer'' (German for "development number"))</ref> Salvarsan was the first organic antisyphilitic, and a great improvement over the inorganic ] compounds that had been used previously. It was distributed as a yellow, crystalline, ] powder that was highly unstable in air.<ref>{{cite web |url=http://chestofbooks.com/health/materia-medica-drugs/American-Medical-Association/A-Handbook-of-Useful-Drugs/Salvarsan-Salvarsan-N-N-R.html |title=A Handbook of Useful Drugs |accessdate=2010-08-17 |quote= |publisher=] |year=1913}}</ref> This significantly complicated administration, as the drug had to be dissolved in several hundred milliliters of distilled, sterile water with minimal exposure to air to produce a solution suitable for injection. Some of the side effects including rashes, liver damage, and risks of life and limb<ref>http://protomag.com/assets/paul-ehrlich-and-the-salvarsan-wars</ref> attributed to Salvarsan were thought to be caused by improper handling and administration, causing Ehrlich, who worked assiduously to standardize practices, to observe, "the step from the laboratory to the patient's bedside ... is extraordinarily arduous and fraught with danger." <ref name=acs /> | ||
| This instability may be due to the fact that the true structure of the compound was not confirmed until 2004. | |||
| Ehrlich originally proposed that Salvarsan's structure was of two double-bonded arsenic atoms, each bonded to an aminophenol group. This became the subject of much debate over the years, particularly because of this unlikely double bond between the arsenic atoms. Scientists found that Salvarsan is in fact is a mixture of three- and five-membered cyclic arsenic species.<ref>http://www.rsc.org/chemistryworld/2013/04/salvarsan-podcast</ref> | |||
| Ehrlich's laboratory developed a more soluble (but slightly less effective) arsenical compound, ] (neoarsphenamine), which was easier to prepare, and it became available in 1912. Less severe side-effects such as nausea and vomiting were still common. An additional problem was that both Salvarsan and Neosalvarsan had to be stored in sealed vials under a ] atmosphere to prevent oxidation These arsenical compounds were supplanted as treatments for syphilis in the 1940s by ].<ref>http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2790789/</ref> | Ehrlich's laboratory developed a more soluble (but slightly less effective) arsenical compound, ] (neoarsphenamine), which was easier to prepare, and it became available in 1912. Less severe side-effects such as nausea and vomiting were still common. An additional problem was that both Salvarsan and Neosalvarsan had to be stored in sealed vials under a ] atmosphere to prevent oxidation These arsenical compounds were supplanted as treatments for syphilis in the 1940s by ].<ref>http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2790789/</ref> | ||
| After leaving Erlich's laboratory, Hata continued parallel investigation of the new medicines in ].<ref>{{cite journal|author=Izumi, Yoshio; and Isozumi, Kazuo|year=2001|url=http://www.kjm.keio.ac.jp/past/50/2/91.pdf|format=free download pdf|title=Modern Japanese medical history and the European influence|journal=Keio Journal of Medicine|volume=50|pages=91–99|pmid=11450598|issue=2|doi=10.2302/kjm.50.91}}</ref> | After leaving Erlich's laboratory, Hata continued parallel investigation of the new medicines in ].<ref>{{cite journal|author=Izumi, Yoshio; and Isozumi, Kazuo|year=2001|url=http://www.kjm.keio.ac.jp/past/50/2/91.pdf|format=free download pdf|title=Modern Japanese medical history and the European influence|journal=Keio Journal of Medicine|volume=50|pages=91–99|pmid=11450598|issue=2|doi=10.2302/kjm.50.91}}</ref> | ||
| ==Mechanism== | |||
| The ] that causes syphilis is a ], '']''. Arsphenamine is not toxic to spirochetes until it has been converted to an active form by the body. | |||
| ==Structure== | ==Structure== | ||
| In terms of its molecular structure, Salvarsan's has long been assumed to feature an As=As ], akin to the N=N linkage in ]. However, in 2005, an extensive ] Salvarsan was shown in fact to be a mixture consisting of cyclo-As<sub>3</sub> and cyclo-As<sub>5</sub> species.<ref>http://www.rsc.org/chemistryworld/2013/04/salvarsan-podcast</ref>.<ref name="pmid15624113">{{cite journal |author=Lloyd NC, Morgan HW, Nicholson BK, Ronimus RS |title=The composition of Ehrlich's salvarsan: resolution of a century-old debate |journal=Angew. Chem. Int. Ed. Engl. |volume=44 |issue=6 |pages=941–4 |year=2005 |pmid=15624113 |doi=10.1002/anie.200461471}}</ref><ref name=acs/> | |||
| ==See also== | ==See also== | ||
Revision as of 13:21, 30 October 2014
Arsphenamine, also known as Salvarsan and compound 606, is a drug that was introduced at the beginning of the 1910s as the first effective treatment for syphilis, and was also used to treat trypanosomiasis. This organoarsenic compound was the first modern chemotherapeutic agent.
History
Sahachiro Hata and Paul Ehrlich discovered the antisyphilitic activity of this compound in 1909 in Erlich's laboratory, during a survey of hundreds of newly synthesized organic arsenical compounds. Ehrlich had theorized that by screening many compounds, a drug could be discovered with antimicrobial activity without killing the human patient. Ehrlich's team began their search for such a "magic bullet" among chemical derivatives of the dangerously toxic drug atoxyl. This project was the first organized team effort to optimize the biological activity of a lead compound through systematic chemical modifications, the basis for nearly all modern pharmaceutical research. The target of Arsphenamine is the bacterium Treponema pallidum, a spirochete that causes syphilis.
Arsphenamine was originally called "606" because it was the sixth in the sixth group of compounds synthesized for testing; it was marketed by Hoechst AG under the trade name Salvarsan in 1910. Salvarsan was the first organic antisyphilitic, and a great improvement over the inorganic mercury compounds that had been used previously. It was distributed as a yellow, crystalline, hygroscopic powder that was highly unstable in air. This significantly complicated administration, as the drug had to be dissolved in several hundred milliliters of distilled, sterile water with minimal exposure to air to produce a solution suitable for injection. Some of the side effects including rashes, liver damage, and risks of life and limb attributed to Salvarsan were thought to be caused by improper handling and administration, causing Ehrlich, who worked assiduously to standardize practices, to observe, "the step from the laboratory to the patient's bedside ... is extraordinarily arduous and fraught with danger."
Ehrlich's laboratory developed a more soluble (but slightly less effective) arsenical compound, Neosalvarsan (neoarsphenamine), which was easier to prepare, and it became available in 1912. Less severe side-effects such as nausea and vomiting were still common. An additional problem was that both Salvarsan and Neosalvarsan had to be stored in sealed vials under a nitrogen atmosphere to prevent oxidation These arsenical compounds were supplanted as treatments for syphilis in the 1940s by penicillin.
After leaving Erlich's laboratory, Hata continued parallel investigation of the new medicines in Japan.
Structure
In terms of its molecular structure, Salvarsan's has long been assumed to feature an As=As double bond, akin to the N=N linkage in ]. However, in 2005, an extensive mass spectral analysis Salvarsan was shown in fact to be a mixture consisting of cyclo-As3 and cyclo-As5 species..
See also
- Dr. Ehrlich's Magic Bullet, 1940 film about Ehrlich's quest to find a cure for syphilis.
References
- ^ Lloyd NC, Morgan HW, Nicholson BK, Ronimus RS (2005). "The composition of Ehrlich's salvarsan: resolution of a century-old debate". Angew. Chem. Int. Ed. Engl. 44 (6): 941–4. doi:10.1002/anie.200461471. PMID 15624113.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gibaud, Stéphane; Jaouen, Gérard (2010). "Arsenic - based drugs: from Fowler's solution to modern anticancer chemotherapy". Topics in Organometallic Chemistry. Topics in Organometallic Chemistry. 32: 1–20. doi:10.1007/978-3-642-13185-1_1. ISBN 978-3-642-13184-4.
{{cite journal}}: External link in|journal= - ^ "Salvarsan". Chemical & Engineering News. Retrieved 2010-02-01.
- In Germany, it was the practice to designate compounds by their development number. Another compound known commonly in Germany by its number is parathion, which was the 605th compound to be developed in search for insecticide. It is commonly known as E605 (E stands for Entwicklungsnummer (German for "development number"))
- "A Handbook of Useful Drugs". American Medical Association. 1913. Retrieved 2010-08-17.
- http://protomag.com/assets/paul-ehrlich-and-the-salvarsan-wars
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2790789/
- Izumi, Yoshio; and Isozumi, Kazuo (2001). "Modern Japanese medical history and the European influence" (free download pdf). Keio Journal of Medicine. 50 (2): 91–99. doi:10.2302/kjm.50.91. PMID 11450598.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - http://www.rsc.org/chemistryworld/2013/04/salvarsan-podcast