| Revision as of 18:34, 23 January 2007 view sourceGiftlite (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers39,612 edits rv← Previous edit | Latest revision as of 18:34, 29 December 2024 view source Warrenmck (talk | contribs)Extended confirmed users1,750 edits forgot a word | ||
| Line 1: | Line 1: | ||
| {{Short description|Form of carbon}} | |||
| {{featured article}} | |||
| {{ |
{{About|the mineral|the gemstone|Diamond (gemstone)|other uses}} | ||
| {{Featured article}} | |||
| ] | |||
| {{Pp|small=yes}} | |||
| <!--First paragraph | |||
| {{Pp-move-indef}} | |||
| Chemistry and Material Properties, Industry Size--> | |||
| {{Use American English|date=March 2024}} | |||
| '''Diamond''' is the hardest known natural material (third-hardest known material below ] and ]), and is the more costly of the two best known forms (or '']'') of ], whose ] and high ] of ] make it useful for industrial applications and ]. (The other equally well known ] is ].) Diamonds are specifically renowned as a ] with superlative physical qualities — they make excellent ]s because they can be scratched only by other diamonds, ], ], or ], which also means they hold a polish extremely well and retain ]. About 130 million ] (26,000 kg) are mined annually, with a total value of nearly ]9 ].{{fact}} About 100 tons are synthesized annually.<ref>{{cite journal|last = Yarnell|first=Amanda|title=The Many Facets of Man-Made Diamonds|journal=Chemical and Engineering News|volume=82|issue=5|pages=26-31|publisher=American Chemical Society|date=2004|url=http://pubs.acs.org/cen/coverstory/8205/8205diamonds.html|id =ISSN 0009-2347|accessdate=2006-10-03 }}</ref> | |||
| {{Use mdy dates|date=March 2024}} | |||
| {{Infobox mineral | |||
| |name = Diamond | |||
| |category = ] | |||
| |boxwidth = | |||
| |boxbgcolor = #7da7d9 | |||
| |image = File:Diamond-dimd15a.jpg | |||
| |imagesize = 260px | |||
| |alt = A triangular prism-shaped diamond | |||
| |caption = A natural diamond crystal | |||
| |formula = ] | |||
| | IMAsymbol = Dia<ref>{{Cite journal| vauthors = Warr LN |date=2021|title=IMA–CNMNC approved mineral symbols|journal=Mineralogical Magazine|volume=85|issue=3|pages=291–320|doi=10.1180/mgm.2021.43|bibcode=2021MinM...85..291W|s2cid=235729616|doi-access=free| issn=0026-461X}}</ref> | |||
| |molweight = {{val|12.01|ul=g/mol}} | |||
| |strunz = 1.CB.10a | |||
| |dana = 1.3.6.1 | |||
| |color = Typically yellow, brown, or gray to colorless. Less often blue, green, black, translucent white, pink, violet, orange, purple, and red. | |||
| |habit = ] | |||
| |system = ] | |||
| |class = Hexoctahedral (m{{Overline|3}}m) <br/>]: (4/m {{Overline|3}} 2/m) | |||
| |symmetry = ''F''d{{overline|3}}m (No. 227) | |||
| |twinning = Spinel law common (yielding "macle") | |||
| |cleavage = 111 (perfect in four directions) | |||
| |fracture = Irregular/Uneven | |||
| |mohs = 10 (defining mineral) | |||
| |luster = ] | |||
| |polish = Adamantine | |||
| |refractive = 2.418 (at 500 nm) | |||
| |opticalprop = Isotropic | |||
| |birefringence = None | |||
| |dispersion = 0.044 | |||
| |pleochroism = None | |||
| |streak = Colorless | |||
| |melt = ] | |||
| |gravity = {{val|3.52|0.01}} | |||
| |density = {{val|3.5|-|3.53|ul=g/cm3}} | |||
| {{val|3500|-|3530|ul=kg/m3}} | |||
| |diaphaneity = ] to subtransparent to translucent | |||
| |references =<ref name=mindat/><ref>{{cite web|publisher=WebMineral|title=Diamond|url=http://webmineral.com/data/Diamond.shtml|access-date=July 7, 2009|archive-date=January 7, 2019|archive-url=https://web.archive.org/web/20190107071332/http://webmineral.com/data/Diamond.shtml|url-status=live}}</ref> | |||
| |SMILES = C1(C2(C7))C3(C89)C(C4(C0))C5CC1C(C1)C(C5(C5))C36C3(C21)C(C78)C(C1)C(C90)C6(C54)CC1C3 | |||
| |Jmol = C1(C2(C7))C3(C89)C(C4(C0))C5CC1C(C1)C(C5(C5))C36C3(C21)C(C78)C(C1)C(C90)C6(C54)CC1C3 | |||
| }} | |||
| ] | |||
| '''Diamond''' is a ] with its atoms arranged in a ] called ]. Diamond as a form of carbon is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as ] is the ] form of carbon at ], but diamond is ] and converts to it at a negligible rate under those conditions. Diamond has the highest ] and ] of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that ]s can subject materials to pressures found deep in the Earth. | |||
| <!--Second paragraph: Etymology and Applications--> | |||
| The name “diamond” derives from the ] ''adamas'' (αδάμας; “invincible”). They have been treasured as ]s since their use as religious ]s in ] at least 2,500 years ago— and usage in ]s and ] tools also dates to early human history.{{fact}} Popularity of diamonds has risen since the 19th century because of increased supply, improved cutting and polishing techniques, growth in the world economy, and innovative and successful advertising campaigns. They are commonly judged by the “four Cs”: ''carat'', ''clarity'', ''color'', and ''cut''. Although ]s are produced each year at nearly four times the rate of natural diamonds, the vast majority of synthetic diamonds produced are small imperfect diamonds suitable only for industrial-grade use.{{fact}} | |||
| Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are ] and ]). Small numbers of ] or impurities (about one per million of lattice atoms) can color a diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a very high ] and a relatively high ]. | |||
| <!--Third paragraph: Mining and Distribution--> | |||
| Roughly 49% of diamonds originate from central and southern ], although significant sources of the mineral have been discovered in ], ], ], ], and ]. | |||
| They are mined from ] and ] ]s, which brought to the surface the diamond crystals from deep in the Earth where the high pressure and temperature enables the formation of the crystals. The mining and distribution of natural diamonds are subjects of frequent controversy such as with concerns over the sale of '']s'' by African ] groups. There are also allegations that the ] misuses its dominance in the industry to control supply and manipulate price via ] practices, although in recent years the company's market share has dropped to below 60%.{{fact}} | |||
| Most natural diamonds have ages between 1 billion and 3.5 billion years. Most were formed at depths between {{convert|150|and|250|km}} in the Earth's ], although a few have come from as deep as {{convert|800|km}}. Under high pressure and temperature, carbon-containing fluids dissolved various minerals and replaced them with diamonds. Much more recently (hundreds to tens of million years ago), they were carried to the surface in ]s and deposited in ]s known as ]s and ]s. | |||
| ==Material properties== | |||
| {{main|Material properties of diamond}} | |||
| {{seealso|Crystallographic defects in diamond}} | |||
| A diamond is a ] ] of ] bonded carbon atoms. Diamonds have been adapted for many uses because of the material's exceptional physical characteristics. Most notable are its extreme ] of diamond, its high ] index, and high thermal conductivity. | |||
| ]s can be grown from high-purity carbon under high pressures and temperatures or from ] gases by ] (CVD). Natural and synthetic diamonds are most commonly distinguished using optical techniques or thermal conductivity measurements. | |||
| ===Mechanical properties=== | |||
| Main article: Material properties of diamond | |||
| See also: Crystallographic defects in diamond | |||
| == Properties == | |||
| A diamond is a transparent crystal of tetrahedrally bonded carbon atoms. Diamonds have been adapted for many uses because of the material's exceptional physical characteristics. Most notable are its extreme hardness of diamond, its high dispersion index, and high thermal conductivity. | |||
| {{Main|Material properties of diamond}} | |||
| Diamond is a solid form of pure carbon with its atoms arranged in a crystal. Solid carbon comes in different forms known as ]s depending on the type of chemical bond. The two most common ] are diamond and ]. In graphite, the bonds are sp<sup>2</sup> ] and the atoms form in planes, with each bound to three nearest neighbors, 120 degrees apart. In diamond, they are sp<sup>3</sup> and the atoms form tetrahedra, with each bound to four nearest neighbors.<ref>{{cite book | vauthors = Delhaes P |chapter=Polymorphism of carbon | veditors = Delhaes P |title=Graphite and precursors |date=2000 |publisher=Gordon & Breach |isbn=978-90-5699-228-6 |pages=1–24}}</ref><ref>{{cite book | vauthors = Pierson HO |title=Handbook of carbon, graphite, diamond, and fullerenes: properties, processing, and applications |date=2012 |publisher=Noyes Publications |isbn=978-0-8155-1739-9 |pages=40–41}}</ref> Tetrahedra are rigid, the bonds are strong, and, of all known substances, diamond has the greatest number of atoms per unit volume, which is why it is both the hardest and the least ].<ref>{{cite book | vauthors = Angus JC |chapter=Structure and thermochemistry of diamond |pages=9–30 | veditors = Paoletti A, Tucciarone A |title=The physics of diamond |date=1997 |publisher=IOS Press |isbn=978-1-61499-220-2}}</ref><ref name=ChemThermo>{{cite book | vauthors = Rock PA |title=Chemical Thermodynamics |date=1983 |publisher=University Science Books |isbn=978-1-891389-32-0 |pages=257–260}}</ref> It also has a high density, ranging from 3150 to 3530 kilograms per cubic metre (over three times the density of water) in natural diamonds and 3520 kg/m{{sup|3}} in pure diamond.<ref name=mindat>{{cite web |publisher=Mindat |title=Diamond |url=https://www.mindat.org/min-1282.html |access-date=July 7, 2009 |archive-date=May 6, 2009 |archive-url=https://web.archive.org/web/20090506083109/http://www.mindat.org/min-1282.html |url-status=live }}</ref> In graphite, the bonds between nearest neighbors are even stronger, but the bonds between parallel adjacent planes are weak, so the planes easily slip past each other. Thus, graphite is much softer than diamond. However, the stronger bonds make graphite less flammable.<ref>{{cite journal |vauthors=Gray T |title=Gone in a Flash |url=https://www.popsci.com/diy/article/2009-08/burn-diamonds-torch-and-liquid-oxygen/ |journal=Popular Science |date=October 8, 2009 |access-date=October 31, 2018 |archive-date=March 7, 2020 |archive-url=https://web.archive.org/web/20200307065622/https://www.popsci.com/diy/article/2009-08/burn-diamonds-torch-and-liquid-oxygen/ |url-status=live }}</ref> | |||
| Diamonds have been adopted for many uses because of the material's exceptional physical characteristics. It has the highest ] and the highest sound velocity. It has low adhesion and friction, and its coefficient of ] is extremely low. Its optical transparency extends from the ] to the deep ] and it has high ]. It also has high electrical resistance. It is chemically inert, not reacting with most corrosive substances, and has excellent biological compatibility.<ref>{{cite book | vauthors = Chen Y, Zhang L |title=Polishing of diamond materials: mechanisms, modeling and implementation |url=https://archive.org/details/polishingdiamond00chen |url-access=limited |date=2013 |publisher=Springer Science & Business Media |isbn=978-1-84996-408-1 |pages=–2}}</ref> | |||
| ====Crystal structure==== | |||
| === Thermodynamics === | |||
| ] of the diamond crystal structure.]] | |||
| ] of carbon]] | |||
| Diamonds typically crystallize in the face-centered ](] <math>Fd\bar{3}m</math>) and consist of ] bonded carbon atoms.<ref name="AMNH">American Museum of Natural History. . Retrieved March 9, 2005 </ref> The ] of diamond has a two atom basis at (0,0,0) and (1/4,1/4,1/4), which means half of the atoms are at lattice points and the other half are offset by (1/4,1/4,1/4), where 1 is the length of a side of the unit cell. Diamond's ] is 3.52 g·cm<sup>−3</sup>. | |||
| The equilibrium pressure and temperature conditions for a transition between graphite and diamond are well established theoretically and experimentally. The equilibrium pressure varies linearly with temperature, between {{val|1.7|ul=GPa}} at {{val|0|u=K}} and {{val|12|u=GPa}} at {{val|5000|u=K}} (the diamond/graphite/liquid ]).<ref name=Bundy>{{cite journal | vauthors = Bundy P, Bassett WA, Weathers MS, Hemley RJ, Mao HK, Goncharov AF |title=The pressure-temperature phase and transformation diagram for carbon; updated through 1994 |journal=Carbon |date=1996 |volume=34 |issue=2 |pages=141–153 |doi=10.1016/0008-6223(96)00170-4|bibcode=1996Carbo..34..141B }}</ref><ref>{{cite book| vauthors = Wang CX, Yang GW |chapter=Thermodynamic and kinetic approaches of diamond and related nanomaterials formed by laser ablation in liquid| veditors = Yang G |title=Laser ablation in liquids: principles and applications in the preparation of nanomaterials |date=2012 |publisher=Pan Stanford |isbn=978-981-4241-52-6 |pages=164–165}}</ref> However, the phases have a wide region about this line where they can coexist. At ], {{convert|20|C|K}} and {{convert|1|atm|MPa}}, the stable phase of carbon is graphite, but diamond is ] and its rate of conversion to graphite is negligible.<ref name=ChemThermo/> However, at temperatures above about {{val|4500|u=K}}, diamond rapidly converts to graphite. Rapid conversion of graphite to diamond requires pressures well above the equilibrium line: at {{val|2000|u=K}}, a pressure of {{val|35|u=GPa}} is needed.<ref name=Bundy/> | |||
| Above the graphite–diamond–liquid carbon triple point, the melting point of diamond increases slowly with increasing pressure; but at pressures of hundreds of GPa, it decreases.<ref>{{cite journal | vauthors = Wang X, Scandolo S, Car R | title = Carbon phase diagram from ab initio molecular dynamics | journal = Physical Review Letters | volume = 95 | issue = 18 | pages = 185701 | date = October 2005 | pmid = 16383918 | doi = 10.1103/PhysRevLett.95.185701 | bibcode = 2005PhRvL..95r5701W }}</ref> At high pressures, ] and ] have a BC8 ] crystal structure, and a similar structure is predicted for carbon at high pressures. At {{val|0|u=K}}, the transition is predicted to occur at {{val|1100|u=GPa}}.<ref>{{cite journal | vauthors = Correa AA, Bonev SA, Galli G | title = Carbon under extreme conditions: phase boundaries and electronic properties from first-principles theory | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 103 | issue = 5 | pages = 1204–1208 | date = January 2006 | pmid = 16432191 | pmc = 1345714 | doi = 10.1073/pnas.0510489103 | doi-access = free | bibcode = 2006PNAS..103.1204C }}</ref> | |||
| The tetrahedral arrangement of atoms is the source of many of diamond’s properties. The carbon atoms in ], the other major ] of carbon, display a different (nontetrahedral) connectivity and as a result shows dramatically different physical characteristics: graphite is a soft, dark gray, opaque mineral. Other elements of the ] such as ] crystalize like diamond. | |||
| Results published in an article in the scientific journal '']'' in 2010 suggest that, at ultra-high pressures and temperatures (about 10 million atmospheres or 1 TPa and 50,000 °C), diamond melts into a metallic fluid. The extreme conditions required for this to occur are present in the ]s ] and ]. Both planets are made up of approximately 10 percent carbon and could hypothetically contain oceans of liquid carbon. Since large quantities of metallic fluid can affect the magnetic field, this could serve as an explanation as to why the geographic and magnetic poles of the two planets are unaligned.<ref>{{cite news |title=Diamond oceans possible on Uranus, Neptune | vauthors = Bland E |newspaper=Discovery News |date=January 15, 2010| archive-url=https://web.archive.org/web/20120311163132/http://news.discovery.com/space/diamond-oceans-jupiter-uranus.html | archive-date=March 11, 2012|url=http://news.discovery.com/space/diamond-oceans-jupiter-uranus.html |access-date=January 16, 2010}}</ref><ref>{{cite journal |title=Diamond: Molten under pressure |vauthors=Silvera I |journal=Nature Physics |volume=6 |pages=9–10 |year=2010 |issue=1 |bibcode=2010NatPh...6....9S |url=http://nrs.harvard.edu/urn-3:HUL.InstRepos:9121282 |doi=10.1038/nphys1491 |s2cid=120836330 |access-date=November 9, 2020 |archive-date=July 30, 2022 |archive-url=https://web.archive.org/web/20220730040414/https://dash.harvard.edu/handle/1/9121282 |url-status=live }}</ref> | |||
| ] is a ] of diamond (and a distinct mineral species) that crystallizes with hexagonal symmetry. It is rarely found in nature but is characteristic of ]s. A ]line variety of diamond is called ]. A colorless, grey to black diamond with a tiny radial structure is a ]. | |||
| === |
=== Crystal structure === | ||
| {{See also|Crystallographic defects in diamond}} | |||
| ]]] | |||
| ] | |||
| Diamond is the ] natural material known, scoring 10 on the relative ]<ref name="AMNH"/> and having an absolute hardness value of between 90, 167, and 231 ]s in various tests. Diamond's hardness has been known since antiquity, and is the source of its name. However, ], an ] of ] first synthesized in 2005, are now believed to be even harder than diamond.<ref>{{cite web | url = http://www.azonano.com/news.asp?newsID=1407 | title = Aggregated Diamond Nanorods, The Hardest Material Known to Man | accessmonthday = September 2 | accessyear = 2006 | work = Nanotechnology News | date = 2005-09-14 | publisher = AZoNano.com}}</ref> | |||
| The most common crystal structure of diamond is called ]. It is formed of ]s (see the figure) stacked together. Although there are 18 atoms in the figure, each corner atom is shared by eight unit cells and each atom in the center of a face is shared by two, so there are a total of eight atoms per unit cell.<ref>{{cite book | vauthors = Rajendran V |title=Materials science |date=2004 |publisher=Tata McGraw-Hill Pub |isbn=978-0-07-058369-6 |page=2.16}}</ref> The length of each side of the unit cell is denoted by ''a'' and is 3.567 ]s.<ref name=Ashcroft>{{cite book | vauthors = Ashcroft NW, Mermin ND |title=Solid state physics |date=1976 |publisher=Holt, Rinehart and Winston |isbn=978-0-03-083993-1 |page= |url-access=registration |url=https://archive.org/details/solidstatephysic00ashc/page/76 }}</ref> | |||
| The nearest neighbor distance in the diamond lattice is 1.732''a''/4 where ''a'' is the lattice constant, usually given in Angstrøms as ''a'' = 3.567 Å, which is 0.3567 nm. | |||
| The hardest diamonds in the world are from the ] area in ], ]. These diamonds are generally small, perfect to semiperfect octahedra, and are used to polish other diamonds. Their hardness is considered to be a product of the crystal growth form, which is single stage growth crystal. Most other diamonds show more evidence of multiple growth stages, which produce inclusions, flaws, and defect planes in the crystal lattice all of which affect their hardness. <ref name="Taylor">Taylor, W.R., Lynton A.J. & Ridd, M., (1990) ''American Mineralogist'', 75, pp. 1290-1310</ref> | |||
| A diamond cubic lattice can be thought of as two interpenetrating ] lattices with one displaced by {{frac|1|4}} of the diagonal along a cubic cell, or as one lattice with two atoms associated with each lattice point.<ref name=Ashcroft/> Viewed from a {{math|<1 1 1>}} ], it is formed of layers stacked in a repeating ABCABC ... pattern. Diamonds can also form an ABAB ... structure, which is known as hexagonal diamond or ], but this is far less common and is formed under different conditions from cubic carbon.<ref>{{cite book |chapter=Molecular models of porous carbons| vauthors = Bandosz TJ, Biggs MJ, Gubbins KE, Hattori Y, Iiyama T, Kaneko T, Pikunic J, Thomson K | veditors = Radovic LR |title=Chemistry and physics of carbon |volume=28 |date=2003 |publisher=Marcel Dekker |isbn=978-0-8247-0987-7 |pages=46–47}}</ref> | |||
| The hardness of diamonds contributes to its suitability as a ]. Because it can only be scratched by other diamonds, it maintains its polish extremely well, keeping its luster over long periods of time. Unlike many other gems, it is well-suited to daily wear because of its resistance to scratching—perhaps contributing to its popularity as the preferred gem in an ] or ], which are often worn every day. | |||
| === Crystal habit === | |||
| Industrial use of diamonds has historically been associated with their hardness; this property makes diamond the ideal material for cutting and grinding tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial adaptations of this ability include diamond-tipped drill bits and saws, or use of diamond powder as an ]. Industrial-grade diamonds are either unsuitable for use as gems or synthetically produced, which lowers their value and makes their use economically feasible. Industrial applications, especially as ]s and ] tools, also date to ancient times.{{fact}} | |||
| ] | |||
| Diamonds occur most often as ] or rounded ] and ] octahedra known as '']s''. As diamond's crystal structure has a cubic arrangement of the atoms, they have many ]s that belong to a ], octahedron, ], ], or ]. The crystals can have rounded-off and unexpressive edges and can be elongated. Diamonds (especially those with rounded crystal faces) are commonly found coated in ''nyf'', an opaque gum-like skin.<ref>{{cite book | vauthors = Webster R, Read PG |title=Gems: Their sources, descriptions and identification |edition=5th |page=17 |publisher=] |location=Great Britain |year=2000 |isbn=978-0-7506-1674-4}}</ref> | |||
| Some diamonds contain opaque fibers. They are referred to as ''opaque'' if the fibers grow from a clear substrate or ''fibrous'' if they occupy the entire crystal. Their colors range from yellow to green or gray, sometimes with cloud-like white to gray impurities. Their most common shape is cuboidal, but they can also form octahedra, dodecahedra, macles, or combined shapes. The structure is the result of numerous impurities with sizes between 1 and 5 microns. These diamonds probably formed in kimberlite magma and sampled the volatiles.<ref name=Cartigny>{{cite journal | vauthors = Cartigny P, Palot M, Thomassot E, Harris JW |title=Diamond Formation: A Stable Isotope Perspective |journal=Annual Review of Earth and Planetary Sciences |date=May 30, 2014 |volume=42 |issue=1 |pages=699–732 |doi=10.1146/annurev-earth-042711-105259 |bibcode=2014AREPS..42..699C|doi-access=free }}</ref> | |||
| ====Electrical Conductivity==== | |||
| Other specialized applications also exist or are being developed, including use as ]s: some blue diamonds are natural semiconductors, in contrast to most other diamonds, which are excellent electrical ]s.<ref name="AMNH"/> | |||
| Diamonds can also form polycrystalline aggregates. There have been attempts to classify them into groups with names such as ], ], stewartite, and framesite, but there is no widely accepted set of criteria.<ref name=Cartigny/> ], a type in which the diamond grains were ] (fused without melting by the application of heat and pressure), is black in color and tougher than single crystal diamond.<ref>{{cite journal | vauthors = Fukura S, Nakagawa T, Kagi H |title=High spatial resolution photoluminescence and Raman spectroscopic measurements of a natural polycrystalline diamond, carbonado |journal=Diamond and Related Materials |date=November 2005 |volume=14 |issue=11–12 |pages=1950–1954 |doi=10.1016/j.diamond.2005.08.046 |bibcode=2005DRM....14.1950F}}</ref> It has never been observed in a volcanic rock. There are many theories for its origin, including formation in a star, but no consensus.<ref name=Cartigny/><ref>{{cite journal | vauthors = Mohammad G, Siddiquei MM, Abu El-Asrar AM | title = Poly (ADP-ribose) polymerase mediates diabetes-induced retinal neuropathy | journal = Mediators of Inflammation | volume = 2013 | issue = 2 | pages = 510451 | year = 2006 | pmid = 24347828 | doi = 10.1086/510451 | pmc = 3857786 | arxiv = physics/0608014 | s2cid = 59405368 | bibcode = 2006ApJ...653L.153G }}</ref><ref>{{cite web |title=Diamonds from Outer Space: Geologists Discover Origin of Earth's Mysterious Black Diamonds |url=https://www.nsf.gov/news/news_summ.jsp?cntn_id=108270&org=NSF |publisher=] |date=January 8, 2007 |access-date=October 28, 2007 |archive-date=December 9, 2007 |archive-url=https://web.archive.org/web/20071209203456/http://www.nsf.gov/news/news_summ.jsp?cntn_id=108270&org=NSF |url-status=live }}</ref> | |||
| ====Toughness==== | |||
| Toughness relates to a material's ability to resist breakage from forceful impact. The ] of natural diamond has been measured as 3.4 <math>MPa\sqrt{m}</math><ref>{{cite journal|last = Field|first=J E|title=Strength and Fracture Properties of Diamond|journal=Philosophical Magazine A|volume=43|issue=3|pages=595-618|publisher=Taylor and Francis Ltd|date=1981|url=http://md1.csa.com/partners/viewrecord.php?requester=gs&collection=TRD&recid=CA6004428WC&recid=0004618SO&q=&uid=789421821&setcookie=yes|accessdate=2006-21-11 }}</ref> | |||
| , which is good compared to other gemstones, but poor compared to most engineering materials. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamond is therefore more fragile in some orientations than others. | |||
| === |
=== Mechanical === | ||
| ==== Hardness ==== | |||
| Diamonds can occur in nearly any colour, though yellow and brown are by far the most common.<ref name="AMNH"/> "Black" diamonds are not truly black, but rather contain numerous dark inclusions that give the gems their dark appearance. When the colour is saturated enough in yellow or brown diamonds, a stone may be referred to as a ''fancy coloured diamond'' by the gem trade, otherwise they are graded for colour in the '']'', of white diamonds.{{fact}} Coloured diamonds contain impurities or structural defects that cause the colouration, while pure or nearly pure diamonds are transparent and colourless. Most diamond impurities replace a carbon atom in the ], known as a ]. The most common impurity, ], causes a slight to intense yellow colouration depending upon the type and concentration of nitrogen present.<ref name="AMNH"/> The ] (GIA) classifies low saturation yellow and brown diamonds as diamonds in the ''normal colour range'', and applies a grading scale from 'D' (colourless) to 'Z' (light yellow). The GIA names diamonds that have more colour than 'Z' colour, ''fancy'' colour diamonds, as well as any other colour than yellow or brown.{{fact}} | |||
| ]er.]] | |||
| Diamond is the hardest material on the ] ]. To conduct the ] ], samples of materials are struck with a pyramid of standardized dimensions using a known force – a diamond crystal is used for the pyramid to permit a wide range of materials to be tested. From the size of the resulting indentation, a Vickers hardness value for the material can be determined. Diamond's great hardness relative to other materials has been known since antiquity, and is the source of its name. This does not mean that it is infinitely hard, indestructible, or unscratchable.<ref>{{Cite web|date=December 16, 2015|title=Diamonds Are Indestructible, Right?|url=https://dominionjewelers.com/diamonds-are-indestructible-right/|access-date=October 31, 2020|website=Dominion Jewelers|language=en-US|archive-date=September 26, 2020|archive-url=https://web.archive.org/web/20200926001227/https://dominionjewelers.com/diamonds-are-indestructible-right/|url-status=live}}</ref> Indeed, diamonds can be scratched by other diamonds<ref>{{cite journal|vauthors=Seal M |title=The abrasion of diamond |journal=Proceedings of the Royal Society A |volume=248 |issue=1254 |date=November 25, 1958 |pages=379–393 |doi=10.1098/rspa.1958.0250|bibcode=1958RSPSA.248..379S }}</ref> and worn down over time even by softer materials, such as vinyl ]s.<ref>{{cite web |vauthors=Weiler HD |title=The wear and care of records and styli |orig-date=1954 |date=April 13, 2021 |via=Shure Applications Engineering |url=https://service.shure.com/s/article/stylus-wear-and-record-wear?language=en_US |access-date=August 25, 2024 |archive-date=March 26, 2023 |archive-url=https://web.archive.org/web/20230326031532/https://service.shure.com/s/article/stylus-wear-and-record-wear?language=en_US |url-status=live }}</ref> | |||
| Diamond hardness depends on its purity, crystalline perfection, and orientation: hardness is higher for flawless, pure crystals oriented to the ] direction (along the longest diagonal of the cubic diamond lattice).<ref>{{cite book|pages=142–147|url=https://books.google.com/books?id=jtC1mUFZfQcC&pg=PA143|title=Properties, Growth and Applications of Diamond|vauthors=Neves AJ, Nazaré MH|publisher=]|year=2001|isbn=978-0-85296-785-0|access-date=November 9, 2020|archive-date=February 19, 2023|archive-url=https://web.archive.org/web/20230219072829/https://books.google.com/books?id=jtC1mUFZfQcC&pg=PA143|url-status=live}}</ref> Therefore, whereas it might be possible to scratch some diamonds with other materials, such as ], the hardest diamonds can only be scratched by other diamonds and ]. | |||
| ===Electromagnetic properties=== | |||
| ====Optical properties==== | |||
| Diamonds exhibit a high ] of visible light.<ref name="AMNH"/> This strong ability to split white light into its component colors is an important aspect of diamond's attraction as a gemstone, giving it impressive ] action that results in so-called ''fire'' in a well-cut stone. The ] of a diamond, its ''adamantine'' brilliance, is a consequence of refractive index of 2.417 (at 589.3 ]),<ref name="AMNH"/> which allows ] to occur easily. | |||
| The hardness of diamond contributes to its suitability as a gemstone. Because it can only be scratched by other diamonds, it maintains its polish extremely well. Unlike many other gems, it is well-suited to daily wear because of its resistance to scratching—perhaps contributing to its popularity as the preferred gem in ] or ]s, which are often worn every day. | |||
| Some diamonds exhibit ] of various colors (predominantly blue) under long wave ] radiation. Most diamonds show no fluorescence although colored diamonds show a wider range of fluorescence than the blue fluorescence normally observed in white diamonds. Nearly all diamonds fluoresce bluish-white, yellow, or green under shorter ] radiation. X-ray screening is used extensively in mining to separate the diamond-bearing from the non-fluorescing waste rock.{{cite needed}} | |||
| The hardest natural diamonds mostly originate from the ] and ] fields located in the ] area in ], Australia. These diamonds are generally small, perfect to semiperfect octahedra, and are used to polish other diamonds. Their hardness is associated with the ] form, which is single-stage crystal growth. Most other diamonds show more evidence of multiple growth stages, which produce inclusions, flaws, and defect planes in the crystal lattice, all of which affect their hardness. It is possible to treat regular diamonds under a combination of high pressure and high temperature to produce diamonds that are harder than the diamonds used in hardness gauges.<ref>{{cite magazine|vauthors=Boser U|title=Diamonds on Demand|url=http://www.smithsonianmag.com/science-nature/diamonds-on-demand.html|magazine=]|volume=39|issue=3|pages=52–59|year=2008|access-date=June 13, 2009|archive-date=March 2, 2012|archive-url=https://web.archive.org/web/20120302163915/http://www.smithsonianmag.com/science-nature/diamonds-on-demand.html|url-status=dead}}</ref> | |||
| ====Thermal properties==== | |||
| Unlike most electrical insulators, diamond is a good conductor of heat because of the strong covalent bonding within the crystal. Most natural blue diamonds contain ] atoms which replace carbon atoms in the crystal matrix, and also have high thermal conductivity. Specially, purified synthetic diamond has the highest ] (2000–2500 W/(m·K), five times more than copper) of any known solid at room temperature. Because diamond has such high thermal conductance it is used in semiconductor manufacture to prevent silicon and other semiconducting materials from overheating.<ref name="AMNH"/> The ] of diamond is 5.4 - 6.4 eV. | |||
| Diamonds cut glass, but this does not positively identify a diamond because other materials, such as quartz, also lie above glass on the ] and can also cut it. Diamonds can scratch other diamonds, but this can result in damage to one or both stones. Hardness tests are infrequently used in practical gemology because of their potentially destructive nature.<ref name=read/> The extreme hardness and high value of diamond means that gems are typically polished slowly, using painstaking traditional techniques and greater attention to detail than is the case with most other gemstones;<ref name="hazen">{{cite book|url=https://books.google.com/books?id=fNJQok6N9_MC&pg=PA7|pages=7–10|title=The diamond makers| vauthors = Hazen RM |publisher=Cambridge University Press|year=1999|isbn=978-0-521-65474-6}}</ref> these tend to result in extremely flat, highly polished facets with exceptionally sharp facet edges. Diamonds also possess an extremely high refractive index and fairly high dispersion. Taken together, these factors affect the overall appearance of a polished diamond and most ]s still rely upon skilled use of a ] (magnifying glass) to identify diamonds "by eye".<ref>{{cite book|url=https://books.google.com/books?id=Jm3FwBiHaI4C&pg=PA37|pages=34–37|title=Synthetic, Imitation and Treated Gemstones| vauthors = O'Donoghue M |publisher=Gulf Professional |year= 1997|isbn=978-0-7506-3173-0}}</ref> | |||
| ==Natural history== | |||
| ===Formation=== | |||
| Diamonds are formed by prolonged exposure of carbon-bearing materials to high ] and ]. On ], the formation of diamonds is possible because there are regions deep within the Earth that are at a high enough pressure and temperature that the formation of diamonds is ] favorable.<ref name="AMNH"/> Beneath thick ] ( in the lithospheric mantle of ]s), diamonds form starting at depths of about 150 kilometers (90 miles),<ref name="AMNH"/> where pressure is roughly 5 ]s and the temperature is around 1200 degrees Celsius (2200 degrees Fahrenheit). Diamonds cannot form beneath ] because the lithosphere is too thin to reach the pressures required for diamond stability. Long periods of exposure to the high pressures and temperatures in cratons allow diamond crystals to grow larger. | |||
| ==== Toughness ==== | |||
| ] | |||
| Somewhat related to hardness is another mechanical property ''toughness'', which is a material's ability to resist breakage from forceful impact. The ] of natural diamond has been measured as 50–65 ]·m<sup>1/2</sup>.{{contradiction inline|reason=Unit of toughness as given at the article on toughness is newton-metres per cubic metre, dimensionally equivalent to newtons per square metre i.e. pascals. What is this factor of m^{1/2} doing here? Should we actually be talking about and linking to ] (which unfortunately doesn't have a discussion of units)?|date=October 2023}}<ref>{{cite book| vauthors = Lee J, Novikov NV |title=Innovative superhard materials and sustainable coatings for advanced manufacturing|url=https://books.google.com/books?id=EXGcDYj8HvEC&pg=PA102|page=102|publisher=Springer|year=2005|isbn=978-0-8493-3512-9}}</ref><ref>{{cite book| vauthors = Marinescu ID, Tönshoff HK, Inasaki I |title=Handbook of ceramic grinding and polishing|url=https://books.google.com/books?id=QCvqtRJJ4XwC&pg=PA21|page=21|publisher=William Andrew|year=2000|isbn=978-0-8155-1424-4}}</ref> This value is good compared to other ceramic materials, but poor compared to most engineering materials such as engineering alloys, which typically exhibit toughness over 80{{nbsp}}MPa·m<sup>1/2</sup>. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamond has a ] and is therefore more fragile in some orientations than others. ] use this attribute to cleave some stones before faceting them.<ref name=harlow/> "Impact toughness" is one of the main indexes to measure the quality of synthetic industrial diamonds. | |||
| ==== Yield strength ==== | |||
| Through studies of carbon ] ratios (similar to the methodology used in ], except with the stable isotopes C-12 and C-13), it has been shown that the carbon found in diamonds comes from both inorganic and organic sources. Some diamonds, known as '']'', are formed from inorganic carbon originally found deep in the Earth's ]. In contrast, '']'' diamonds contain organic carbon from organic ] that has been pushed down from the surface of the Earth's ] through ] (see ]) before transforming into diamond.<ref name="AMNH"/> These two different source carbons have measurably different <sup>13</sup>C:<sup>12</sup>C ratios. Diamonds that have come to the Earth's surface are generally very old, ranging from under 1 ] to 3.3 billion years old. | |||
| Diamond has compressive yield strength of 130–140{{nbsp}}GPa.<ref>{{cite journal | vauthors = Eremets MI, Trojan IA, Gwaze P, Huth J, Boehler R, Blank VD |title=The strength of diamond |journal=Applied Physics Letters |date=October 3, 2005 |volume=87 |issue=14 |pages=141902 |doi=10.1063/1.2061853|bibcode=2005ApPhL..87n1902E}}</ref> This exceptionally high value, along with the hardness and transparency of diamond, are the reasons that ] cells are the main tool for high pressure experiments.<ref name=Dubrovinsky>{{cite journal | vauthors = Dubrovinsky L, Dubrovinskaia N, Prakapenka VB, Abakumov AM | title = Implementation of micro-ball nanodiamond anvils for high-pressure studies above 6 Mbar | journal = Nature Communications | volume = 3 | issue = 1 | pages = 1163 | date = October 23, 2012 | pmid = 23093199 | pmc = 3493652 | doi = 10.1038/ncomms2160 | bibcode = 2012NatCo...3.1163D }}</ref> These anvils have reached pressures of {{val|600|u=GPa}}.<ref name="Wogan2012">{{Cite web |vauthors=Wogan T |publisher=Nature Communications |url=http://physicsworld.com/cws/article/news/2012/nov/02/improved-diamond-anvil-cell-allows-higher-pressures-than-ever-before |title=Improved diamond anvil cell allows higher pressures than ever before |work=] |date=November 2, 2012 |access-date=July 1, 2022 |archive-date=January 2, 2018 |archive-url=https://web.archive.org/web/20180102123446/http://physicsworld.com/cws/article/news/2012/nov/02/improved-diamond-anvil-cell-allows-higher-pressures-than-ever-before |url-status=live }}</ref> Much higher pressures may be possible with ] diamonds.<ref name=Dubrovinsky/><ref name="Wogan2012"/> | |||
| ==== Elasticity and tensile strength ==== | |||
| Diamonds occur most often as ] or rounded ] and ] octahedra known as ''macles'' or ''maccles''. As diamond's crystal structure has a cubic arrangement of the atoms, they have many ]s that belong to a ], ], ], ] or ]. The crystals can have rounded off and unexpressive edges and can be elongated. Sometimes they are found grown together or form double "twinned" crystals grown together at the surfaces of the octahedron. This is all due to the conditions in which they form. Diamonds (especially those with rounded crystal faces) are commonly found coated in ''nyf'', an opaque gum-like skin.<ref>Webster, Robert, and Read, Peter G. (Ed.) (2000). ''Gems: Their sources, descriptions and identification'' (5th ed.), p. 17. Butterworth-Heinemann, Great Britain. ISBN 0-7506-1674-1.</ref> | |||
| Usually, attempting to deform bulk diamond crystal by tension or bending results in brittle fracture. However, when single crystalline diamond is in the form of micro/nanoscale wires or needles (~100–300{{nbsp}}nanometers in diameter, micrometers long), they can be elastically stretched by as much as 9–10 percent tensile strain without failure,<ref>{{cite journal | vauthors = Dang C, Chou JP, Dai B, Chou CT, Yang Y, Fan R, Lin W, Meng F, Hu A, Zhu J, Han J, Minor AM, Li J, Lu Y | display-authors = 6 | title = Achieving large uniform tensile elasticity in microfabricated diamond | journal = Science | volume = 371 | issue = 6524 | pages = 76–78 | date = January 2021 | pmid = 33384375 | doi = 10.1126/science.abc4174 | doi-access = | bibcode = 2021Sci...371...76D | s2cid = 229935085 }}</ref> with a maximum local tensile stress of about {{nowrap|89–98 GPa}},<ref>{{cite journal | vauthors = Banerjee A, Bernoulli D, Zhang H, Yuen MF, Liu J, Dong J, Ding F, Lu J, Dao M, Zhang W, Lu Y, Suresh S | display-authors = 6 | title = Ultralarge elastic deformation of nanoscale diamond | journal = Science | volume = 360 | issue = 6386 | pages = 300–302 | date = April 2018 | pmid = 29674589 | doi = 10.1126/science.aar4165 | doi-access = | bibcode = 2018Sci...360..300B | s2cid = 5047604 }}</ref> very close to the theoretical limit for this material.<ref>{{cite journal | vauthors = LLorca J | title = On the quest for the strongest materials | journal = Science | volume = 360 | issue = 6386 | pages = 264–265 | date = April 2018 | pmid = 29674578 | doi = 10.1126/science.aat5211 | arxiv = 2105.05099 | s2cid = 4986592 | bibcode = 2018Sci...360..264L }}</ref> | |||
| === Electrical conductivity === | |||
| Diamonds can also form in other natural high-pressure, high-temperature events. Very small diamonds, known as ''microdiamonds'' or ''nanodiamonds'', have been found in ]s where ]s strike the Earth and create shock zones of high pressure and temperature where diamond formation can occur. Microdiamonds are now used as one indicator of ancient ] impact sites.<ref name="AMNH"/> | |||
| Other specialized applications also exist or are being developed, including use as ]s: some ]s are natural semiconductors, in contrast to most diamonds, which are excellent ]. The conductivity and blue color originate from boron impurity. Boron substitutes for carbon atoms in the diamond lattice, donating a hole into the ].<ref name="boron">{{cite journal | vauthors = Collins AT |title=The Optical and Electronic Properties of Semiconducting Diamond |journal=] |volume=342 |pages=233–244 |year=1993 |doi=10.1098/rsta.1993.0017 |issue=1664 |bibcode=1993RSPTA.342..233C |s2cid=202574625}}</ref> | |||
| Substantial conductivity is commonly observed in nominally ] diamond grown by ]. This conductivity is associated with ]-related species adsorbed at the surface, and it can be removed by ] or other surface treatments.<ref>{{cite journal | vauthors = Landstrass MI, Ravi KV |title=Resistivity of chemical vapor deposited diamond films |journal=] |volume=55 |pages=975–977 |year=1989 |doi=10.1063/1.101694 |issue=10 |bibcode=1989ApPhL..55..975L}}</ref><ref>{{cite journal | vauthors = Zhang W, Ristein J, Ley L | title = Hydrogen-terminated diamond electrodes. II. Redox activity | journal = Physical Review E | volume = 78 | issue = 4 Pt 1 | pages = 041603 | date = October 2008 | pmid = 18999435 | doi = 10.1103/PhysRevE.78.041603 | bibcode = 2008PhRvE..78d1603Z }}</ref> | |||
| === Surfacing === | |||
| ] | |||
| Diamond-bearing rock is brought close to the surface through deep-origin ] eruptions. The ] for such a volcano must originate at a depth where diamonds can be formed,<ref name="AMNH"/> 150 km (90 miles) deep or more (three times or more the depth of source magma for most volcanoes); this is a relatively rare occurrence. These typically small surface volcanic craters extend downward in formations known as ]s.<ref name="AMNH"/> The pipes contain material that was transported toward the surface by volcanic action, but was not ejected before the volcanic activity ceased. During eruption these pipes are open to the surface, resulting in open circulation; many ]s of surface rock and even wood and/or ]s are found in volcanic pipes. Diamond-bearing volcanic pipes are closely related to the oldest, coolest regions of continental crust (]s). This is because cratons are very thick, and their ] mantle extends to great enough depth that diamonds are stable. Not all pipes contain diamonds, and even fewer contain enough diamonds to make mining economically viable. | |||
| Thin needles of diamond can be made to vary their electronic ] from the normal 5.6 eV to near zero by selective mechanical deformation.<ref>{{cite journal | vauthors = Shi Z, Dao M, Tsymbalov E, Shapeev A, Li J, Suresh S | title = Metallization of diamond | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 117 | issue = 40 | pages = 24634–24639 | date = October 2020 | pmid = 33020306 | pmc = 7547227 | doi = 10.1073/pnas.2013565117 | doi-access = free | bibcode = 2020PNAS..11724634S }}</ref> | |||
| The magma in volcanic pipes is usually one of two characteristic types, which cool into ] known as either ] or ].<ref name="AMNH"/> The magma itself does not contain diamond; instead, it acts as an elevator that carries deep-formed rocks (xenoliths), minerals (]s), and fluids upward. These rocks are characteristically rich in ]-bearing ], ], and ] minerals<ref name="AMNH"/> which are often altered to ] by heat and fluids during and after eruption. Certain ''indicator minerals'' typically occur within diamondiferous kimberlites and are used as mineralogic tracers by prospectors, who follow the indicator trail back to the volcanic pipe which may contain diamonds. These minerals are rich in ] (Cr) or ] (Ti), elements which impart bright colors to the minerals. The most common indicator minerals are chromian ]s (usually bright red Cr-], and occasionally green ]-series garnets), eclogitic garnets, orange Ti-pyrope, red high-Cr ]s, dark ], bright green Cr-], glassy green ], black ], and ].<ref name="AMNH"/> Kimberlite deposits are known as ''blue ground'' for the deeper serpentinized part of the deposits, or as ''yellow ground'' for the near surface ] ] and carbonate ] and ] portion. | |||
| High-purity diamond wafers 5 cm in diameter exhibit perfect resistance in one direction and perfect conductance in the other, creating the possibility of using them for quantum data storage. The material contains only 3 parts per million of nitrogen. The diamond was grown on a stepped substrate, which eliminated cracking.<ref>{{Cite web | vauthors = Irving M |date=April 28, 2022 |title=Two-inch diamond wafers could store a billion Blu-Ray's worth of data |url=https://newatlas.com/electronics/2-inch-diamond-wafers-quantum-memory-billion-blu-rays/ |access-date=April 29, 2022 |website=New Atlas |language=en-US}}</ref> | |||
| Once diamonds have been transported to the surface by magma in a volcanic pipe, they may ] out and be distributed over a large area. A volcanic pipe containing diamonds is known as a ''primary source'' of diamonds. ''Secondary sources'' of diamonds include all areas where a significant number of diamonds, eroded out of their kimberlite or lamproite matrix, accumulate because of water or wind action. These include ] deposits and deposits along existing and ancient shorelines, where loose diamonds tend to accumulate because of their approximate size and density. Diamonds have also rarely been found in deposits left behind by glaciers (notably in ] and ]); however, in contrast to alluvial deposits, glacial deposits are not known to be of significant concentration and are therefore not viable commercial sources of diamond. | |||
| === Surface property === | |||
| Diamonds can also be brought to the surface through certain processes which may occur when two ]s collide and deeply formed rock is thrust to the surface,{{fact}} although this phenomenon is less understood and currently assumed to be uncommon. | |||
| Diamonds are naturally ] and ], which means the diamonds' surface cannot be wet by water, but can be easily wet and stuck by oil. This property can be utilized to extract diamonds using oil when making synthetic diamonds. However, when diamond surfaces are chemically modified with certain ions, they are expected to become so ] that they can stabilize multiple layers of ] at ].<ref>{{cite journal | vauthors = Wissner-Gross AD, Kaxiras E | title = Diamond stabilization of ice multilayers at human body temperature | journal = Physical Review E | volume = 76 | issue = 2 Pt 1 | pages = 020501 | date = August 2007 | pmid = 17929997 | doi = 10.1103/physreve.76.020501 | url = http://www.alexwg.org/link?url=http%3A%2F%2Fwww.alexwg.org%2Fpublications%2FPhysRevERapidComm_76-020501.pdf | url-status = live | s2cid = 44344503 | bibcode = 2007PhRvE..76b0501W | archive-url = https://web.archive.org/web/20110724214405/http://www.alexwg.org/publications/PhysRevERapidComm_76-020501.pdf | archive-date = July 24, 2011 }}</ref> | |||
| The surface of diamonds is partially oxidized. The oxidized surface can be reduced by heat treatment under hydrogen flow. That is to say, this heat treatment partially removes oxygen-containing functional groups. But diamonds (sp<sup>3</sup>C) are unstable against high temperature (above about {{convert|400|C}}) under atmospheric pressure. The structure gradually changes into sp<sup>2</sup>C above this temperature. Thus, diamonds should be reduced below this temperature.<ref>{{cite journal | vauthors = Fujimoto A, Yamada Y, Koinuma M, Sato S | title = Origins of sp(3)C peaks in C1s X-ray Photoelectron Spectra of Carbon Materials | journal = Analytical Chemistry | volume = 88 | issue = 12 | pages = 6110–6114 | date = June 2016 | pmid = 27264720 | doi = 10.1021/acs.analchem.6b01327 | doi-access = free }}</ref> | |||
| ==Gemological characteristics== | |||
| The most familiar usage of diamonds today is as gemstones used for ]. This usage dates back into ] and predates other uses.{{fact}} The ] of white light into ]s, is the primary gemological characteristic of gem diamonds. In the ], experts in the field of '']'' have developed methods of grading diamonds and other gemstones based on the characteristics most important to their value as a gem. Four characteristics, known informally as the ''four Cs'', are now commonly used as the basic descriptors of diamonds: these are ''carat'', ''cut'', ''color'', and ''clarity''. | |||
| === Chemical stability === | |||
| Most gem diamonds are traded on the wholesale market based on single values for each of the four Cs; for example knowing that a diamond is rated as 1.5 carats, VS2 clarity, F color, excellent cut round brilliant, is enough to reasonably establish an expected price range. More detailed information from within each characteristic is used to determine actual market value for individual stones. Consumers who purchase individual diamonds are often advised to use the four Cs to pick the diamond that is "right" for them. | |||
| At room temperature, diamonds do not react with any chemical reagents including strong acids and bases. | |||
| In an atmosphere of pure oxygen, diamond has an ] that ranges from {{convert|690|C}} to {{convert|840|C}}; smaller crystals tend to burn more easily. It increases in temperature from red to white heat and burns with a pale blue flame, and continues to burn after the source of heat is removed. By contrast, in air the combustion will cease as soon as the heat is removed because the oxygen is diluted with nitrogen. A clear, flawless, transparent diamond is completely converted to carbon dioxide; any impurities will be left as ash.<ref>{{cite book | vauthors = Bauer M |title=Precious Stones | volume = 1 |date=2012 |publisher=Dover Publications |isbn=978-0-486-15125-0 |pages=115–117}}</ref> Heat generated from cutting a diamond will not ignite the diamond,<ref>{{cite web |title=Diamond Care and Cleaning Guide |url=https://www.gia.edu/diamond-care-cleaning |publisher=Gemological Institute of America |access-date=August 1, 2019 |language=en |archive-date=August 1, 2019 |archive-url=https://web.archive.org/web/20190801170616/https://www.gia.edu/diamond-care-cleaning |url-status=live }}</ref> and neither will a cigarette lighter,<ref>{{cite web |vauthors=Jones C |title=Diamonds are Flammable! How to Safeguard Your Jewelry |url=http://www.dmia.net/diamonds-are-flammable/ |website=DMIA |access-date=August 1, 2019 |date=August 27, 2016 |archive-date=August 1, 2019 |archive-url=https://web.archive.org/web/20190801170616/http://www.dmia.net/diamonds-are-flammable/ |url-status=live }}</ref> but house fires and blow torches are hot enough. Jewelers must be careful when molding the metal in a diamond ring.<ref>{{cite web |vauthors=Baird CS |title=Can you light diamond on fire? |url=https://wtamu.edu/~cbaird/sq/2014/03/27/can-you-light-diamond-on-fire/ |website=Science Questions with Surprising Answers |access-date=August 1, 2019 |archive-date=August 1, 2019 |archive-url=https://web.archive.org/web/20190801170618/https://wtamu.edu/~cbaird/sq/2014/03/27/can-you-light-diamond-on-fire/ |url-status=live }}</ref> | |||
| Other characteristics not described by the four Cs influence the value or appearance of a gem diamond. These characteristics include physical characteristics such as the presence of ], as well as data on a diamond's history including its source and which gemological institute performed evaluation services on the diamond. ''Cleanliness'' also dramatically affects a diamond's beauty. | |||
| Diamond powder of an appropriate grain size (around 50{{nbsp}}microns) burns with a shower of sparks after ignition from a flame. Consequently, ]s based on ] powder can be prepared. The resulting sparks are of the usual red-orange color, comparable to charcoal, but show a very linear trajectory which is explained by their high density.<ref>{{cite journal| vauthors = Lederle F, Koch J, Hübner EG |title=Colored Sparks|journal=European Journal of Inorganic Chemistry|date=February 21, 2019|volume=2019|issue=7|pages=928–937|doi=10.1002/ejic.201801300|s2cid=104449284}}</ref> Diamond also reacts with fluorine gas above about {{convert|700|C}}. | |||
| There are three{{fact}} major non-profit gemological associations which grade and provide reports on diamonds. While carat weight and cut angles are mathematically defined, the clarity and color are judged by the trained human eye and are therefore open to slight variance in interpretation. | |||
| * ] (GIA) was the first laboratory to issue modern diamond reports,{{fact}} and is held in high regard amongst gemologists for its consistent, conservative grading. | |||
| * ] (AGS) is not as widely recognized nor as old as the GIA but garners a high reputation. The AGS employs a number system for grading cut quality, color grade, and clarity. The highest grade being '0', and the lowest being '10'. | |||
| * ] (HRD) Official certification laboratory of the Belgian diamond industry. ISO9002 certified. Very popular in Europe where it is recognized as a legal document. | |||
| === |
=== Color === | ||
| {{Main|Diamond color}} | |||
| The ''] weight'' measures the mass of a diamond. One carat is defined as a fifth of a gram, or exactly 200 ]s (about 0.007 ]). The ''point'' unit—equal to one one-hundredth of a carat (0.01 carat, or 2 mg)—is commonly used for diamonds of less than one carat. All else being equal, the value of a diamond increases ]ly in relation to carat weight, since larger diamonds are both rarer and more desirable for use as gemstones. A review of comparable diamonds available for purchase in September 2005 demonstrates this effect (approximate prices for round cut, G color, VS2 diamonds with "1A" cut grade, as listed on http://www.pricescope.com): <!-- When updating, round to nearest $500 --> | |||
| ] in ]]] | |||
| ]]] | |||
| Diamond has a wide ] of {{val|5.5|ul=eV}} corresponding to the deep ] wavelength of 225{{nbsp}}nanometers. This means that pure diamond should transmit visible light and appear as a clear colorless crystal. Colors in diamond originate from lattice defects and impurities. The diamond crystal lattice is exceptionally strong, and only atoms of ], ], and ] can be introduced into diamond during the growth at significant concentrations (up to atomic percents). Transition metals ] and ], which are commonly used for growth of synthetic diamond by high-pressure high-temperature techniques, have been detected in diamond as individual atoms; the maximum concentration is 0.01% for nickel<ref>{{cite journal | vauthors = Collins AT, Kanda H, Isoya J, Ammerlaan CA, Van Wyk JA |title=Correlation between optical absorption and EPR in high-pressure diamond grown from a nickel solvent catalyst |journal=Diamond and Related Materials |volume=7 |pages=333–338 |year=1998 |doi=10.1016/S0925-9635(97)00270-7 |issue=2–5 |bibcode=1998DRM.....7..333C }}</ref> and even less for cobalt. Virtually any element can be introduced to diamond by ion implantation.<ref>{{cite journal |doi=10.1103/PhysRevB.61.12909 |title=Vibronic spectra of impurity-related optical centers in diamond |year=2000 | vauthors = Zaitsev AM |journal=Physical Review B |volume=61 |pages=12909–12922 |issue=19 |bibcode=2000PhRvB..6112909Z}}</ref> | |||
| Nitrogen is by far the most common impurity found in gem diamonds and is responsible for the yellow and brown color in diamonds. Boron is responsible for the blue color.<ref>{{cite journal| vauthors = Walker J |title=Optical absorption and luminescence in diamond|journal=Reports on Progress in Physics|volume=42|pages=1605–1659|year=1979|doi=10.1088/0034-4885/42/10/001|issue=10|bibcode=1979RPPh...42.1605W|url=http://accreditedgemologists.org/lightingtaskforce/OpticalAbsorptionand.pdf |archive-url=https://web.archive.org/web/20150906142645/http://accreditedgemologists.org/lightingtaskforce/OpticalAbsorptionand.pdf |archive-date=September 6, 2015 |url-status=live|citeseerx=10.1.1.467.443|s2cid=250857323 }}</ref> Color in diamond has two additional sources: irradiation (usually by alpha particles), that causes the color in green diamonds, and ] of the diamond crystal lattice. Plastic deformation is the cause of color in some brown<ref>{{cite journal| vauthors = Hounsome LS, Jones R, Shaw MJ, Briddon PR, Öberg S, Briddon P, Öberg S |title=Origin of brown coloration in diamond |journal=] |volume=73 |page=125203|year=2006|doi=10.1103/PhysRevB.73.125203 |issue=12|bibcode=2006PhRvB..73l5203H}}</ref> and perhaps pink and red diamonds.<ref>{{cite book| vauthors = Wise RW |title=Secrets Of The Gem Trade, The Connoisseur's Guide To Precious Gemstones|publisher=Brunswick House Press|pages=223–224|year=2001|isbn=978-0-9728223-8-1}}</ref> In order of increasing rarity, yellow diamond is followed by brown, colorless, then by blue, green, black, pink, orange, purple, and red.<ref name=harlow/> "Black", or ], diamonds are not truly black, but rather contain numerous dark inclusions that give the gems their dark appearance. Colored diamonds contain impurities or structural defects that cause the coloration, while pure or nearly pure diamonds are transparent and colorless. Most diamond impurities replace a carbon atom in the ], known as a ]. The most common impurity, nitrogen, causes a slight to intense yellow coloration depending upon the type and concentration of nitrogen present.<ref name=harlow/> The ] (GIA) classifies low saturation yellow and brown diamonds as diamonds in the ''normal color range'', and applies a grading scale from "D" (colorless) to "Z" (light yellow). Yellow diamonds of high color saturation or a different color, such as pink or blue, are called ''fancy colored'' diamonds and fall under a different grading scale.<ref name=harlow/> | |||
| {| class="infobox" style="border-collapse: collapse;" border="2" | |||
| |- style="background-color: #cccccc;" | |||
| !Carat size | |||
| !Cost per carat (US$) | |||
| !Total cost (US$) | |||
| |- | |||
| |0.5 carat (50 points) | |||
| |align="right"|3,000 | |||
| |align="right"|1,500 | |||
| |- | |||
| |1.0 carat | |||
| |align="right"|6,500 | |||
| |align="right"|6,500 | |||
| |- | |||
| |1.5 carats | |||
| |align="right"|8,500 | |||
| |align="right"|12,750 | |||
| |- | |||
| |2.0 carats | |||
| |align="right"|13,000 | |||
| |align="right"|26,000 | |||
| |- | |||
| |3.0 carats | |||
| |align="right"|17,000 | |||
| |align="right"|51,000 | |||
| |- | |||
| |5.0 carats | |||
| |align="right"|23,000 | |||
| |align="right"|115,000 | |||
| |} | |||
| In 2008, the ], a {{convert|35.56|carat|g|adj=on}} ] once belonging to the King of Spain, fetched over US$24 million at a Christie's auction.<ref>{{cite news |vauthors=Khan U |title=Blue-grey diamond belonging to King of Spain has sold for record 16.3{{nbsp}}GBP |url=https://www.telegraph.co.uk/culture/3703861/Blue-grey-diamond-belonging-to-King-of-Spain-has-sold-for-record-16.3m.html |work=] |location=London |date=December 10, 2008 |access-date=March 31, 2010 |archive-date=February 7, 2009 |archive-url=https://web.archive.org/web/20090207212758/http://www.telegraph.co.uk/culture/3703861/Blue-grey-diamond-belonging-to-King-of-Spain-has-sold-for-record-16.3m.html |url-status=live }}</ref> In May 2009, a {{convert|7.03|carat|g|adj=on}} ] fetched the highest price per carat ever paid for a diamond when it was sold at auction for 10.5 million Swiss francs (6.97 million euros, or US$9.5 million at the time).<ref>{{cite news|vauthors=Nebehay S|title=Rare blue diamond sells for record $9.5 million|url=https://www.reuters.com/article/artsNews/idUSTRE54B6O020090512|work=Reuters|date=May 12, 2009|access-date=May 13, 2009|archive-date=May 16, 2009|archive-url=https://web.archive.org/web/20090516234031/http://www.reuters.com/article/artsNews/idUSTRE54B6O020090512|url-status=live}}</ref> That record was, however, beaten the same year: a {{convert|5|carat|g|adj=on}} vivid pink diamond was sold for US$10.8 million in Hong Kong on December 1, 2009.<ref>{{cite news|url=https://www.reuters.com/article/idUSTRE5B02P620091201|title=Vivid pink diamond sells for record $10.8 million|work=Reuters|date=December 1, 2009|vauthors=Pomfret J|access-date=July 1, 2017|archive-date=December 2, 2020|archive-url=https://web.archive.org/web/20201202103252/https://www.reuters.com/article/idUSTRE5B02P620091201|url-status=live}}</ref> | |||
| The price per carat does not increase smoothly with increasing size. Instead, there are sharp jumps around milestone carat weights, as demand is much higher for diamonds weighing just more than a milestone than for those weighing just less. As an example, a 0.95 carat diamond may have a significantly lower price per carat than a comparable 1.05 carat diamond, because of differences in demand. | |||
| === Clarity === | |||
| A weekly diamond price list, the ] is published by ], CEO of Rapaport Group of New York, for different diamond cuts, clarity and weights.<ref>, </ref> It is currently considered the ] retail price baseline. Jewelers often trade diamonds at negotiated discounts off the ] price (e.g., "R -3%"). | |||
| Clarity is one of the 4C's (color, clarity, cut and carat weight) that helps in identifying the quality of diamonds. The ] (GIA) developed 11 clarity scales to decide the quality of a diamond for its sale value. The GIA clarity scale spans from Flawless (FL) to included (I) having internally flawless (IF), very, very slightly included (VVS), very slightly included (VS) and slightly included (SI) in between. Impurities in natural diamonds are due to the presence of natural minerals and oxides. The clarity scale grades the diamond based on the color, size, location of impurity and quantity of clarity visible under 10x magnification.<ref>{{cite journal | vauthors = Cowing MD |year=2014 |title=Objective ciamond clarity grading |journal=Journal of Gemmology |volume=34 |number=4 |pages=316–332 |doi=10.15506/JoG.2014.34.4.316 |url=https://acagemlab.com/wp-content/uploads/2019/01/JoG2014_34_4_Cowing_Obj_Diamond_Clarity-1.pdf |archive-url=https://web.archive.org/web/20210418052002/https://acagemlab.com/wp-content/uploads/2019/01/JoG2014_34_4_Cowing_Obj_Diamond_Clarity-1.pdf |archive-date=April 18, 2021 |url-status=live |access-date=September 19, 2021}}</ref> Inclusions in diamond can be extracted by optical methods. The process is to take pre-enhancement images, identifying the inclusion removal part and finally removing the diamond facets and noises.<ref>{{cite journal | vauthors = Wang W, Cai L | title = Inclusion extraction from diamond clarity images based on the analysis of diamond optical properties | journal = Optics Express | volume = 27 | issue = 19 | pages = 27242–27255 | date = September 2019 | pmid = 31674589 | doi = 10.1364/OE.27.027242 | bibcode = 2019OExpr..2727242W | s2cid = 203141270 | doi-access = free }}</ref> | |||
| === Fluorescence === | |||
| In the wholesale trade of gem diamonds, carat is often used in denominating lots of diamonds for sale. For example, a buyer may place an order for 100 carats of 0.5 carat, D–F, VS2-SI1, excellent cut diamonds, indicating he wishes to purchase 200 diamonds (100 carats total mass) of those approximate characteristics. Because of this, diamond prices (particularly among wholesalers and other industry professionals) are often quoted per carat, rather than per stone. | |||
| ] (top) and normal light (bottom)]] | |||
| Between 25% and 35% of natural diamonds exhibit some degree of fluorescence when examined under invisible long-wave ultraviolet light or higher energy radiation sources such as X-rays and lasers.<ref>{{Cite web |date=March 27, 2018 |title=Fact Checking Diamond Fluorescence: 11 Myths Dispelled |url=https://4cs.gia.edu/en-us/blog/fact-checking-diamond-fluorescence-myths-dispelled/ |access-date=June 6, 2022 |website=GIA 4Cs |language=en-US |archive-date=March 24, 2022 |archive-url=https://web.archive.org/web/20220324052612/https://4cs.gia.edu/en-us/blog/fact-checking-diamond-fluorescence-myths-dispelled/ |url-status=live }}</ref> Incandescent lighting will not cause a diamond to fluoresce. Diamonds can fluoresce in a variety of colors including blue (most common), orange, yellow, white, green and very rarely red and purple. Although the causes are not well understood, variations in the atomic structure, such as the number of nitrogen atoms present are thought to contribute to the phenomenon. | |||
| === Thermal conductivity === | |||
| ''Total carat weight'' (t.c.w.) is a phrase used to describe the total mass of diamonds or other gemstone in a piece of jewelry, when more than one gemstone is used. Diamond solitaire earrings, for example, are usually quoted in t.c.w. when placed for sale, indicating the mass of the diamonds in both earrings and not each individual diamond. T.c.w. is also widely used for diamond necklaces, bracelets and other similar jewelry pieces. | |||
| Diamonds can be identified by their high thermal conductivity (900–{{val|2320|u=W·m{{Sup|−1}}·K{{Sup|−1}}}}).<ref>{{cite journal | vauthors = Wei L, Kuo PK, Thomas RL, Anthony TR, Banholzer WF | title = Thermal conductivity of isotopically modified single crystal diamond | journal = Physical Review Letters | volume = 70 | issue = 24 | pages = 3764–3767 | date = June 1993 | pmid = 10053956 | doi = 10.1103/PhysRevLett.70.3764 | bibcode = 1993PhRvL..70.3764W }}</ref> Their high ] is also indicative, but other materials have similar refractivity. | |||
| == |
== Geology == | ||
| Diamonds are extremely rare, with concentrations of at most parts per billion in source rock.<ref name=Cartigny/> Before the 20th century, most diamonds were found in ]s. Loose diamonds are also found along existing and ancient ]lines, where they tend to accumulate because of their size and density.<ref name=AMNH>{{cite book| vauthors = Erlich EI, Hausel WD |title=Diamond deposits: origin, exploration, and history of discovery|date=2002|publisher=Society for Mining, Metallurgy, and Exploration|location=Littleton, CO|isbn=978-0-87335-213-0}}</ref>{{rp|149}} Rarely, they have been found in ] (notably in ] and ]), but these deposits are not of commercial quality.<ref name=AMNH/>{{rp|19}} These types of deposit were derived from localized igneous ] through ] and ] by ] or ].<ref name=Shirey2013>{{cite journal | vauthors = Shirey SB, Shigley JE |title=Recent Advances in Understanding the Geology of Diamonds |journal=Gems & Gemology |date=December 1, 2013 |volume=49 |issue=4 |pages=188–222 |doi=10.5741/GEMS.49.4.188 |doi-access=free}}</ref> | |||
| {{main|Diamond clarity}} | |||
| Clarity is a measure of internal defects of a diamond called ''inclusions''. Inclusions may be crystals of a foreign material or another diamond crystal, or structural imperfections such as tiny cracks that can appear whitish or cloudy. The number, size, color, relative location, orientation, and visibility of inclusions can all affect the relative clarity of a diamond. The ] (GIA) and other organizations have developed systems to grade clarity, which are based on those inclusions which are visible to a trained professional when a diamond is viewed under 10x magnification. | |||
| Most diamonds come from the ], and most of this section discusses those diamonds. However, there are other sources. Some blocks of the crust, or ]s, have been buried deep enough as the crust thickened so they experienced ]. These have evenly distributed ''microdiamonds'' that show no sign of transport by magma. In addition, when meteorites strike the ground, the shock wave can produce high enough temperatures and pressures for ''microdiamonds'' and '']'' to form.<ref name=Shirey2013/> Impact-type microdiamonds can be used as an indicator of ancient impact craters.<ref>{{cite book|title=The Mantle and Core|vauthors=Carlson RW|url=https://books.google.com/books?id=1clZ4ABsfoAC&pg=PA248|page=248|publisher=Elsevier|year=2005|isbn=978-0-08-044848-0|access-date=November 9, 2020|archive-date=November 9, 2023|archive-url=https://web.archive.org/web/20231109173351/https://books.google.com/books?id=1clZ4ABsfoAC&pg=PA248#v=onepage&q&f=false|url-status=live}}</ref> ] in Russia may have the world's largest diamond deposit, estimated at trillions of carats, and formed by an asteroid impact.<ref>{{Cite journal|volume=23|pages=3–12| vauthors = Deutsch A, Masaitis VL, Langenhorst F, Grieve RA |title=Popigai, Siberia—well preserved giant impact structure, national treasury, and world's geological heritage|journal=Episodes|year=2000|issue=1|doi=10.18814/epiiugs/2000/v23i1/002|doi-access=free}}</ref> | |||
| Diamonds become increasingly rare when considering higher clarity gradings. Only about 20 percent of all diamonds mined have a clarity rating high enough for the diamond to be considered appropriate for use as a gemstone; the other 80 percent are relegated to industrial use. Of that top 20 percent, a significant portion contains one or more visible inclusions. Those that do not have a visible inclusion are known as "eye-clean" and are preferred by most buyers, although visible inclusions can sometimes be hidden under the setting in a piece of jewelry. | |||
| A common misconception is that diamonds form from highly compressed ]. Coal is formed from buried prehistoric plants, and most diamonds that have been dated are far older than the first ]. It is possible that diamonds can form from coal in ]s, but diamonds formed in this way are rare, and the carbon source is more likely ] rocks and organic carbon in sediments, rather than coal.<ref>{{cite web |url=http://geology.com/articles/diamonds-from-coal/ |title=How do diamonds form? They don't form from coal! | vauthors = King H |date=2012 |work=Geology and Earth Science News and Information |publisher=geology.com |access-date=June 29, 2012 |archive-url=https://web.archive.org/web/20131030014537/http://geology.com/articles/diamonds-from-coal/ |archive-date=October 30, 2013 |url-status=live}}</ref><ref>{{cite journal |title=10 common scientific misconceptions |vauthors=Pak-Harvey A |journal=The Christian Science Monitor |date=October 31, 2013 |url=http://www.csmonitor.com/Science/2013/1031/10-common-scientific-misconceptions/Diamonds-form-from-pressurized-coal |access-date=August 30, 2017 |archive-date=January 6, 2017 |archive-url=https://web.archive.org/web/20170106012033/http://www.csmonitor.com/Science/2013/1031/10-common-scientific-misconceptions/Diamonds-form-from-pressurized-coal |url-status=live }}</ref> | |||
| Most inclusions present in gem-quality diamonds do not affect the diamonds' performance or structural integrity. However, large clouds can affect a diamond's ability to transmit and scatter light. Large cracks close to or breaking the surface may reduce a diamond's resistance to fracture. | |||
| === Surface distribution === | |||
| Diamonds are graded by the major societies on a scale ranging from flawless to imperfect. | |||
| ]s of the world. The pink and orange areas are ] and ], which together constitute cratons.]] | |||
| Diamonds are far from evenly distributed over the Earth. A rule of thumb known as Clifford's rule states that they are almost always found in ]s on the oldest part of ]s, the stable cores of continents with typical ages of 2.5{{nbsp}}billion years or more.<ref name=Shirey2013/><ref>{{cite book | vauthors = Pohl WL |title=Economic Geology: Principles and Practice |date=2011 |publisher=John Wiley & Sons |isbn=978-1-4443-9486-3}}</ref>{{rp |314}} However, there are exceptions. The ] in ], the largest producer of diamonds by weight in the world, is located in a ''mobile belt'', also known as an '']'',<ref>{{cite encyclopedia | vauthors = Allaby M |title=mobile belt |encyclopedia=A dictionary of geology and earth sciences |date=2013 |publisher=Oxford University Press |location=Oxford |isbn=978-0-19-174433-4 |edition=4th}}</ref> a weaker zone surrounding the central craton that has undergone compressional tectonics. Instead of ], the host rock is ]. Lamproites with diamonds that are not economically viable are also found in the United States, India, and Australia.<ref name=Shirey2013/> In addition, diamonds in the ] of the ] in ] and microdiamonds in the ] are found in a type of rock called ].<ref name=Shirey2013/> | |||
| ===Color=== | |||
| {{main|Diamond color}} | |||
| ] | |||
| ]. Its deep blue coloration is caused by trace amounts of ] in the diamond.]] | |||
| A chemically pure and structurally perfect diamond is perfectly transparent with no ], or '''color'''. However, in reality almost no gem-sized natural diamonds are absolutely perfect. The color of a diamond may be affected by chemical impurities and/or structural defects in the ]. Depending on the hue and intensity of a diamond's coloration, a diamond's color can either detract from or enhance its value. For example, most white diamonds are discounted in price as more yellow hue is detectable, while intense pink or blue diamonds (such as the ]) can be dramatically more valuable. | |||
| ]s can be found in narrow (1 to 4 meters) dikes and sills, and in pipes with diameters that range from about 75 m to 1.5 km. Fresh rock is dark bluish green to greenish gray, but after exposure rapidly turns brown and crumbles.<ref>{{cite book| vauthors = Kjarsgaard BA |chapter=Kimberlite pipe models: significance for exploration| veditors = Milkereit B |title=Proceedings of Exploration 07: Fifth Decennial International Conference on Mineral Exploration|date=2007|publisher=], 2007|pages=667–677|chapter-url=http://www.dmec.ca/ex07-dvd/E07/pdfs/46.pdf |archive-url=https://web.archive.org/web/20121224053731/http://www.dmec.ca/ex07-dvd/E07/pdfs/46.pdf |archive-date=December 24, 2012 |url-status=live|access-date=March 1, 2018}}</ref> It is hybrid rock with a chaotic mixture of small minerals and rock fragments (]) up to the size of watermelons. They are a mixture of ]s and ]s (minerals and rocks carried up from the lower crust and mantle), pieces of surface rock, altered minerals such as ], and new minerals that crystallized during the eruption. The texture varies with depth. The composition forms a continuum with ]s, but the latter have too much oxygen for carbon to exist in a pure form. Instead, it is locked up in the mineral ] ({{chem|]|]|]|3}}).<ref name=Shirey2013/> | |||
| Most diamonds used as gemstones are basically transparent with little tint, or ''white diamonds''. The most common impurity, ], replaces a small proportion of carbon atoms in a diamond's structure and causes a yellowish to brownish tint. This effect is present in almost all white diamonds; in only the rarest diamonds is the coloration due to this effect undetectable. The GIA has developed a rating system for color in white diamonds, from "D" to "Z" (with D being "colorless" and Z having a bright yellow coloration), which has been widely adopted in the industry and is universally recognized, superseding several older systems once used in different countries. The GIA system uses a benchmark set of either natural diamonds of known color grade, along with standardized and carefully controlled lighting conditions are also. Precision-crafted ] master sets are sometimes used in the trade, however the GIA has found these sets to be inaccurate. {{fact}} Diamonds with higher color grades are rarer, in higher demand, and therefore more expensive, than lower color grades. Oddly enough, diamonds graded Z are also rare, and the bright yellow color is also highly valued. Diamonds graded D-F are considered "colorless", G-J are considered "near-colorless", K-M are "slightly colored". N-Y usually appear light yellow or brown. | |||
| All three of the diamond-bearing rocks (kimberlite, lamproite and lamprophyre) lack certain minerals (] and ]) that are incompatible with diamond formation. In ], ] is large and conspicuous, while lamproite has Ti-] and lamprophyre has ] and ]. They are all derived from magma types that erupt rapidly from small amounts of melt, are rich in ] and ], and are less ] than more common mantle melts such as ]. These characteristics allow the melts to carry diamonds to the surface before they dissolve.<ref name=Shirey2013/> | |||
| In contrast to yellow or brown hues, diamonds of other colors are much rarer and more valuable. While even a pale pink or blue hue may increase the value of a diamond, more intense coloration is usually considered more desirable and commands the highest prices. A variety of impurities and structural imperfections cause different colors in diamonds, including yellow, pink, blue, red, green, brown, and other hues. Diamonds with unusual or intense coloration are sometimes labeled "fancy" by the diamond industry. Intense yellow coloration is considered one of the fancy colors, and is separate from the color grades of white diamonds. Gemologists have developed rating systems for fancy colored diamonds, but they are not in common use because of the relative rarity of colored diamonds. | |||
| === |
=== Exploration === | ||
| ] | |||
| {{main|Diamond cut}} | |||
| ] is the art and science of creating a gem-quality diamond out of mined rough. The ''cut'' of a diamond describes the manner in which a diamond has been shaped and polished from its beginning form as a rough stone to its final gem proportions. The cut of a diamond describes the quality of workmanship and the angles to which a diamond is cut. Often diamond cut is confused with "shape." | |||
| ] pipes can be difficult to find. They weather quickly (within a few years after exposure) and tend to have lower topographic relief than surrounding rock. If they are visible in outcrops, the diamonds are never visible because they are so rare. In any case, kimberlites are often covered with vegetation, sediments, soils, or lakes. In modern searches, ] such as ]s, ], and ], help identify promising regions to explore. This is aided by isotopic dating and modeling of the geological history. Then surveyors must go to the area and collect samples, looking for kimberlite fragments or ''indicator minerals''. The latter have compositions that reflect the conditions where diamonds form, such as extreme melt depletion or high pressures in ]s. However, indicator minerals can be misleading; a better approach is ], where the compositions of minerals are analyzed as if they were in equilibrium with mantle minerals.<ref name=Shirey2013/> | |||
| There are mathematical guidelines for the angles and length ratios at which the diamond is supposed to be cut in order to reflect the maximum amount of light. Round brilliant diamonds, the most common, are guided by these specific guidelines, though fancy cut stones are not able to be as accurately guided by mathematical specifics. | |||
| Finding kimberlites requires persistence, and only a small fraction contain diamonds that are commercially viable. The only major discoveries since about 1980 have been in Canada. Since existing mines have lifetimes of as little as 25 years, there could be a shortage of new natural diamonds in the future.<ref name=Shirey2013/> | |||
| The techniques for cutting diamonds have been developed over hundreds of years, with perhaps the greatest achievements made in 1919 by ] and gem enthusiast ]. He developed the ] by calculating the ideal shape to return and scatter light when a diamond is viewed from above. The modern round brilliant has 57 facets (polished faces), counting 33 on the ''crown'' (the top half), and 24 on the ''pavilion'' (the lower half). The girdle is the thin middle part. The function of the crown is to diffuse light into various colors and the pavilion's function to reflect light back through the top of the diamond. | |||
| === Ages === | |||
| Tolkowsky defines the ideal dimensions to have: | |||
| Diamonds are dated by analyzing inclusions using the decay of radioactive isotopes. Depending on the elemental abundances, one can look at the decay of ], ], ], ], or ]. Those found in kimberlites have ages ranging from {{nowrap|1 to 3.5 billion years}}, and there can be multiple ages in the same kimberlite, indicating multiple episodes of diamond formation. The kimberlites themselves are much younger. Most of them have ages between tens of millions and 300 million years old, although there are some older exceptions (Argyle, ] and Wawa). Thus, the kimberlites formed independently of the diamonds and served only to transport them to the surface.<ref name=Cartigny/><ref name=Shirey2013/> Kimberlites are also much younger than the cratons they have erupted through. The reason for the lack of older kimberlites is unknown, but it suggests there was some change in mantle chemistry or tectonics. No kimberlite has erupted in human history.<ref name=Shirey2013/> | |||
| * Table percentage (table diameter divided by overall diameter) = 53% | |||
| * Depth percentage (Overall depth divided by the overall diameter) = 59.3% | |||
| * Pavilion Angle (Angle between the girdle and the pavilion) = 40.75° | |||
| * Crown Angle (Angle between the girdle and the crown) = 34.5° | |||
| * Pavilion Depth (Depth of pavilion divided by overall diameter) = 43.1% | |||
| * Crown Depth (Depth of crown divided by crown diameter) = 16.2% | |||
| === Origin in mantle === | |||
| The culet is the tiny point or facet at the bottom of the diamond. This should be a negligible diameter, otherwise light leaks out of the bottom. Tolkowsky's ideal dimensions did not include a girdle. However, a thin girdle is required in reality in order to prevent the diamond from easily chipping in the setting. A normal girdle should be about 1%–2% of the overall diameter. | |||
| ] with centimeter-size ] crystals]] | |||
| ] | |||
| Most gem-quality diamonds come from depths of 150–250 km in the ]. Such depths occur below cratons in ''mantle keels'', the thickest part of the lithosphere. These regions have high enough pressure and temperature to allow diamonds to form and they are not convecting, so diamonds can be stored for billions of years until a kimberlite eruption samples them.<ref name=Shirey2013/> | |||
| Host rocks in a mantle keel include ] and ], two type of ]. The most dominant rock type in the ], peridotite is an ] consisting mostly of the minerals ] and ]; it is low in ] and high in ]. However, diamonds in peridotite rarely survive the trip to the surface.<ref name=Shirey2013/> Another common source that does keep diamonds intact is ], a ] rock that typically forms from ] as an oceanic plate plunges into the mantle at a ].<ref name=Cartigny/> | |||
| The further the diamond's characteristics are from Tolkowsky's ideal, the less light will be reflected. However, there is a small range in which the diamond can be considered "ideal." Today, because of the relative importance of carat weight in society, many diamonds are often intentionally cut poorly to increase carat weight. There is a financial premium for a diamond that weighs the magical 1.0 carat, so often the girdle is made thicker or the depth is increased. Neither of these tactics make the diamond appear any bigger, and they greatly reduce the sparkle of the diamond. So a poorly cut 1.0 carat diamond may have the same diameter and appear as large as a 0.85 carat diamond. The depth percentage is the overall quickest indication of the quality of the cut of a round brilliant. "Ideal" round brilliant diamonds should not have a depth percentage greater than 62.5%. Another quick indication is the overall diameter. Typically a round brilliant 1.0 carat diamond should have a diameter of about 6.5 mm. Mathematically, the diameter in millimeters of a round brilliant should approximately equal 6.5 times the ] of carat weight, or 11.1 times the cube root of gram weight, or 1.4 times the cube root of point weight. | |||
| A smaller fraction of diamonds (about 150 have been studied) come from depths of 330–660 km, a region that includes the ]. They formed in eclogite but are distinguished from diamonds of shallower origin by inclusions of ] (a form of ] with excess silicon). A similar proportion of diamonds comes from the lower mantle at depths between 660 and 800 km.<ref name=Cartigny/> | |||
| "ideal" cuts can be controversial as the definition brilliance and beauty is very subjective. | |||
| Diamond is thermodynamically stable at high pressures and temperatures, with the phase transition from ] occurring at greater temperatures as the pressure increases. Thus, underneath continents it becomes stable at temperatures of 950{{nbsp}}degrees Celsius and pressures of 4.5 gigapascals, corresponding to depths of 150{{nbsp}}kilometers or greater. In subduction zones, which are colder, it becomes stable at temperatures of 800 °C and pressures of 3.5{{nbsp}}gigapascals. At depths greater than 240 km, iron–nickel metal phases are present and carbon is likely to be either dissolved in them or in the form of ]s. Thus, the deeper origin of some diamonds may reflect unusual growth environments.<ref name=Cartigny/><ref name=Shirey2013/> | |||
| Tolkowsky's mathematical model is now superseded by the GIA Facetware software that is the culmination of 20 years of studies on diamond cuts. | |||
| In 2018 the first known natural samples of a phase of ice called ] were found as inclusions in diamond samples. The inclusions formed at depths between 400 and 800 km, straddling the upper and lower mantle, and provide evidence for water-rich fluid at these depths.<ref>{{cite journal| vauthors = Cartier K |title=Diamond Impurities Reveal Water Deep Within the Mantle|journal=Eos|date=April 2, 2018|volume=99|doi=10.1029/2018EO095949|doi-access=free}}</ref><ref name=Perkins>{{cite journal|vauthors=Perkins S|title=Pockets of water may lie deep below Earth's surface|journal=Science|date=March 8, 2018|url=https://www.science.org/content/article/pockets-water-may-lay-deep-below-earth-s-surface|access-date=June 30, 2022|archive-date=March 8, 2018|archive-url=https://web.archive.org/web/20180308220310/http://www.sciencemag.org/news/2018/03/pockets-water-may-lay-deep-below-earth-s-surface|url-status=live}}</ref> | |||
| New diamond cuts are now all the rage in the diamond industry as for example a design invented in 2003 and called the Genesis cut. This cut differs in shape from the more traditional cuts in its concave surfaces and angles and resembles a 4-pointed star. | |||
| === Carbon sources === | |||
| ] | |||
| The mantle has roughly one billion ] of carbon (for comparison, the atmosphere-ocean system has about 44,000 gigatonnes).<ref>{{cite book | vauthors = Lee CA, Jiang H, Dasgupta R, Torres M |chapter=A Framework for Understanding Whole-Earth Carbon Cycling |pages=313–357 |doi=10.1017/9781108677950.011 | veditors = Orcutt BN, Daniel I, Dasgupta R |title=Deep carbon: past to present |date=2019 |publisher=Cambridge University Press |isbn=978-1-108-67795-0|s2cid=210787128 }}</ref> Carbon has two ], ] and ], in a ratio of approximately 99:1 by mass.<ref name=Shirey2013/> This ratio has a wide range in meteorites, which implies that it also varied a lot in the early Earth. It can also be altered by surface processes like ]. The fraction is generally compared to a standard sample using a ratio ] expressed in parts per thousand. Common rocks from the mantle such as basalts, carbonatites, and kimberlites have ratios between −8 and −2. On the surface, organic sediments have an average of −25 while carbonates have an average of 0.<ref name=Cartigny/> | |||
| Populations of diamonds from different sources have distributions of δ<sup>13</sup>C that vary markedly. Peridotitic diamonds are mostly within the typical mantle range; eclogitic diamonds have values from −40 to +3, although the peak of the distribution is in the mantle range. This variability implies that they are not formed from carbon that is ''primordial'' (having resided in the mantle since the Earth formed). Instead, they are the result of tectonic processes, although (given the ages of diamonds) not necessarily the same tectonic processes that act in the present.<ref name=Shirey2013/> Diamond-forming carbon originates in the top ≈700 kilometers (430 mi) of the upper mantle closest to the surface known as the ].<ref name=Cartigny/> | |||
| ====Shape==== | |||
| Diamonds do not show all of their beauty as rough stones; instead, they must be cut and polished to exhibit the characteristic fire and brilliance that diamond gemstones are known for. Diamonds are cut into a variety of shapes that are generally designed to accentuate these features. | |||
| === Formation and growth === | |||
| Diamonds which are not cut to the specifications of Tolkowsky's round brilliant shape (or subsequent variations) are known as "fancy cuts." Popular fancy cuts include the ''baguette'' (from the French, meaning ''rod'' or ]), ''marquise'', ''princess'' (square outline), ''heart'', ''briolette'' (a form of the rose cut), and ''pear'' cuts. Newer cuts that have been introduced into the jewelry industry are the "cushion" "radiant"(similar to princess cuts, but with rounded edges instead of square edges) and "ascher" cuts. Many fancy colored diamonds are now being cut according to these new styles. Generally speaking, these "fancy cuts" are not held to the same strict standards as Tolkowsky-derived round brilliants and there are less specific mathematical guidelines of angles which determine a well-cut stone. Cuts are influenced heavily by fashion: the baguette cut—which accentuates a diamond's luster and downplays its fire—was all the rage during the ] period, whereas the princess cut—which accentuates a diamond's fire rather than its luster—is currently gaining popularity. The princess cut is also popular amongst diamond cutters: of all the cuts, it wastes the least of the original crystal. The past decades have seen the development of new diamond cuts, often based on a modification of an existing | |||
| ] | |||
| cut. Some of these include extra facets. These newly developed cuts are viewed by many as more of an attempt at brand differentiation by diamond sellers, than actual improvements to the state of the art. | |||
| Diamonds in the mantle form through a '']'' process where a C–O–H–N–S fluid or melt dissolves minerals in a rock and replaces them with new minerals. (The vague term C–O–H–N–S is commonly used because the exact composition is not known.) Diamonds form from this fluid either by reduction of oxidized carbon (e.g., CO<sub>2</sub> or CO<sub>3</sub>) or oxidation of a reduced phase such as ].<ref name=Cartigny/> | |||
| Using probes such as polarized light, ], and ], a series of growth zones can be identified in diamonds. The characteristic pattern in diamonds from the lithosphere involves a nearly concentric series of zones with very thin oscillations in luminescence and alternating episodes where the carbon is resorbed by the fluid and then grown again. Diamonds from below the lithosphere have a more irregular, almost polycrystalline texture, reflecting the higher temperatures and pressures as well as the transport of the diamonds by convection.<ref name=Shirey2013/> | |||
| ====Quality==== | |||
| The quality of a diamond's cut is widely considered the most important of the four Cs in determining the beauty of a diamond; indeed, it is commonly acknowledged that a well-cut diamond can appear to be of greater carat weight, and have clarity and color appear to be of better grade than they actually are. The skill with which a diamond is cut determines its ability to reflect and refract light. | |||
| === Transport to the surface === | |||
| In addition to carrying the most importance to a diamond's quality as a gemstone, the cut is also the most difficult to quantitatively judge. A number of factors, including proportion, ], and the relative angles of various facets, are determined by the quality of the cut and can affect the performance of a diamond. A poorly cut diamond with facets cut only a few degrees out of alignment can result in a poorly performing stone. For a round brilliant cut, there is a balance between "brilliance" and "fire." When a diamond is cut for too much "fire," it looks like a ], which gives off much more "fire" than real diamond. A well-executed round brilliant cut should reflect light upwards and make the diamond appear white when viewed from the top. An inferior cut will produce a stone that appears dark at the center and in some extreme cases the ring settings may show through the top of the diamond as shadows. | |||
| ] | |||
| Geological evidence supports a model in which kimberlite magma rises at 4–20 meters per second, creating an upward path by ] of the rock. As the pressure decreases, a vapor phase ] from the magma, and this helps to keep the magma fluid. At the surface, the initial eruption explodes out through fissures at high speeds (over {{cvt|200|m/s|mph}}). Then, at lower pressures, the rock is eroded, forming a pipe and producing fragmented rock (]). As the eruption wanes, there is ] phase and then metamorphism and hydration produces ]s.<ref name=Shirey2013/> | |||
| === Double diamonds === | |||
| Several different theories on the "ideal" proportions of a diamond have been and continue to be advocated by various owners of patents on machines to view how well a diamond is cut. These advocate a shift away from grading cut by the use of various angles and proportions toward measuring the performance of a cut stone. A number of specially modified viewers and machines have been developed toward this end. They included the FireScope, a.k.a. SymmetriScope or IdealScope (tests for light leakage, light return and proportions), Hearts and Arrows Viewer (test for "]" characteristic pattern observable on stones exhibiting high symmetry), (tests for direct light performance results of a diamond), Isee2 Beauty evaluator (tests for diffused light performance results for round or octagonal diamonds), and ASET (test for AGS cut grade). These viewers and machines often help sellers to demonstrate the light performance results of the diamond in addition to the traditional 4 Cs. These machines are seen as a marketing tool rather than having much scientific value. | |||
| In rare cases, diamonds have been found that contain a cavity within which is a second diamond. The first double diamond, the ], was found by ] in ], Russia, in 2019.<ref>{{cite web|url=https://www.nationalgeographic.com/science/article/rare-diamond-diamond-found-siberia|title=Bizarre 'nesting doll' diamond found inside another diamond|publisher=]|vauthors=Wei-Haas M|date=October 10, 2019|access-date=November 27, 2021|archive-date=November 27, 2021|archive-url=https://web.archive.org/web/20211127050127/https://www.nationalgeographic.com/science/article/rare-diamond-diamond-found-siberia|url-status=dead}}</ref> Another one was found in the ] in ] in 2021.<ref>{{cite news|url=https://www.abc.net.au/news/2021-11-26/ellendale-discovery-comes-as-race-to-restart-production-heats-up/100648088|title=Rare 'double diamond' discovery comes as race to restart mothballed Ellendale mine heats up|publisher=]|vauthors=Fowler C|date=November 26, 2021|access-date=November 27, 2021|archive-date=November 26, 2021|archive-url=https://web.archive.org/web/20211126223633/https://www.abc.net.au/news/2021-11-26/ellendale-discovery-comes-as-race-to-restart-production-heats-up/100648088|url-status=live}}</ref> | |||
| === In space === | |||
| The GIA has developed a set of criteria for grading the cut of round brilliant stones that is now the standard in the diamond industry and is called Facetware. | |||
| {{Main|Extraterrestrial diamonds}} | |||
| Although diamonds on ] are rare, they are very common in space. In ]s, about three percent of the carbon is in the form of ]s, having diameters of a few nanometers. Sufficiently small diamonds can form in the cold of space because their lower ] makes them more stable than graphite. The isotopic signatures of some nanodiamonds indicate they were formed outside the Solar System in stars.<ref>{{cite journal| vauthors = Tielens AG |title=The molecular universe|journal=Reviews of Modern Physics|date=July 12, 2013|volume=85|issue=3|pages=1021–1081|doi=10.1103/RevModPhys.85.1021|bibcode=2013RvMP...85.1021T}}</ref> | |||
| High pressure experiments predict that large quantities of diamonds condense from ] into a "diamond rain" on the ice giant planets ] and ].<ref>{{cite journal | vauthors = Kerr RA | title = Neptune may crush methane into diamonds | journal = Science | volume = 286 | issue = 5437 | pages = 25 | date = October 1999 | pmid = 10532884 | doi = 10.1126/science.286.5437.25a | s2cid = 42814647 }}</ref><ref>{{cite journal| vauthors = Scandolo S, Jeanloz R |author-link2=Raymond Jeanloz |title=The Centers of Planets: In laboratories and computers, shocked and squeezed matter turns metallic, coughs up diamonds and reveals Earth's white-hot center|journal=American Scientist|date=November–December 2003|volume=91|issue=6|pages=516–525|jstor=27858301|bibcode=2003AmSci..91..516S|doi=10.1511/2003.38.905|s2cid=120975663 }}</ref><ref>{{cite news |vauthors=Kaplan S |title=It rains solid diamonds on Uranus and Neptune |url=https://www.washingtonpost.com/news/speaking-of-science/wp/2017/08/25/it-rains-solid-diamonds-on-uranus-and-neptune/ |access-date=October 16, 2017 |newspaper=] |date=August 25, 2017 |archive-date=August 27, 2017 |archive-url=https://web.archive.org/web/20170827011901/https://www.washingtonpost.com/news/speaking-of-science/wp/2017/08/25/it-rains-solid-diamonds-on-uranus-and-neptune/ |url-status=live }}</ref> Some extrasolar planets may be almost entirely composed of diamond.<ref>{{cite news|last1=Max Planck Institute for Radio Astronomy|title=A planet made of diamond|url=http://www.astronomy.com/news/2011/08/a-planet-made-of-diamond|access-date=September 25, 2017|work=Astronomy magazine|date=August 25, 2011|archive-date=May 14, 2023|archive-url=https://web.archive.org/web/20230514174530/https://astronomy.com/news/2011/08/a-planet-made-of-diamond|url-status=live}}</ref> | |||
| ====The cutting process==== | |||
| {{main|Diamond cutting}} | |||
| Diamonds may exist in carbon-rich stars, particularly ]s. One theory for the origin of ], the toughest form of diamond, is that it originated in a white dwarf or ].<ref>{{cite journal | vauthors = Heaney PJ, de Vicenzi EP |title=Strange Diamonds: the Mysterious Origins of Carbonado and Framesite |journal=Elements |volume=1 |pages=85–89 |year=2005 |doi=10.2113/gselements.1.2.85 |issue=2|bibcode=2005Eleme...1...85H }}</ref><ref>{{cite journal | vauthors = Shumilova T, Tkachev S, Isaenko S, Shevchuk S, Rappenglück M, Kazakov V |title=A "diamond-like star" in the lab. Diamond-like glass |journal=Carbon |date=April 2016 |volume=100 |pages=703–709 |doi=10.1016/j.carbon.2016.01.068|doi-access=free |bibcode=2016Carbo.100..703S }}</ref> Diamonds formed in stars may have been the first minerals.<ref>{{cite news |vauthors=Wei-Haas M |title=Life and Rocks May Have Co-Evolved on Earth |url=http://www.smithsonianmag.com/science-nature/life-and-rocks-may-have-co-evolved-on-earth-180957807/ |access-date=September 26, 2017 |work=] |language=en |archive-date=September 2, 2017 |archive-url=https://web.archive.org/web/20170902203717/http://www.smithsonianmag.com/science-nature/life-and-rocks-may-have-co-evolved-on-earth-180957807/ |url-status=live }}</ref> | |||
| ] | |||
| == Industry == | |||
| The process of shaping a rough diamond into a polished gemstone is both an art and a science. The choice of cut is often decided by the original shape of the rough stone, location of the inclusions and flaws to be eliminated, the preservation of the weight, popularity of certain shapes amongst consumers and many other considerations. The round brilliant cut is preferred when the crystal is an octahedron, as often two stones may be cut from one such crystal. Oddly shaped crystals such as macles are more likely to be cut in a ''fancy cut''—that is, a cut other than the round brilliant—which the particular crystal shape lends itself to. | |||
| {{See also|Diamonds as an investment|List of countries by diamond production|Clean Diamond Trade Act}} | |||
| ] diamond set in a ring]] | |||
| The most familiar uses of diamonds today are as gemstones used for ], and as industrial abrasives for cutting hard materials. The markets for gem-grade and industrial-grade diamonds value diamonds differently. | |||
| Even with modern techniques, the cutting and polishing of a diamond crystal always results in a dramatic loss of weight; rarely is it less than 50%. Sometimes the cutters compromise and accept lesser proportions and symmetry in order to avoid inclusions or to preserve the carat rating. Since the per carat price of diamond shifts around key milestones (such as 1.00 carat), many one-carat diamonds are the result of compromising "Cut" for "Carat." Some jewelry experts advise consumers to buy a 0.99 carat diamond for its better price or buy a 1.10 carat diamond for its better cut, avoiding a 1.00 carat diamond which is more likely to be a poorly cut stone. | |||
| === |
=== Gem-grade diamonds === | ||
| {{Main|Diamond (gemstone)}} | |||
| In the gem trade the term light performance is used to describe how well a polished diamond will return light to the viewer. There are three light properties which are described in relation to light performance; brilliance, fire, and scintillation. Brilliance refers to the white light reflections from the external and internal facet surfaces. Fire refers to the spectral colors which are produced as a result of the diamond dispersing the white light. Scintillation refers to the small flashes of light that are seen when the diamond, light source or the viewer is moved. A diamond that is cut and polished to produce a high level of these qualities is said to be high in ''light performance''. | |||
| The ] of white light into ]s is the primary gemological characteristic of gem diamonds. In the 20th century, experts in gemology developed methods of grading diamonds and other gemstones based on the characteristics most important to their value as a gem. Four characteristics, known informally as the ''four Cs'', are now commonly used as the basic descriptors of diamonds: these are its mass in '']'' (a carat being equal to 0.2{{nbsp}}grams), '']'' (quality of the cut is graded according to ], ] and ]), '']'' (how close to white or colorless; for fancy diamonds how intense is its hue), and '']'' (how free is it from ]). A large, flawless diamond is known as a ].<ref>{{cite book|url=https://books.google.com/books?id=DIWEi5Hg93gC&pg=PA42|page=42|vauthors=Hesse RW|title=Jewelrymaking through history|publisher=Greenwood Publishing Group|year=2007|isbn=978-0-313-33507-5|access-date=November 9, 2020|archive-date=November 9, 2023|archive-url=https://web.archive.org/web/20231109173258/https://books.google.com/books?id=DIWEi5Hg93gC&pg=PA42|url-status=live}}</ref> | |||
| ===Cleaning=== | |||
| {{main|Jewelry cleaning}} | |||
| Although it is not one of the four Cs, ''cleanliness'' affects a diamond's beauty as much as any of the four Cs. A clean diamond is more brilliant and fiery than the same diamond when it is "dirty." Dirt or grease on the top of a diamond reduces its luster. Water, dirt, or grease on the bottom of a diamond interferes with the diamond's brilliance and fire. Even a thin film absorbs some light that could have been reflected to the person looking at the diamond. Colored dye or smudges can affect the perceived color of a diamond. Historically, some jewelers' stones were misgraded because of smudges on the girdle, or dye on the culet. Current practice is to clean a diamond thoroughly before grading its color. | |||
| A large trade in gem-grade diamonds exists. Although most gem-grade diamonds are sold newly polished, there is a well-established market for resale of polished diamonds (e.g. pawnbroking, auctions, second-hand jewelry stores, diamantaires, bourses, etc.). One hallmark of the trade in gem-quality diamonds is its remarkable concentration: wholesale trade and diamond cutting is limited to just a few locations; in 2003, 92% of the world's diamonds were cut and polished in ], ].<ref>{{cite news| vauthors = Adiga A |title=Uncommon Brilliance |url=http://www.time.com/time/magazine/article/0,9171,501040419-610100,00.html|archive-url=https://web.archive.org/web/20070310173327/http://www.time.com/time/magazine/article/0,9171,501040419-610100,00.html|url-status=dead|archive-date=March 10, 2007|magazine=]|date=April 12, 2004|access-date=November 3, 2008}}</ref> Other important centers of diamond cutting and trading are the ] in ], where the ] is based, ], the ] in ], the ] in ] and ]. One contributory factor is the geological nature of diamond deposits: several large primary kimberlite-pipe mines each account for significant portions of market share (such as the ] in Botswana, which is a single large-pit mine that can produce between {{convert|12500000|and|15000000|carat|kg}} of diamonds per year<ref>{{cite web |title=Jwaneng|url=http://www.debswana.com/Operations/Pages/Jwaneng.aspx|publisher=Debswana|access-date=March 9, 2012|url-status=dead|archive-url=https://web.archive.org/web/20120317175718/http://www.debswana.com/Operations/Pages/Jwaneng.aspx |archive-date=March 17, 2012}}</ref>). Secondary alluvial diamond deposits, on the other hand, tend to be fragmented amongst many different operators because they can be dispersed over many hundreds of square kilometers (e.g., alluvial deposits in Brazil).{{Citation needed|date=May 2021}} | |||
| Maintaining a clean diamond can sometimes be difficult as jewelry settings can obstruct cleaning efforts and oils, grease, and other ] materials adhere well to a diamond's surface. Some jewelers provide their customers with ]-based cleaning kits; ]s are also popular. | |||
| The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers, the most important being Antwerp, where 80% of all ]s, 50% of all cut diamonds and more than 50% of all rough, cut and industrial diamonds combined are handled.<ref name=India>{{cite book|vauthors=Tichotsky J|title=Russia's Diamond Colony: The Republic of Sakha|url=https://books.google.com/books?id=F7N4G_wxkUYC|page=254|publisher=]|year=2000|isbn=978-90-5702-420-7|access-date=November 9, 2020|archive-date=November 9, 2023|archive-url=https://web.archive.org/web/20231109173259/https://books.google.com/books?id=F7N4G_wxkUYC|url-status=live}}</ref> This makes Antwerp a de facto "world diamond capital".<ref>{{cite news | url = http://www.spiegel.de/international/spiegel/0,1518,416243,00.html | title = Jews Surrender Gem Trade to Indians | work = ] | date = May 15, 2006 | access-date = November 29, 2010 | archive-date = November 26, 2010 | archive-url = https://web.archive.org/web/20101126213945/http://www.spiegel.de/international/spiegel/0,1518,416243,00.html | url-status = live }}</ref> The city of Antwerp also hosts the ], created in 1929 to become the first and biggest diamond bourse dedicated to rough diamonds.<ref>{{cite web |url=https://www.awdc.be/en/20th-century |title=The history of the Antwerp Diamond Center |website=Antwerp World Diamond Center |date=August 16, 2012 |access-date=June 30, 2015 |archive-date=February 22, 2013 |archive-url=https://web.archive.org/web/20130222071114/https://www.awdc.be/en/20th-century |url-status=live }}</ref> Another important diamond center is New York City, where almost 80% of the world's diamonds are sold, including auction sales.<ref name="India" /> | |||
| Cleanliness does not affect the diamond's market value as any competent jeweler will clean the diamond before offering it for sale. However, cleanliness might reflect a diamond's sentimental value: some jewelers have noted a correlation between ring cleanliness and marriage quality . | |||
| The ] company, as the world's largest diamond mining company, holds a dominant position in the industry, and has done so since soon after its founding in 1888 by the British businessman ]. De Beers is currently the world's largest operator of diamond production facilities (mines) and ] for gem-quality diamonds. The Diamond Trading Company (DTC) is a subsidiary of De Beers and markets rough diamonds from De Beers-operated mines. De Beers and its subsidiaries own mines that produce some 40% of annual world diamond production. For most of the 20th century over 80% of the world's rough diamonds passed through De Beers,<ref>{{cite web|url=http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003D0079:EN:HTML|title=Commission Decision of 25 July 2001 declaring a concentration to be compatible with the common market and the EEA Agreement|work=Case No COMP/M.2333 – De Beers/LVMH|publisher=]|year=2003|access-date=February 6, 2009|archive-date=May 12, 2011|archive-url=https://web.archive.org/web/20110512174536/http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003D0079:EN:HTML|url-status=live}}</ref> but by 2001–2009 the figure had decreased to around 45%,<ref>{{cite news |title=Business: Changing facets; Diamonds |url=http://www.economist.com/node/8743058 |newspaper=] |volume=382 |issue=8517 |page=68 |year=2007 |access-date=December 22, 2010 |archive-date=May 12, 2011 |archive-url=https://web.archive.org/web/20110512061729/http://www.economist.com/node/8743058 |url-status=live }}</ref> and by 2013 the company's market share had further decreased to around 38% in value terms and even less by volume.<ref name="idexonline">{{cite web|url=http://www.idexonline.com/portal_FullEditorial.asp?id=38357|title=Certainty in the Diamond Industry? Watch Out For Tipping Points – IDEX's Memo|publisher=idexonline.com|access-date=September 24, 2014|archive-date=January 9, 2015|archive-url=https://web.archive.org/web/20150109023521/http://www.idexonline.com/portal_FullEditorial.asp?id=38357|url-status=dead}}</ref> De Beers sold off the vast majority of its diamond stockpile in the late 1990s – early 2000s<ref>{{cite web|title=The Elusive Sparcle |url=http://www.gjepc.org/solitaire/magazines/Aug05_Sep05/aug05_sep05.aspx?inclpage=Specials§ion_id=3 |publisher=The Gem & Jewellery Export Promotion Council |access-date=April 26, 2009 |url-status=dead |archive-url=https://web.archive.org/web/20090616043101/http://www.gjepc.org/solitaire/magazines/Aug05_Sep05/aug05_sep05.aspx?inclpage=Specials§ion_id=3 |archive-date=June 16, 2009 }}</ref> and the remainder largely represents working stock (diamonds that are being sorted before sale).<ref>{{cite news| vauthors = Even-Zohar C |title=Crisis Mitigation at De Beers|url=http://www.docstoc.com/docs/19770902/Crisis-Mitigation-at-De-Beers|publisher=DIB online|date=November 6, 2008|access-date=April 26, 2009|url-status=dead|archive-url=https://web.archive.org/web/20110512061727/http://www.docstoc.com/docs/19770902/Crisis-Mitigation-at-De-Beers|archive-date=May 12, 2011}}</ref> This was well documented in the press<ref>{{cite web| vauthors = Even-Zohar C |title=De Beers to Halve Diamond Stockpile |url=http://www.allbusiness.com/retail-trade/apparel-accessory-stores-womens-specialty/4224156-1.html |publisher=] |date=November 3, 1999 |access-date=April 26, 2009 |url-status=dead |archive-url=https://web.archive.org/web/20090705101028/http://www.allbusiness.com/retail-trade/apparel-accessory-stores-womens-specialty/4224156-1.html |archive-date=July 5, 2009 }}</ref> but remains little known to the general public. | |||
| ==History== | |||
| Diamonds are thought to have been first recognized and mined in ], where significant alluvial deposits of the stone could then be found. The earliest written reference can be found in the ] text, the ] another ] text, the ], which was completed around 296 BCE and describes diamond's hardness, luster, and dispersion. Diamonds quickly became associated with divinity, being used to decorate religious ]s, and were believed to bring good fortune to those who carried them. Ownership was restricted among various ]s by color, with only kings being allowed to own all colors of diamond. | |||
| As a part of reducing its influence, De Beers withdrew from purchasing diamonds on the open market in 1999 and ceased, at the end of 2008, purchasing Russian diamonds mined by the largest Russian diamond company ].<ref>{{cite web|title=Judgment of the Court of First Instance of 11 July 2007 – Alrosa v Commission|url=http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:C2007/199/70|publisher=EUR-Lex|year=2007|access-date=April 26, 2009|archive-date=December 1, 2017|archive-url=https://web.archive.org/web/20171201043518/http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:C2007/199/70|url-status=live}}</ref> As of January 2011, De Beers states that it only sells diamonds from the following four countries: Botswana, Namibia, South Africa and Canada.<ref>{{cite web |url=http://www.debeersgroup.com/en/Exploration-and-mining/Mining-operations/ |title=Mining operations |publisher=The De Beers Group |year=2007 |access-date=January 4, 2011 |url-status=dead |archive-url=https://web.archive.org/web/20080613143223/http://www.debeersgroup.com/en/Exploration-and-mining/Mining-operations/ |archive-date=June 13, 2008 }}</ref> Alrosa had to suspend their sales in October 2008 due to the ],{{cn|date=February 2023}} but the company reported that it had resumed selling rough diamonds on the open market by October 2009.<ref>{{cite web |url=http://www.eng.alrosa.ru/press_center/releases/2009/10/ |title=Media releases – Media Centre – Alrosa |publisher=Alrosa |date=December 22, 2009 |access-date=January 4, 2011 |url-status=dead |archive-url=https://web.archive.org/web/20130820212115/http://www.eng.alrosa.ru/press_center/releases/2009/10/ |archive-date=August 20, 2013 }}</ref> Apart from Alrosa, other important diamond mining companies include ], which is the world's largest mining company;<ref>{{cite news|url = http://www.abc.net.au/news/stories/2007/08/22/2012367.htm|title = Another record profit for BHP|publisher = ABC News|date = August 22, 2007|access-date = August 23, 2007|archive-date = May 12, 2011|archive-url = https://web.archive.org/web/20110512061741/http://www.abc.net.au/news/stories/2007/08/22/2012367.htm|url-status = live}}</ref> ], the owner of the ] (100%), ] (60%), and ] (78%) diamond mines;<ref>{{cite web|title=Our Companies|work=Rio Tinto web site|publisher=Rio Tinto|url=http://www.riotinto.com/whatweproduce/218_our_companies.asp|access-date=March 5, 2009|url-status=dead|archive-url=https://web.archive.org/web/20130511045232/http://www.riotinto.com/whatweproduce/218_our_companies.asp|archive-date=May 11, 2013}}</ref> and ], the owner of several major diamond mines in Africa. | |||
| In February 2005, a joint ]-U.S. team of ] reported the discovery of four ]-rich stone ceremonial burial ]s originating from China's ] and ] cultures (4000 BCE–2500 BCE) which, because of the axes' specular surfaces, the scientists believe were polished using diamond powder . Although there are diamond deposits now known to exist close to the burial sites, no direct evidence of coeval diamond mining has been found: the researchers came to this conclusion by polishing corundum using various ] abrasives and modern techniques then comparing the results using an ]. At that scale, the surface of the modern diamond-polished corundum closely resembled that of the axes; however, the polishes of the latter were superior. | |||
| ] | |||
| Diamonds were traded to both the east and west of India and were recognized by various cultures for their gemological or industrial uses. In his work ''],'' the ] writer ] noted diamond's ornamental uses, as well as its usefulness to ] because of its hardness. In China, diamonds seem to have been used primarily as ] for engraving ] and drilling holes in beads. Archaeological evidence from ] suggests that diamonds were used as drill tips as early as the 4th century BCE. In ], however, diamonds disappeared for almost 1,000 years following the rise of ] because of two effects: early ]s rejected diamonds because of their earlier use in ]s, and ] traders restricted the flow of trade between Europe and India. | |||
| Further down the supply chain, members of The ] (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent diamond bourses in major cutting centers such as Tel Aviv, Antwerp, Johannesburg and other cities across the US, Europe and Asia.<ref name=harlow/> In 2000, the WFDB and The International Diamond Manufacturers Association established the ] to prevent the trading of diamonds used to fund war and inhumane acts. WFDB's additional activities include sponsoring the ] every two years, as well as the establishment of the ] (IDC) to oversee diamond grading.<ref>{{Cite web |title=Introduction {{!}} IDC |url=https://internationaldiamondcouncil.org/introduction |access-date=October 18, 2022 |website=internationaldiamondcouncil.org |language=en |archive-date=October 18, 2022 |archive-url=https://web.archive.org/web/20221018144626/https://internationaldiamondcouncil.org/introduction |url-status=live }}</ref> | |||
| ] cut (old European).]] | |||
| Once purchased by Sightholders (which is a trademark term referring to the companies that have a three-year supply contract with DTC), diamonds are cut and polished in preparation for sale as gemstones ('industrial' stones are regarded as a by-product of the gemstone market; they are used for abrasives).<ref name=polish>{{cite book | url = https://books.google.com/books?id=fkBJ0HL34WsC&pg=PA297 | pages = 297–299 | title = Africa's silk road | vauthors = Broadman HG, Isik G | publisher = World Bank Publications | year = 2007 | isbn = 978-0-8213-6835-0 | access-date = November 9, 2020 | archive-date = November 9, 2023 | archive-url = https://web.archive.org/web/20231109173401/https://books.google.com/books?id=fkBJ0HL34WsC&pg=PA297#v=onepage&q&f=false | url-status = live }}</ref> The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide.<ref name=polish/> Traditional diamond cutting centers are Antwerp, ], Johannesburg, New York City, and Tel Aviv. Recently, diamond cutting centers have been established in China, India, ], Namibia and Botswana.<ref name=polish/> Cutting centers with lower cost of labor, notably Surat in ], handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems in greater quantities than was previously economically feasible.<ref name="India" /> | |||
| Until the late ], diamonds were most prized in their natural octahedral state, perhaps with the crystal surfaces polished to increase luster and remove foreign material. Around ], the flow of diamonds into Europe increased via ]'s trade network, with most flowing through the ] ports of ], ], and ]. During this time, the ] against cutting diamonds into gem shapes, which was established over 1,000 years earlier in the traditions of India, ended allowing the development of diamond cutting technology to begin in earnest. By 1375, a guild of diamond polishers had been established at ]. Over the following centuries, various diamond cuts were introduced which increasingly demonstrated the fire and brilliance that makes diamonds treasured today: the ''table cut'', the ''briolette'' (around 1476), the ''rose cut'' (mid-16th century), and by the mid-17th century, the ''Mazarin'', the first ] design. In 1919, ] developed an ''ideal'' round brilliant cut design that has set the standard for comparison of modern gems; however, diamond cuts have continued to be refined. | |||
| Diamonds prepared as gemstones are sold on diamond exchanges called '']''. There are 28 registered diamond bourses in the world.<ref>{{cite web|title=Bourse listing|url=http://www.wfdb.com/wfdb-bourses|publisher=World Federation of Diamond Bourses|access-date=February 12, 2012|archive-date=October 25, 2016|archive-url=https://web.archive.org/web/20161025031428/http://wfdb.com/wfdb-bourses|url-status=dead}}</ref> Bourses are the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or sold unset ("loose"). According to the Rio Tinto, in 2002 the diamonds produced and released to the market were valued at US$9 billion as rough diamonds, US$14 billion after being cut and polished, US$28 billion in wholesale diamond jewelry, and US$57 billion in retail sales.<ref>{{cite web|title=North America Diamond Sales Show No Sign of Slowing |url=http://www.awdiamonds.com/article-8.html|publisher=A&W diamonds|access-date=May 5, 2009 |url-status=dead |archive-url=https://web.archive.org/web/20090106185423/http://www.awdiamonds.com/article-8.html|archive-date=January 6, 2009}}</ref> | |||
| The rise in popularity of diamonds as gems seems to have paralleled increasing availability through European history. In the 13th century, King ] established a law that only the king could own diamonds. However, within a century diamonds were popular gems among the moneyed ]ic and merchant classes, and by at latest 1477 had begun to be used in ]s. Popularity continued to rise as new cuts were developed that enhanced the diamond's aesthetic appeal, and has largely continued unabated to this day; diamonds have proven popular with all classes in society as their cost has become within reach. A number of large diamonds have become historically significant objects, as their inclusion in various sets of ] and the purchase, sale, and sometimes theft of notable diamonds, have sometimes become politicized. | |||
| === |
==== Cutting ==== | ||
| {{Main|Diamond cutting|Diamond cut}} | |||
| :{{see also|List of famous diamonds}} | |||
| ] Diamond—an example of unusual diamond cut and jewelry arrangement.]] | |||
| The ], part of the ], was the largest gem-quality rough diamond ever found (1905), at 3,106.75 carats. One of the diamonds cut from it, Cullinan I or the Great Star of Africa, was formerly the largest gem-quality cut diamond at 530.2 carats, but now that title has been taken by ] (1985), a 545.67 carat, yellow-brown diamond. The largest flawless and colorless (grade D) diamond is the ] which weighs 273.85 carats. The ] is the second largest (1990) at 203.04 carats. | |||
| Mined rough diamonds are converted into gems through a multi-step process called "cutting". Diamonds are extremely hard, but also brittle and can be split up by a single blow. Therefore, diamond cutting is traditionally considered as a delicate procedure requiring skills, scientific knowledge, tools and experience. Its final goal is to produce a faceted jewel where the specific angles between the facets would optimize the diamond luster, that is dispersion of white light, whereas the number and area of facets would determine the weight of the final product. The weight reduction upon cutting is significant and can be of the order of 50%.<ref name=x50>{{cite book | url = https://books.google.com/books?id=jPT6JADCqgwC&pg=PA280 | page = 280 | title = Handbook of carbon, graphite, diamond, and fullerenes: properties, processing, and applications | vauthors = Pierson HO | publisher = William Andrew | year = 1993 | isbn = 978-0-8155-1339-1 }}</ref> Several possible shapes are considered, but the final decision is often determined not only by scientific, but also practical considerations. For example, the diamond might be intended for display or for wear, in a ring or a necklace, singled or surrounded by other gems of certain color and shape.<ref name=antique>{{cite book | url = https://books.google.com/books?id=Y84qRt6nz-8C&pg=PA88 | pages = 82–102 | title = Antique jewellery: its manufacture, materials and design | vauthors = James DS | publisher = Osprey Publishing | year = 1998 | isbn = 978-0-7478-0385-0 }}{{Dead link|date=November 2023 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> Some of them may be considered as classical, such as ], ], ], ], ] diamonds, etc. Some of them are special, produced by certain companies, for example, ], ], ] diamonds, etc.<ref>{{cite web|url=http://www.kristallsmolensk.com/backstage/benchmarks/shapes/|title=The Classical and Special Shapes of Diamonds|publisher=kristallsmolensk.com|access-date=July 14, 2015|archive-date=July 14, 2015|archive-url=https://web.archive.org/web/20150714175156/http://www.kristallsmolensk.com/backstage/benchmarks/shapes/|url-status=live}}</ref> | |||
| ], an ] at the ], believes that the galaxy's largest diamond is the core of the ] ''BPM 37093''. Observations indicate that the core is a diamond crystal 4000 km in diameter . | |||
| The most time-consuming part of the cutting is the preliminary analysis of the rough stone. It needs to address a large number of issues, bears much responsibility, and therefore can last years in case of unique diamonds. The following issues are considered: | |||
| == The diamond industry == | |||
| * The hardness of diamond and its ability to cleave strongly depend on the crystal orientation. Therefore, the crystallographic structure of the diamond to be cut is analyzed using ] to choose the optimal cutting directions. | |||
| ] diamond set in a ring.]] | |||
| * Most diamonds contain visible non-diamond inclusions and crystal flaws. The cutter has to decide which flaws are to be removed by the cutting and which could be kept. | |||
| {{See also|Diamonds as an investment}} | |||
| * Splitting a diamond with a hammer is difficult, a well-calculated, angled blow can cut the diamond, piece-by-piece, but it can also ruin the diamond itself. Alternatively, it can be cut with a ], which is a more reliable method.<ref name=antique/><ref>{{cite book | url = https://books.google.com/books?id=X3qe9jzYUAQC&pg=PA984 | pages = 984–992 | title = Handbook of industrial diamonds and diamond films | vauthors = Prelas MA, Popovici G, Bigelow LK | publisher = CRC Press | year = 1998 | isbn = 978-0-8247-9994-6 | access-date = November 9, 2020 | archive-date = November 9, 2023 | archive-url = https://web.archive.org/web/20231109173351/https://books.google.com/books?id=X3qe9jzYUAQC&pg=PA984#v=onepage&q&f=false | url-status = live }}</ref> | |||
| The diamond industry can be broadly separated into two basically distinct categories: one dealing with gem-grade diamonds and another for industrial-grade diamonds. While a large trade in both types of diamonds exists, the two markets act in dramatically different ways. | |||
| After initial cutting, the diamond is shaped in numerous stages of polishing. Unlike cutting, which is a responsible but quick operation, polishing removes material by gradual erosion and is extremely time-consuming. The associated technique is well developed; it is considered as a routine and can be performed by technicians.<ref>{{cite journal | url = https://books.google.com/books?id=i9kDAAAAMBAJ&pg=PA760 | pages = 760–764 | title = Gem Cutting | journal = ] | year = 1940 | volume = 74 | issue = 5 | issn = 0032-4558 | access-date = November 9, 2020 | archive-date = November 9, 2023 | archive-url = https://web.archive.org/web/20231109173258/https://books.google.com/books?id=i9kDAAAAMBAJ&pg=PA760#v=onepage&q&f=false | url-status = live }}</ref> After polishing, the diamond is reexamined for possible flaws, either remaining or induced by the process. Those flaws are concealed through various ] techniques, such as repolishing, crack filling, or clever arrangement of the stone in the jewelry. Remaining non-diamond inclusions are removed through laser drilling and filling of the voids produced.<ref name=read>{{cite book | url = https://books.google.com/books?id=t-OQO3Wk-JsC&pg=PA166 | pages = 165–166 | title = Gemmology | vauthors = Read PG | publisher = Butterworth-Heinemann | year = 2005 | isbn = 978-0-7506-6449-3 | access-date = November 9, 2020 | archive-date = November 9, 2023 | archive-url = https://web.archive.org/web/20231109173801/https://books.google.com/books?id=t-OQO3Wk-JsC&pg=PA166#v=onepage&q&f=false | url-status = live }}</ref> | |||
| ===Gem diamond industry=== | |||
| A large trade in ]-grade diamonds exists. Unlike ]s such as ] or ], gem diamonds do not trade as a ]: there is a substantial mark-up in the sale of diamonds, and there is not a very active market for resale of diamonds. One hallmark of the trade in gem-quality diamonds is its remarkable concentration: wholesale trade and diamond cutting is limited to a few locations (most importantly ], ], ], ], ] and ]), and a single company—]—controls a significant proportion of the trade in diamonds.{{fact}} They are based in ], ] and ], ]. | |||
| ==== Marketing ==== | |||
| The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers (the most important being ]).{{fact}} The De Beers company, as the world's largest diamond miner holds a clearly dominant position in the industry, and has done so since soon after its founding in 1888 by the British imperialist ]. De Beers owns or controls a significant portion of the world's rough diamond production facilities (]) and distribution channels for gem-quality diamonds. The company and its subsidiaries own mines that produce some 40 percent of annual world diamond production. At one time it was thought over 80 percent of the world's rough diamonds passed through the ] (DTC, a subsidiary of ]) in ], but presently the figure is estimated at less than 50 percent. De Beers used its ] position to establish strict price controls, and market diamonds directly to consumers in world markets.{{fact}} | |||
| ] | |||
| Marketing has significantly affected the image of diamond as a valuable commodity. | |||
| The ] is acknowledged as one of the most successful and innovative campaigns in history. ], the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and opened up new markets, even in countries where no diamond tradition had existed before. N.W. Ayer's multifaceted marketing campaign included ], advertising the diamond itself rather than the De Beers brand, and building associations with celebrities and royalty. This coordinated campaign has lasted decades and continues today; it is perhaps best captured by the ] "a diamond is forever". The De Beers account is now handled by the firm JWT, formerly known as J. Walter Thompson.{{fact}} | |||
| ], the advertising firm retained by ] in the mid-20th century, succeeded in reviving the American diamond market and the firm created new markets in countries where no diamond tradition had existed before. N. W. Ayer's marketing included ], advertising focused on the diamond product itself rather than the De Beers brand, and associations with celebrities and royalty. Without advertising the De Beers brand, De Beers was advertising its competitors' diamond products as well,<ref>{{cite web | url = http://www.diamonds.net/news/NewsItem.aspx?ArticleID=33243 | title = Keep the Diamond Dream Alive | vauthors = Rapaport M | work = Rapaport Magazine | publisher = Diamonds.net | access-date = September 9, 2012 | archive-date = September 13, 2012 | archive-url = https://web.archive.org/web/20120913214013/http://www.diamonds.net/news/NewsItem.aspx?ArticleID=33243 | url-status = live }}</ref> but this was not a concern as De Beers dominated the diamond market throughout the 20th century. De Beers' market share dipped temporarily to second place in the global market below Alrosa in the aftermath of the global economic crisis of 2008, down to less than 29% in terms of carats mined, rather than sold.<ref name="jckonline.com">{{cite web |author=JCK Staff |url=http://www.jckonline.com/2011/01/26/10-things-rocking-industry |title=10 Things Rocking the Industry |work=JCK |publisher=Jckonline.com |date=January 26, 2011 |access-date=September 9, 2012 |url-status=dead |archive-url=https://web.archive.org/web/20130107102249/http://www.jckonline.com/2011/01/26/10-things-rocking-industry |archive-date=January 7, 2013 }}</ref> The campaign lasted for decades but was effectively discontinued by early 2011. De Beers still advertises diamonds, but the advertising now mostly promotes its own brands, or licensed product lines, rather than completely "generic" diamond products.<ref name="jckonline.com"/> The campaign was perhaps best captured by the slogan "]".<ref name=sell /> This slogan is now being used by De Beers Diamond Jewelers,<ref>{{cite web | vauthors = Bates R |url=http://www.jckonline.com/blogs/cutting-remarks/2011/01/14/interview-forevermark-ceo |title=Interview with Forevermark CEO |work=JCK |publisher=Jckonline.com |date=January 14, 2011 |access-date=September 9, 2012 |url-status=dead |archive-url=https://web.archive.org/web/20121128004942/http://www.jckonline.com/blogs/cutting-remarks/2011/01/14/interview-forevermark-ceo |archive-date=November 28, 2012 }}</ref> a jewelry firm which is a 50/50% joint venture between the De Beers mining company and ], the luxury goods conglomerate. | |||
| Further down the supply chain, members of The ] (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent ]s in major cutting centres such as ], ], ] and other cities across the USA, Europe and Asia. | |||
| Brown-colored diamonds constituted a significant part of the diamond production, and were predominantly used for industrial purposes. They were seen as worthless for jewelry (not even being assessed on the ] scale). After the development of Argyle diamond mine in Australia in 1986, and marketing, brown diamonds have become acceptable gems.<ref>{{cite book|url=https://books.google.com/books?id=_WI86J88ydAC&pg=PA34|page=34|title=The nature of diamonds| vauthors = Harlow GE |publisher=Cambridge University Press|year=1998|isbn=978-0-521-62935-5}}</ref><ref>{{cite book|url=https://books.google.com/books?id=zNicdkuulE4C&pg=PA416 |page=416|title=Industrial minerals & rocks| vauthors = Kogel JE |publisher= Society for Mining, Metallurgy, and Exploration (U.S.)|year=2006|isbn=978-0-87335-233-8}}</ref> The change was mostly due to the numbers: the Argyle mine, with its {{convert|35000000|carat|kg}} of diamonds per year, makes about one-third of global production of natural diamonds;<ref>{{cite web|access-date=August 4, 2009 |url=http://www.costellos.com.au/diamonds/industry.html |title=The Australian Diamond Industry |url-status=dead |archive-url=https://web.archive.org/web/20090716170624/http://www.costellos.com.au/diamonds/industry.html |archive-date=July 16, 2009 }}</ref> 80% of Argyle diamonds are brown.<ref>{{cite book | url = https://books.google.com/books?id=068-M3xrDSQC&pg=PT158 | page = 158 | title = Diamond deposits: origin, exploration, and history of discovery | vauthors = Erlich E, Hausel DW | publisher = SME | year = 2002 | isbn = 978-0-87335-213-0 }}</ref> | |||
| In 2000, the WFDB and The ] established the ] to prevent the trading of diamonds used to fund war and inhumane acts. | |||
| === Industrial-grade diamonds === | |||
| WFDB's additional activities also include sponsoring the ] every two years, as well as the establishment of the '']'' (IDC) to oversee diamond grading. However, due to the dominance of such labs as The ] (GIA), compliance with IDC rules is mostly confined to smaller laboratories.{{fact}} | |||
| ] with synthetic diamond blade]] | |||
| ] blade with tiny diamonds shown embedded in the metal]] | |||
| ]]] | |||
| Industrial diamonds are valued mostly for their hardness and thermal conductivity, making many of the gemological characteristics of diamonds, such as the ], irrelevant for most applications. Eighty percent of mined diamonds (equal to about {{convert|135000000|carat|kg}} annually) are unsuitable for use as gemstones and are used industrially.<ref>{{cite web|url=http://www.minerals.net/mineral/diamond.aspx|title=Diamond: The mineral Diamond information and pictures|publisher=minerals.net|access-date=September 24, 2014|archive-date=October 23, 2014|archive-url=https://web.archive.org/web/20141023195127/http://www.minerals.net/mineral/diamond.aspx|url-status=live}}</ref> In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; in 2014, {{convert|4500000000|carat|kg}} of synthetic diamonds were produced, 90% of which were produced in China. Approximately 90% of diamond ] is currently of synthetic origin.<ref name=usgs>{{cite web|title=Industrial Diamonds Statistics and Information|url=http://minerals.usgs.gov/minerals/pubs/commodity/diamond/|work=]|access-date=May 5, 2009|archive-date=May 6, 2009|archive-url=https://web.archive.org/web/20090506221551/http://minerals.usgs.gov/minerals/pubs/commodity/diamond/|url-status=live}}</ref> | |||
| === Industrial diamond industry === | |||
| The market for industrial-grade diamonds operates much differently from its gem-grade counterpart. Industrial diamonds are valued mostly for their hardness and heat conductivity, making many of the gemological characteristics of diamond, including clarity and color, mostly irrelevant. This helps explain why 80% of mined diamonds (equal to about 100 million carats or 20,000 kg annually), unsuitable for use as gemstones and known as '']'', are destined for industrial use. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; another 3 billion carats (600 metric tons) of synthetic diamond is produced annually for industrial use—nearly 25 times the mass of natural diamonds mined over the same period.{{fact}} | |||
| The boundary between gem-quality diamonds and industrial diamonds is poorly defined and partly depends on market conditions (for example, if demand for polished diamonds is high, some lower-grade stones will be polished into low-quality or small gemstones rather than being sold for industrial use). Within the category of industrial diamonds, there is a sub-category comprising the lowest-quality, mostly opaque stones, which are known as ].<ref name=spear>{{cite book| vauthors = Spear KE, Dismukes JP |title=Synthetic Diamond: Emerging CVD Science and Technology|url=https://books.google.com/books?id=RR5HF25DB7UC|page=628|publisher=]–]|year=1994|isbn=978-0-471-53589-8}}</ref> | |||
| The dominant industrial use of diamond is in cutting, drilling, grinding, and polishing. Most uses of diamonds in these technologies do not require large diamonds; in fact, most diamonds that are gem-quality except for their small size, can find an industrial use. Diamonds are embedded in drill tips or saw blades, or ground into a powder for use in grinding and polishing applications. Specialized applications include use in laboratories as containment for high pressure experiments (see ]), high-performance ], and limited use in specialized ]s. | |||
| Industrial use of diamonds has historically been associated with their hardness, which makes diamond the ideal material for cutting and grinding tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial applications of this property include diamond-tipped ]s and saws, and the use of diamond powder as an ]. Less expensive industrial-grade diamonds (bort) with more flaws and poorer color than gems, are used for such purposes.<ref>{{cite book| vauthors = Holtzapffel C |title=Turning And Mechanical Manipulation |url= https://archive.org/details/turningandmecha01holtgoog|publisher=Holtzapffel & Co |pages= |year=1856|isbn=978-1-879335-39-4}}</ref> Diamond is not suitable for machining ] ]s at high speeds, as carbon is soluble in iron at the high temperatures created by high-speed machining, leading to greatly increased wear on diamond tools compared to alternatives.<ref>{{cite journal| vauthors = Coelho RT, Yamada S, Aspinwall DK, Wise ML |title=The application of polycrystalline diamond (PCD) tool materials when drilling and reaming aluminum-based alloys including MMC|journal=International Journal of Machine Tools and Manufacture|volume=35|issue=5|pages=761–774|year=1995|doi=10.1016/0890-6955(95)93044-7}}</ref> | |||
| With the continuing advances being made in the production of synthetic diamonds, future applications are beginning to become feasible. Garnering much excitement is the possible use of diamond as a ] suitable to build ]s from, or the use of diamond as a ] in ]. Significant research efforts in ], ], and the ] are under way to capitalize on the potential offered by diamond's unique material properties, combined with increased quality and quantity of supply starting to become available from synthetic diamond manufacturers.{{fact}} | |||
| Specialized applications include use in laboratories as containment for ] (see ]), high-performance ], and limited use in specialized ]s.<ref name=spear/> With the continuing advances being made in the production of synthetic diamonds, future applications are becoming feasible. The high ] of diamond makes it suitable as a ] for integrated circuits in ].<ref>{{cite journal | vauthors = Sakamoto M, Endriz JG, Scifres DR |title=120 W CW output power from monolithic AlGaAs (800 nm) laser diode array mounted on diamond heatsink|journal=] |volume=28 |issue=2 |pages=197–199 |year=1992 |doi=10.1049/el:19920123 |bibcode=1992ElL....28..197S}}</ref> | |||
| === Diamond supply chain === | |||
| {{See also|List of diamond mines}} | |||
| === Mining === | |||
| The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world. In fact, the amount of power which De Beers has consolidated historically prevented it from direct trade with the ], as its trade practices led to an ] for violating ] (the case was settled in 2004).{{fact}} The concentration of power only loosens at the retail level, where diamonds are sold by a limited number of distributors, known as ]s, to jewelers around the world. | |||
| {{See also|List of diamond mines|Exploration diamond drilling}} | |||
| Approximately {{convert|130000000|carat|kg}} of diamonds are mined annually, with a total value of nearly US$9 billion, and about {{convert|100000|kg|abbr=on}} are synthesized annually.<ref name=yarnell>{{cite journal|vauthors=Yarnell A|title=The Many Facets of Man-Made Diamonds|url=http://pubs.acs.org/cen/coverstory/8205/8205diamonds.html|journal=]|volume=82|issue=5|pages=26–31|year=2004|doi=10.1021/cen-v082n005.p026|access-date=October 3, 2006|archive-date=October 28, 2008|archive-url=https://web.archive.org/web/20081028181945/http://pubs.acs.org/cen/coverstory/8205//8205diamonds.html|url-status=live}}</ref> | |||
| ].]] | |||
| Roughly 49% of diamonds originate from ] and ], although significant sources of the mineral have been discovered in ], ], ], ], and ].<ref name=usgs/> They are mined from kimberlite and lamproite volcanic pipes, which can bring diamond crystals, originating from deep within the Earth where high pressures and temperatures enable them to form, to the surface. The mining and distribution of natural diamonds are subjects of frequent controversy such as concerns over the sale of '']s'' or ''conflict diamonds'' by African ] groups.<ref name=conflict>{{cite web|title=Conflict Diamonds |url=https://www.un.org/peace/africa/Diamond.html |publisher=United Nations |date=March 21, 2001 |access-date=May 5, 2009 |url-status=dead |archive-url=https://web.archive.org/web/20100309083348/http://www.un.org/peace/africa/Diamond.html |archive-date=March 9, 2010 }}</ref> The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world. | |||
| ==== Sources ==== | |||
| Historically diamonds were known to be found only in alluvial deposits in ]; India led the world in diamond production from the time of their discovery in approximately the 9th century BCE to the mid-18th century AD, but the commercial potential of these sources has been exhausted. The first non-Indian diamond source was found in ] in 1725. While no commercial diamond production exists in the ], ] and ] are the only states to have a verifiable source of diamonds. Today, most commercially viable diamond deposits are in ], notably in ], ], ], the ], ], ] and ] . There are also commercial deposits being actively mined in the ] of ], ] (mostly in ], for example ] and ]), Brazil, and in Northern and Western ]. Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes. | |||
| Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care is required not to destroy larger diamonds, and then sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of ], after which the final sorting steps are done by hand. Before the use of ]s became commonplace,<ref name=x50/> the separation was done with grease belts; diamonds have a stronger tendency to stick to grease than the other minerals in the ore.<ref name=harlow>{{cite book| vauthors = Harlow GE |title=The nature of diamonds|pages=223, 230–249|url=https://books.google.com/books?id=_WI86J88ydAC&pg=PA223|publisher=]|year=1998|isbn=978-0-521-62935-5}}</ref> | |||
| ==== Conflict Diamonds ==== | |||
| {{main|Blood diamond}} | |||
| In some of the more politically unstable central African and west African countries, revolutionary groups have taken control of diamond mines, using proceeds from diamond sales to finance their operations. Diamonds sold through this process are known as ''conflict diamonds'' or ''blood diamonds''. In response to public concerns that their diamond purchases were contributing to war and human rights abuses in central Africa and west Africa, the ], the diamond industry and diamond-trading nations introduced the ] in 2002, which is aimed at ensuring that conflict diamonds do not become intermixed with the diamonds not controlled by such rebel groups.{{fact}} The Kimberley Process provides documentation and certification of diamond exports from producing countries to ensure that the proceeds of sale are not being used to fund criminal or revolutionary activities.{{fact}} Although the Kimberley Process has been highly successful in limiting the number of conflict diamonds entering the market, conflict diamonds smuggled to market continue to persist to some degree (approx. 1% of diamonds traded today are possible conflict diamonds<ref> World Diamond Council website - DiamondFacts.org, accessed November 5, 2006</ref> ). According to the 2006 book, two major flaws still hinder the effectiveness of the Kimberley Process: the relative ease of smuggling diamonds across African borders and given phony histories, and the violent nature of diamond mining in nations which are not in a technical state of war and whose diamonds are therefore considered "clean." | |||
| ]'s Udachnaya diamond mine]] | |||
| Diamonds from Canada are considered 100% Conflict free. The Canadian Government has setup a body known as Canadian Diamond Code of Conduct: () to help authenticate Canadian Diamonds. This is the most stringent tracking system of diamonds in the world. One of the brands that follow this strict tracking system is called Eskimo Diamonds. | |||
| ] | |||
| Historically, diamonds were found only in ]s in ] and ] of the ] delta in ].<ref>{{cite book| vauthors = Catelle WR |title=The Diamond|publisher=John Lane Co.|year=1911|page=159}}</ref> India led the world in diamond production from the time of their discovery in approximately the 9th century BC<ref name=hershey/><ref>{{cite book| vauthors = Ball V |chapter=1|title=Diamonds, Gold and Coal of India|url=https://archive.org/details/diamondscoalgold00ballrich |page=|publisher=Trübner & Co|location=London|year=1881}} Ball was a geologist in British service.</ref> to the mid-18th century AD, but the commercial potential of these sources had been exhausted by the late 18th century and at that time India was eclipsed by Brazil where the first non-Indian diamonds were found in 1725.<ref name=hershey/> Currently, one of the most prominent Indian mines is located at ].<ref>{{cite news|url=http://www.9newz.com/mail-today-biggest-diamond-found-in-panna|title=Biggest diamond found in Panna|date=July 1, 2010|publisher=Mail Today|url-status=dead|archive-url=https://web.archive.org/web/20110707071636/http://www.9newz.com/mail-today-biggest-diamond-found-in-panna|archive-date=July 7, 2011}}</ref> | |||
| Currently, gem production totals nearly 30 million carats (6,000 kg) of cut and polished stones annually, and over 100 million carats (20,000 kg) of mined diamonds are sold for industrial use each year, as are about 100,000 kg of synthesized diamond. In 2003, this constituted total production of nearly US$9 ] in value.{{fact}} | |||
| Diamond extraction from primary deposits (kimberlites and lamproites) started in the 1870s after the discovery of the ] in South Africa.<ref>{{cite book | title = Encyclopedia of African history | vauthors = Shillington K | page = 767 | url = https://books.google.com/books?id=Ftz_gtO-pngC&pg=PA767 | publisher = CRC Press | isbn = 978-1-57958-453-5 | year = 2005 | access-date = November 9, 2020 | archive-date = November 9, 2023 | archive-url = https://web.archive.org/web/20231109173804/https://books.google.com/books?id=Ftz_gtO-pngC&pg=PA767#v=onepage&q&f=false | url-status = live }}</ref> Production has increased over time and now an accumulated total of {{convert|4500000000|carat|kg}} have been mined since that date.<ref name=giasummer2007>{{cite journal| vauthors = Janse AJ |title=Global Rough Diamond Production Since 1870|journal=Gems & Gemology|volume=43|pages=98–119|year=2007|doi=10.5741/GEMS.43.2.98|issue=2}}</ref> Twenty percent of that amount has been mined in the last five years, and during the last 10 years, nine new mines have started production; four more are waiting to be opened soon. Most of these mines are located in Canada, Zimbabwe, Angola, and one in Russia.<ref name=giasummer2007/> | |||
| ==== Mining ==== | |||
| Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care has to be taken in order to prevent larger diamonds from being destroyed in this process and subsequently the particles are sorted by density. Nowadays, the diamonds are located in the diamond-rich density fraction with the help of X-ray fluorescence,{{fact}} after which the final sorting steps are done by hand. Before the use of X-rays became commonplace, the separation was done with grease belts;{{fact}} diamonds have a stronger tendency to stick to grease than the other minerals in the ore. | |||
| In the U.S., diamonds have been found in ], ], ], Wyoming, and ].<ref name=DGemGLorenz>{{cite journal | vauthors = Lorenz V |title=Argyle in Western Australia: The world's richest diamantiferous pipe; its past and future |journal=Gemmologie, Zeitschrift der Deutschen Gemmologischen Gesellschaft |volume=56 |issue=1–2 |pages=35–40 |year=2007}}</ref><ref name=Montana>{{cite web |title=Microscopic diamond found in Montana |url=http://www.montanastandard.com/articles/2004/10/18/featuresbusiness/hjjfijicjbhdjc.txt | vauthors = Cooke S |work=] |date=October 17, 2004 |access-date=May 5, 2009 |url-status=dead |archive-url=https://web.archive.org/web/20050121085707/http://www.montanastandard.com/articles/2004/10/18/featuresbusiness/hjjfijicjbhdjc.txt |archive-date=January 21, 2005}}</ref> In 2004, the discovery of a microscopic diamond in the U.S. led to the January 2008 bulk-sampling of ]s in a remote part of Montana. The ] in ] is open to the public, and is the only mine in the world where members of the public can dig for diamonds.<ref name=Montana/> | |||
| ==== Distribution ==== | |||
| The ], or DTC, is a subsidiary of De Beers and markets rough diamonds produced both by De Beers mines and other mines from which it purchases rough diamond production. DTC performs sophisticated sorting of rough diamonds into over 16,000 categories,{{fact}} and then sells bulk lots of rough diamonds to a limited number of sightholders a few times a year. | |||
| Today, most commercially viable diamond deposits are in Russia (mostly in ], for example ] and ]), ], Australia (] and ]) and the ].<ref>{{cite web| vauthors = Marshall S, Shore J |title=The Diamond Life|url=http://gnn.tv/videos/2/The_Diamond_Life|publisher=]|year=2004|access-date=March 21, 2007|archive-url=https://web.archive.org/web/20070126235556/http://gnn.tv/videos/2/The_Diamond_Life|archive-date=January 26, 2007}}</ref> In 2005, Russia produced almost one-fifth of the global diamond output, according to the ]. Australia boasts the richest diamantiferous pipe, with production from the Argyle diamond mine reaching peak levels of 42{{nbsp}}metric tons per year in the 1990s.<ref name=DGemGLorenz/><ref>{{cite journal| vauthors = Shigley JE, Chapman J, Ellison RK |year=2001|title=Discovery and Mining of the Argyle Diamond Deposit, Australia|journal=Gems & Gemology |volume=37|issue=1|pages=26–41 |url=http://www.argylediamonds.com.au/docs/gems_and_gemology.pdf|access-date=February 20, 2010|doi=10.5741/GEMS.37.1.26|url-status=dead|archive-url=https://web.archive.org/web/20090930095856/http://www.argylediamonds.com.au/docs/gems_and_gemology.pdf|archive-date=September 30, 2009}}</ref> There are also commercial deposits being actively mined in the ] of Canada and Brazil.<ref name=usgs/> Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes. | |||
| Once purchased by sightholders, diamonds are cut and polished in preparation for sale as gemstones. The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide. Traditional diamond cutting centers are ], ], ], ], and ]. Recently, diamond cutting centers have been established in ], ], and ]. Cutting centers with lower ], notably ] in Gujarat, India, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in ] or ]. Demonstrating this, India produces 90% of all cut and polished diamonds by number, but only 55% by value.{{fact}} The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems than was previously economically feasible. | |||
| ==== Political issues ==== | |||
| Diamonds which have been prepared as gemstones are sold on diamond exchanges called ''bourses''. There are 24 registered diamond bourses.{{fact}} This is the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or as is increasingly popular, sold unset ("loose"). According to the Rio Tinto Group, in 2002 the diamonds produced and released to the market were valued at US$9 billion as rough diamonds, US$14 billion after being cut and polished, US$28 billion in wholesale diamond ], and retail sales of US$57 billion. | |||
| {{Main|Kimberley Process|Blood diamond|Child labour in the diamond industry}} | |||
| {{wikibooks|Development Cooperation Handbook|Stories/Unsustainable Growth|Unsustainable Growth}} | |||
| ] | |||
| In some of the more politically unstable central African and west African countries, revolutionary groups have taken control of ], using proceeds from diamond sales to finance their operations. Diamonds sold through this process are known as ''conflict diamonds'' or ''blood diamonds''.<ref name=conflict/> | |||
| ===Synthetics, Simulants, and enhancements=== | |||
| {{main|Synthetic diamond|Diamond simulants|Diamond enhancement}} | |||
| It is important to distinguish that a ] is a true diamond created by a technological process, whereas a ] is defined as a non-diamond material that is used to simulate the appearance of a true diamond. | |||
| In response to public concerns that their diamond purchases were contributing to war and ] in ] and ] Africa, the ], the diamond industry and diamond-trading nations introduced the ] in 2002.<ref name=kimb>{{cite book|url=https://books.google.com/books?id=hWrEcl2ydzEC&pg=PA305|pages=305–313|title=Resource politics in Sub-Saharan Africa|vauthors=Basedau M, Mehler A|year=2005|publisher=GIGA-Hamburg|isbn=978-3-928049-91-7|access-date=November 9, 2020|archive-date=November 9, 2023|archive-url=https://web.archive.org/web/20231109173806/https://books.google.com/books?id=hWrEcl2ydzEC&pg=PA305#v=onepage&q&f=false|url-status=live}}</ref> The Kimberley Process aims to ensure that conflict diamonds do not become intermixed with the diamonds not controlled by such rebel groups. This is done by requiring diamond-producing countries to provide proof that the money they make from selling the diamonds is not used to fund criminal or revolutionary activities. Although the Kimberley Process has been moderately successful in limiting the number of conflict diamonds entering the market, some still find their way in. According to the International Diamond Manufacturers Association, conflict diamonds constitute 2–3% of all diamonds traded.<ref>{{cite book|title=World Federation of Diamond Bourses (WFDB) and International Diamond Manufacturers Association: Joint Resolution of 19 July 2000|url=https://books.google.com/books?id=fnRnyS7I9cYC&pg=PA334|publisher=World Diamond Council|date=July 19, 2000|access-date=November 5, 2006|isbn=978-90-04-13656-4|archive-date=November 9, 2023|archive-url=https://web.archive.org/web/20231109173808/https://books.google.com/books?id=fnRnyS7I9cYC&pg=PA334#v=onepage&q&f=false|url-status=live}}</ref> Two major flaws still hinder the effectiveness of the Kimberley Process: (1) the relative ease of smuggling diamonds across African borders, and (2) the violent nature of diamond mining in nations that are not in a technical state of war and whose diamonds are therefore considered "clean".<ref name=kimb/> | |||
| The gemological and industrial uses of diamond have created a large demand for rough stones. A portion of this demand is now being met by ]s, artificially-made diamonds which have similar properties to natural diamonds. This process has historically produced industrial-grade diamonds, but synthetic diamond producers have recently begun to produce diamonds with high enough quality to penetrate the gem diamond market. Diamonds have been manufactured synthetically for over fifty years.{{fact}} | |||
| The Canadian Government has set up a body known as the Canadian Diamond Code of Conduct<ref>{{cite web |title=Voluntary Code of Conduct For Authenticating Canadian Diamond Claims |url=http://www.canadiandiamondcodeofconduct.ca/images/EN_CDCC_Committee_Procedures.pdf |publisher=Canadian Diamond Code Committee|year=2006|access-date=October 30, 2007|archive-date=February 29, 2012|archive-url=https://web.archive.org/web/20120229233018/http://www.canadiandiamondcodeofconduct.ca/images/EN_CDCC_Committee_Procedures.pdf|url-status=dead}}</ref> to help authenticate Canadian diamonds. This is a stringent tracking system of diamonds and helps protect the "conflict free" label of Canadian diamonds.<ref>{{cite journal| vauthors = Kjarsgaard BA, Levinson AA |title=Diamonds in Canada|journal=Gems and Gemology|volume=38|issue=3|pages=208–238|year=2002|doi=10.5741/GEMS.38.3.208|doi-access=free}}</ref> | |||
| A diamond's gem quality, which is not as dependent on material properties as industrial applications, has invited both imitation and the invention of procedures to enhance the gemological properties of natural diamonds. Materials which have similar gemological characteristics to diamond but are not mined or synthetic diamond are known as ''diamond simulants''. The most familiar diamond simulant to most consumers is ] (commonly abbreviated as CZ); recently ] has also gained cachet as a popular diamond simulant. Both CZ and moissanite are synthetically produced for use as a diamond simulant. Diamond enhancements are specific treatments, performed on natural diamonds (usually those already cut and polished into a gem), which are designed to better the gemological characteristics of the stone in one or more ways. These include laser drilling to remove inclusions, application of sealants to fill cracks, treatments to improve a white diamond's color grade, and treatments to give fancy color to a white diamond. | |||
| Mineral resource exploitation in general causes irreversible environmental damage, which must be weighed against the socio-economic benefits to a country.<ref>A meta-analysis of the environmental impact specific to diamond mining is in {{Cite report | vauthors = Oluleye G | title = Environmental Impacts of Mined Diamonds |publisher=Imperial College London Consultants |url=https://www.imperial-consultants.co.uk/wp-content/uploads/2021/02/Final-report-Environmental-Impacts-of-Mined-Diamonds-updated-8-21.pdf |archive-url=https://web.archive.org/web/20211203093203/https://www.imperial-consultants.co.uk/wp-content/uploads/2021/02/Final-report-Environmental-Impacts-of-Mined-Diamonds-updated-8-21.pdf |archive-date=December 3, 2021 |url-status=live |access-date=July 1, 2022}} <!-- NOTE ON DATE: They don't put a date on the case study's page. The page copyright is 2019, but the upload is dated 2021/02, and the report itself cites a 2020 study. Case study url: https://www.imperial-consultants.co.uk/casestudies/meta-study-environmental-impact-of-diamond-mining/ --> <!-- OLD VERSION: https://www.imperial-consultants.co.uk/wp-content/uploads/2021/02/Final-report-Environmental-Impacts-of-Mined-Diamonds.pdf --></ref> | |||
| Currently, trained gemologists with appropriate equipment are able to distinguish natural diamonds from all synthetic and simulant diamonds, and identify all enhanced natural diamonds. The established natural diamond industry has a vested interest in maintaining the distinction between natural diamonds and other diamonds, and has made significant investments toward that end. However, as manufacturing technology improves, synthetic diamonds may become indistinguishable from natural diamonds, and new techniques for creating and treating simulants (such as coating them with a very thin diamond-like layer of carbon) are making it increasingly difficult to distinguish simulants from real diamonds. | |||
| == Synthetics, simulants, and enhancements == | |||
| ===Symbolism in the Occult=== | |||
| === Synthetics === | |||
| Historically, and in occultist myths, it has been claimed that diamonds possess several ] powers: | |||
| {{Main|Synthetic diamond}} | |||
| * A diamond gives victory to him who carries it bound on his left arm, no matter the number of enemies.<ref name=Lewis>Spence, Lewis. "An Encyclopaedia of Occultism". Published by University Books, Inc., 1960.</ref> | |||
| * ]s, ]s, ]s, all fly before it; hence, it is good for ] and the ].<ref name=Lewis/> | |||
| * It deprives ] and ]s of their virtue (i.e., ability to attract iron).<ref name=Lewis/> | |||
| * Arabic diamonds are said to attract iron greater than a ].<ref name=Lewis/> | |||
| * A diamond's hardiness can only be broken by smearing it with fresh ]'s blood.<ref name=Lewis/> | |||
| ] | |||
| Because of their extraordinary physical properties, diamonds have been used symbolically since near the time of their first discovery. Perhaps the earliest symbolic use of diamonds was as the eyes of ] devotional statues.{{fact}} In Hinduism ] uses Vajrayudham or the thunderbolt as his primary weapon.{{fact}} Vajra is the word for diamond and ayudham means weapon in ]. The diamonds themselves were thought to be endowments from the gods and were therefore cherished. The point at which diamonds began to be associated with divinity is not known, but early texts indicate that it was recognized in ] since at least 400 BCE.{{fact}} It is said the ] believed diamonds were tears of the gods;{{fact}} the ] believed they were splinters of fallen stars.{{fact}} Many long dead cultures have sought to explain diamond's superlative properties through divine or mystical affiliations.{{fact}} | |||
| Synthetic diamonds are diamonds manufactured in a laboratory, as opposed to diamonds mined from the Earth. The gemological and industrial uses of diamond have created a large demand for rough stones. This demand has been satisfied in large part by synthetic diamonds, which have been manufactured by various processes for more than half a century. However, in recent years it has become possible to produce gem-quality synthetic diamonds of significant size.<ref name="AMNH"/> It is possible to make colorless synthetic gemstones that, on a molecular level, are identical to natural stones and so visually similar that only a gemologist with special equipment can tell the difference.<ref name="bain">{{cite web |url=http://www.bain.com/Images/PR_BAIN_REPORT_The_global_diamond_industry.pdf |archive-url=https://web.archive.org/web/20120131062348/http://www.bain.com/Images/PR_BAIN_REPORT_The_global_diamond_industry.pdf |archive-date=January 31, 2012 |url-status=live|title=The Global Diamond Industry: Lifting the Veil of Mystery|publisher=]|access-date=January 14, 2012}}</ref> | |||
| In ], also known as ] (Diamond Vehicle), diamonds are an important symbol,{{fact}} and the ] is one of the most popular texts. | |||
| The majority of commercially available synthetic diamonds are yellow and are produced by so-called ''high-pressure high-temperature'' (]) processes.<ref>{{cite journal| vauthors = Shigley JE, Abbaschian R |title=Gemesis Laboratory Created Diamonds|journal=Gems & Gemology|volume=38|issue=4|pages=301–309|year=2002|doi=10.5741/GEMS.38.4.301|doi-access=free}}</ref> The yellow color is caused by ] impurities. Other colors may also be reproduced such as blue, green or pink, which are a result of the addition of ] or from ] after synthesis.<ref>{{cite journal| vauthors = Shigley JE, Shen AH, Breeding CM, McClure SF, Shigley JE |title=Lab Grown Colored Diamonds from Chatham Created Gems|journal=Gems & Gemology|volume=40|issue=2|pages=128–145|year=2004|doi=10.5741/GEMS.40.2.128|doi-access=free}}</ref> | |||
| In Western culture, diamonds are the traditional emblem of fearlessness and virtue,{{fact}} but have also often associated with power, wealth, crime and misfortune. Today, diamonds are used to symbolize eternity and love, being often seen adorning ]s and sometimes ]s as well. The popularity of this modern tradition can be traced directly to the marketing campaigns of De Beers, starting in 1938.{{fact}} Prior to the ] marketing campaign, engagement rings had no one particular stone associated with them. The first diamond engagement ring can be traced to the marriage of ] (then Archduke of ]) to ] in 1477.{{fact}} Other early examples of betrothal jewels incorporating diamonds include the ''Bridal Crown of Blanche'' (ca. 1370–80){{fact}} and the ''Heftlein'' brooch of Vienna (ca. 1430–40),{{fact}} a pictorial piece depicting a wedding couple. Inaccessibility of diamonds to the vast majority of the population limited the popularity of diamonds as betrothal jewels during this period. | |||
| Another popular method of growing synthetic diamond is ] (CVD). The growth occurs under low pressure (below atmospheric pressure). It involves feeding a mixture of gases (typically {{nowrap|1 to 99 ]}} to ]) into a chamber and splitting them into chemically active ] in a ] ignited by ], ], ], ], or ].<ref>{{cite journal | vauthors = Werner M, Locher R |title=Growth and application of undoped and doped diamond films |journal=Reports on Progress in Physics |volume=61 |pages=1665–1710 |year=1998 |doi=10.1088/0034-4885/61/12/002 |issue=12 |bibcode=1998RPPh...61.1665W|s2cid=250878100 }}</ref> This method is mostly used for coatings, but can also produce single crystals several millimeters in size (see picture).<ref name=yarnell/> | |||
| The ] company further taps modern symbolism by purporting to synthetically convert the carbonized remains of people or pets into "memorial diamonds."{{fact}} However, many people feel very uncomfortable at the thought of wearing the carbonized remains of people as jewelry.{{fact}} | |||
| As of 2010, nearly all 5,000 million carats (1,000{{nbsp}}tonnes) of synthetic diamonds produced per year are for industrial use. Around 50% of the 133 million carats of natural diamonds mined per year end up in industrial use.<ref name="bain"/><ref>{{cite web|url=https://www.cnbc.com/2012/08/27/the-billion-dollar-business-of-diamonds-from-mining-to-retail.html|vauthors=Pisani B|title=The Business of Diamonds, From Mining to Retail|publisher=]|date=August 27, 2012|access-date=September 9, 2017|archive-date=July 7, 2017|archive-url=https://web.archive.org/web/20170707001321/http://www.cnbc.com/id/48782968|url-status=live}}</ref> Mining companies' expenses average 40 to 60 US dollars per carat for natural colorless diamonds, while synthetic manufacturers' expenses average {{nowrap|$2,500 per carat}} for synthetic, gem-quality colorless diamonds.<ref name="bain"/>{{rp|79}} However, a purchaser is more likely to encounter a synthetic when looking for a fancy-colored diamond because only 0.01% of natural diamonds are fancy-colored, while most synthetic diamonds are colored in some way.<ref>{{cite book|url=https://books.google.com/books?id=zNicdkuulE4C&pg=PA428|pages=426–430|title=Industrial Minerals & Rocks|vauthors=Kogel JE|publisher=SME|year=2006|isbn=978-0-87335-233-8|access-date=November 9, 2020|archive-date=November 9, 2023|archive-url=https://web.archive.org/web/20231109173917/https://books.google.com/books?id=zNicdkuulE4C&pg=PA428#v=onepage&q&f=false|url-status=live}}</ref> | |||
| The diamond is the ] for people born in the month of April, and is also used as the symbol of a sixty-year ], such as a ] (see '']'').{{fact}} | |||
| <gallery widths="200px" heights="180px"> | |||
| Diamonds are a common focus of fiction. Notable pieces of fiction include ]'s '']'' (1956), ]'s '']'' (1988), ]'s "The Diamond as Big As the Ritz" (1922), and ]'s '']'' (1995). In addition, diamonds are the subject of various myths and legends. | |||
| File:HPHTdiamonds2.JPG|alt=Six crystals of cubo-octahedral shapes, each about 2 millimeters in diameter. Two are pale blue, one is pale yellow, one is green-blue, one is dark blue and one green-yellow.|Synthetic diamonds of various colors grown by the high-pressure high-temperature technique | |||
| File:Apollo synthetic diamond.jpg|alt=A round, clear gemstone with many facets, the main face being hexagonal, surrounded by many smaller facets.|Colorless gem cut from diamond grown by chemical vapor deposition | |||
| </gallery> | |||
| === Simulants === | |||
| ==References == | |||
| {{Main|Diamond simulant}} | |||
| <div class="references-small"> | |||
| ] | |||
| <references/> | |||
| </div> | |||
| <div class="references-small"> | |||
| * Anderson, Arthur & Judith. . Retrieved September 12, 2005. | |||
| * The Columbia Electronic Encyclopedia, Sixth Edition (2003). "Diamond". Retrieved March 9, 2005 at http://www.answers.com/topic/diamond. | |||
| * Cuellar, Fred. . Diamond Cutters International. Retrieved April 10, 2005. | |||
| * David, Joshua (September 2003). . ''Wired'', issue 11.09. | |||
| * De Beers Group. . Retrieved March 14, 2005. | |||
| * Epstein, Edward Jay (February 1982). (subscription required). ''The Atlantic Monthly''. | |||
| * Epstein, Edward Jay (1982). (Complete book, includes "Chapter 20: Have you ever tried to sell a diamond?") | |||
| * Eppler, W.F. ''Praktische Gemmologie''. Rühle-Diebner-Verlag, 1989 | |||
| * Government of Gujarat (2004). . Retrieved March 14, 2005. | |||
| * Kjarsgaard, B.A. and Levinson, A. A. (2002). Diamonds in Canada. ''Gems & Gemology'', Vol. 38, No. 3, pp. 208–238. | |||
| * Pagel - Theisen, Verena. ''Diamond Grading ABC: the Manual.'' Rubin & Son, Antwerp, Belgium, 2001. ISBN 3-9800434-6-0 | |||
| * Pricescope. . Retrieved September 26, 2005. | |||
| * Sque, Steve (March 8, 2005). . Retrieved March 10, 2005. | |||
| * Taylor, W.R., Lynton A.J. & Ridd, M., (1990) ''American Mineralogist'', 75, pp. 1290-1310. | |||
| * Tolkowsky, Marcel (1919). ''Diamond Design: A Study of the Reflection and Refraction of Light in a Diamond.'' London: E. & F.N. Spon, Ltd. ( as edited by Jasper Paulsen, Seattle, 2001.) | |||
| * Tyson, Peter (November 2000). . Retrieved March 10, 2005. | |||
| * United Nations Department of Public Information (March 21, 2001). . Retrieved March 10, 2005. | |||
| * Weiner, K.L., Hochleitner, R., Weiss, S., Voelstadt H. ''Diamant'', Lapis, München, 1994. | |||
| * Yarnell, Amanda (February 2, 2004). . '']'', vol. 82, no. 5, pp 26–31. | |||
| * American Museum of Natural History. . Retrieved Oct 21,2005. | |||
| * Supercomputing Institute..Retrieved Nov 01,2005. | |||
| * Carnegie Institution..Retrieved Nov 01,2005. | |||
| * Spence, Lewis. "An Encyclopaedia of Occultism". Published by University Books, Inc., 1960. | |||
| * World Diamond Council. . Retrieved November 19, 2006. | |||
| * Wise, Richard W. "Secrets Of The Gem Trade, The Connoisseur's Guide To Precious Gemstones". (2003) Brunswick House Press. | |||
| * Zoellner, Tom. . (2006) St. Martin's Press. | |||
| </div> | |||
| A diamond simulant is a non-diamond material that is used to simulate the appearance of a diamond, and may be referred to as diamante. ] is the most common. The gemstone ] (silicon carbide) can be treated as a diamond simulant, though more costly to produce than cubic zirconia. Both are produced synthetically.<ref>{{cite book| vauthors = O'Donoghue M, Joyner L |title=Identification of gemstones|pages=12–19|publisher=Butterworth-Heinemann|location=Great Britain|year=2003|isbn=978-0-7506-5512-5}}</ref> | |||
| ==External links== | |||
| {{commons|Diamond}} | |||
| {{wiktionary}} | |||
| === Enhancements === | |||
| * | |||
| {{Main|Diamond enhancement}} | |||
| * (Java applet). | |||
| * | |||
| * | |||
| Diamond enhancements are specific treatments performed on natural or synthetic diamonds (usually those already cut and polished into a gem), which are designed to better the gemological characteristics of the stone in one or more ways. These include laser drilling to remove inclusions, application of sealants to fill cracks, treatments to improve a white diamond's color grade, and treatments to give fancy color to a white diamond.<ref>{{cite book|url=https://books.google.com/books?id=kCc80Q4gzSgC&pg=PA115|page=115|title=The diamond formula|vauthors=Barnard AS|publisher=Butterworth-Heinemann|year=2000|isbn=978-0-7506-4244-6|access-date=November 9, 2020|archive-date=November 9, 2023|archive-url=https://web.archive.org/web/20231109173812/https://books.google.com/books?id=kCc80Q4gzSgC&pg=PA115#v=onepage&q&f=false|url-status=live}}</ref> | |||
| <!--Categories--> | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| Coatings are increasingly used to give a diamond simulant such as cubic zirconia a more "diamond-like" appearance. One such substance is ]—an amorphous carbonaceous material that has some physical properties similar to those of the diamond. Advertising suggests that such a coating would transfer some of these diamond-like properties to the coated stone, hence enhancing the diamond simulant. Techniques such as ] should easily identify such a treatment.<ref>{{cite journal| vauthors = Shigley JE |title=Observations on new coated gemstones|journal=Gemmologie: Zeitschrift der Deutschen Gemmologischen Gesellschaft|volume=56|issue=1–2|pages=53–56|year=2007}}</ref> | |||
| {{Link FA|eo}} | |||
| {{Link FA|lv}} | |||
| === Identification === | |||
| <!--Interlanguage links--> | |||
| ] image of a diamond, taken in a ]]] | |||
| ] | |||
| Early diamond identification tests included a scratch test relying on the superior hardness of diamond. This test is destructive, as a diamond can scratch another diamond, and is rarely used nowadays. Instead, diamond identification relies on its superior thermal conductivity. Electronic thermal probes are widely used in the gemological centers to separate diamonds from their imitations. These probes consist of a pair of battery-powered ]s mounted in a fine copper tip. One thermistor functions as a heating device while the other measures the temperature of the copper tip: if the stone being tested is a diamond, it will conduct the tip's thermal energy rapidly enough to produce a measurable temperature drop. This test takes about two to three seconds.<ref>{{cite patent | inventor = Wenckus JF | country = US | number = 4488821 | title = Method and means of rapidly distinguishing a simulated diamond from natural diamond | pubdate = December 18, 1984 | fdate = November 24, 1982 | pridate = 1981-03-03 | assign1 = Ceres Electronics Corporation }}; {{US patent|4488821}}</ref> | |||
| ] | |||
| ] | |||
| Whereas the thermal probe can separate diamonds from most of their simulants, distinguishing between various types of diamond, for example synthetic or natural, irradiated or non-irradiated, etc., requires more advanced, optical techniques. Those techniques are also used for some diamonds simulants, such as silicon carbide, which pass the thermal conductivity test. Optical techniques can distinguish between natural diamonds and synthetic diamonds. They can also identify the vast majority of treated natural diamonds.<ref name=raman>{{cite book|url=https://books.google.com/books?id=W2cSkEsWbSkC&pg=PA387|pages=387–394|title=Raman spectroscopy in archaeology and art history|vauthors=Edwards HG, Chalmers GM|publisher=Royal Society of Chemistry|year=2005|isbn=978-0-85404-522-8|access-date=November 9, 2020|archive-date=November 9, 2023|archive-url=https://web.archive.org/web/20231109174417/https://books.google.com/books?id=W2cSkEsWbSkC&pg=PA387#v=onepage&q&f=false|url-status=live}}</ref> "Perfect" crystals (at the atomic lattice level) have never been found, so both natural and synthetic diamonds always possess characteristic imperfections, arising from the circumstances of their crystal growth, that allow them to be distinguished from each other.<ref name=spot/> | |||
| ] | |||
| ] | |||
| Laboratories use techniques such as spectroscopy, microscopy, and luminescence under shortwave ultraviolet light to determine a diamond's origin.<ref name=raman/> They also use specially made instruments to aid them in the identification process. Two screening instruments are the ''DiamondSure'' and the ''DiamondView'', both produced by the ] and marketed by the GIA.<ref>{{cite web |vauthors=Donahue PJ |title=DTC Appoints GIA Distributor of DiamondSure and DiamondView |url=http://www.professionaljeweler.com/archives/news/2004/041904story.html |work=Professional Jeweler Magazine |date=April 19, 2004 |access-date=March 2, 2009 |archive-date=March 6, 2012 |archive-url=https://web.archive.org/web/20120306220856/http://www.professionaljeweler.com/archives/news/2004/041904story.html |url-status=dead }}</ref> | |||
| ] | |||
| ] | |||
| Several methods for identifying synthetic diamonds can be performed, depending on the method of production and the color of the diamond. CVD diamonds can usually be identified by an orange fluorescence. D–J colored diamonds can be screened through the ]'s<ref>{{cite web|title=SSEF diamond spotter and SSEF illuminator|url=http://dkamhi.com/ssef%20type%20IIa.htm|publisher=SSEF Swiss Gemmological Institute|access-date=May 5, 2009|url-status=dead|archive-url=https://web.archive.org/web/20090627023938/http://dkamhi.com/ssef%20type%20IIa.htm|archive-date=June 27, 2009}}</ref> Diamond Spotter. Stones in the D–Z color range can be examined through the DiamondSure UV/visible spectrometer, a tool developed by De Beers.<ref name=spot>{{cite journal| vauthors = Welbourn C |title=Identification of Synthetic Diamonds: Present Status and Future Developments, Proceedings of the 4th International Gemological Symposium|journal=Gems and Gemology|volume=42|issue=3|pages=34–35|year=2006}}</ref> Similarly, natural diamonds usually have minor imperfections and flaws, such as inclusions of foreign material, that are not seen in synthetic diamonds. | |||
| ] | |||
| ] | |||
| Screening devices based on diamond type detection can be used to make a distinction between diamonds that are certainly natural and diamonds that are potentially synthetic. Those potentially synthetic diamonds require more investigation in a specialized lab. Examples of commercial screening devices are D-Screen (WTOCD / HRD Antwerp), Alpha Diamond Analyzer (Bruker / HRD Antwerp), and D-Secure (DRC Techno). | |||
| ] | |||
| ] | |||
| == Etymology, earliest use and composition discovery == | |||
| ] | |||
| The name ''diamond'' is derived from {{langx|grc|ἀδάμας}} (''adámas''), 'proper, unalterable, unbreakable, untamed', from ] (''a-''), 'not' + {{langx|grc|δαμάω}} (''damáō''), 'to overpower, tame'.<ref>{{cite web |vauthors=Liddell HG, Scott R |title=Adamas |work=A Greek-English Lexicon |url=https://www.perseus.tufts.edu/cgi-bin/ptext?doc=Perseus%3Atext%3A1999.04.0057%3Aentry%3D%231145 |publisher=] |access-date=February 20, 2021 |archive-date=November 9, 2023 |archive-url=https://web.archive.org/web/20231109174330/http://www.perseus.tufts.edu/hopper/invalidquery.jsp?doc=Perseus:text:1999.04.0057:entry=entry=#1145 |url-status=live }}</ref> Diamonds are thought to have been first recognized and mined in ], where significant ]s of the stone could be found many centuries ago along the rivers ], ], and ]. Diamonds have been known in India for at least 3,000{{nbsp}}years but most likely 6,000{{nbsp}}years.<ref name=hershey>{{cite book |url=https://books.google.com/books?id=35eij1e1al8C&pg=PA23 |vauthors=Hershey W |title=The Book of Diamonds |publisher=Hearthside Press |location=New York |year=1940 |pages=22–28 |isbn=978-1-4179-7715-4 |access-date=November 9, 2020 |archive-date=November 9, 2023 |archive-url=https://web.archive.org/web/20231109174422/https://books.google.com/books?id=35eij1e1al8C&pg=PA23 |url-status=live }}</ref> | |||
| ] | |||
| ] | |||
| Diamonds have been treasured as gemstones since their use as ] in ]. Their usage in engraving tools also dates to early ].<ref>{{cite book|author=Pliny the Elder|author-link=Pliny the Elder|title=Natural History: A Selection|publisher=]|page=371|year=2004|isbn=978-0-14-044413-1}}</ref><ref name=ancient_China>{{cite news|title=Chinese made first use of diamond|url=http://news.bbc.co.uk/2/hi/science/nature/4555235.stm|work=BBC News|date=May 17, 2005|access-date=March 21, 2007|archive-date=March 20, 2007|archive-url=https://web.archive.org/web/20070320064349/http://news.bbc.co.uk/2/hi/science/nature/4555235.stm|url-status=live}}</ref> The popularity of diamonds has risen since the 19th century because of increased supply, improved cutting and polishing techniques, growth in the world economy, and innovative and successful advertising campaigns.<ref name=sell>{{cite web|vauthors=Epstein EJ|title=Have You Ever Tried To Sell a Diamond?|url=https://www.theatlantic.com/issues/82feb/8202diamond1.htm|work=]|year=1982|access-date=May 5, 2009|archive-date=May 17, 2008|archive-url=https://web.archive.org/web/20080517125715/http://www.theatlantic.com/issues/82feb/8202diamond1.htm|url-status=live}}</ref> | |||
| ] | |||
| ] | |||
| In 1772, the French scientist ] used a lens to concentrate the rays of the sun on a diamond in an atmosphere of ], and showed that the only product of the combustion was ], proving that diamond is composed of carbon.<ref>See: {{unbulleted list citebundle | |||
| ] | |||
| |1 = {{Citation |vauthors=Lavoisier A |orig-date=1772 (part 2) |date=October 15, 2007 |title=Premier mémoire sur la destruction du diamant par le feu |trans-title=First memoir on the destruction of diamond by fire |work=Histoire de l'Académie royale des sciences, avec les Mémoires de Mathématique & de Physique, tirés des registres de cette Académie |trans-journal=History of the Royal Academy of Sciences, with the Memoirs of Mathematics & Physics, drawn from the records of this academy] |location=Gallica |publisher=Académie des sciences |pages=564–591 |language=fr |issn=1967-4783 |id=ark:/12148/bpt6k35711 |url=http://gallica.bnf.fr/ark:/12148/bpt6k35711/f739.image |access-date=July 1, 2022 |archive-date=May 9, 2022 |archive-url=https://web.archive.org/web/20220509130518/https://gallica.bnf.fr/ark:/12148/bpt6k35711/f739.image |url-status=live }} | |||
| ] | |||
| |2 = {{Citation |vauthors=Lavoisier A |orig-date=1772 (part 2) |date=October 15, 2007 |title=Second mémoire sur la destruction du diamant par le feu |trans-title=Second memoir on the destruction of diamond by fire |work=Histoire de l'Académie royale des sciences, avec les Mémoires de Mathématique & de Physique, tirés des registres de cette Académie |location=Gallica |publisher=Académie des sciences |pages=591–616 |language=fr |issn=1967-4783 |id=ark:/12148/bpt6k35711 |url=http://gallica.bnf.fr/ark:/12148/bpt6k35711/f766.image |access-date=July 1, 2022 |archive-date=July 10, 2022 |archive-url=https://web.archive.org/web/20220710231047/https://gallica.bnf.fr/ark:/12148/bpt6k35711/f766.image |url-status=live }} <!-- catalog-url=http://catalogue.bnf.fr/ark:/12148/cb32786820s --> | |||
| ] | |||
| }}</ref> Later, in 1797, the English chemist ] repeated and expanded that experiment.<ref>{{Cite journal |vauthors=Smithson T |orig-date=December 15, 1797 |title=On the nature of the diamond |journal=Philosophical Transactions of the Royal Society of London |year=1797 |volume=87 |pages=123–127 |doi=10.1098/rstl.1797.0005 |s2cid=186213726 |url=https://books.google.com/books?id=vlBFAAAAcAAJ&pg=PA123 |access-date=July 1, 2022 |doi-access=free |archive-date=February 19, 2023 |archive-url=https://web.archive.org/web/20230219072828/https://books.google.com/books?id=vlBFAAAAcAAJ&pg=PA123 |url-status=live }}</ref> By demonstrating that burning diamond and graphite releases the same amount of gas, he established the chemical equivalence of these substances.<ref name=hazen/> | |||
| ] | |||
| ] | |||
| == See also == | |||
| ] | |||
| {{Portal|Minerals}} | |||
| ] | |||
| * ] | |||
| ] | |||
| * ] | |||
| ] | |||
| * ] | |||
| ] | |||
| ** ] | |||
| ] | |||
| * ] | |||
| ] | |||
| * ] | |||
| ] | |||
| ] | |||
| == Citations == | |||
| ] | |||
| {{Reflist|30em}} | |||
| ] | |||
| ] | |||
| == General and cited references == | |||
| ] | |||
| {{refbegin|30em}} | |||
| ] | |||
| * {{cite book |vauthors=Even-Zohar C |year=2007 |title=From Mine to Mistress: Corporate Strategies and Government Policies in the International Diamond Industry |edition=2nd |publisher=Mining Journal Press |url=http://www.mine2mistress.com/ |access-date=April 18, 2007 |archive-date=March 10, 2020 |archive-url=https://web.archive.org/web/20200310081831/http://www.mine2mistress.com/ |url-status=live }} | |||
| ] | |||
| * {{cite book | vauthors = Davies G |year=1994 |title=Properties and growth of diamond |publisher=INSPEC |isbn=978-0-85296-875-8}} | |||
| ] | |||
| * {{cite book | vauthors = O'Donoghue M |title=Gems |publisher=Elsevier |year=2006 |isbn=978-0-7506-5856-0}} | |||
| ] | |||
| * {{cite book | vauthors = O'Donoghue M, Joyner L |year=2003 |title=Identification of gemstones |publisher=Butterworth-Heinemann |location=Great Britain |isbn=978-0-7506-5512-5}} | |||
| ] | |||
| * {{cite book | vauthors = Feldman A, Robins LH |year=1991 |title=Applications of Diamond Films and Related Materials |publisher=Elsevier|isbn=978-1-48329124-6}} | |||
| ] | |||
| * {{cite book | vauthors = Field JE |year=1979 |title=The Properties of Diamond |publisher=Academic Press |location=London |isbn=978-0-12-255350-9}} | |||
| ] | |||
| * {{cite book | vauthors = Field JE |year=1992 |title=The Properties of Natural and Synthetic Diamond |publisher=Academic Press |location=London |isbn=978-0-12-255352-3}} | |||
| ] | |||
| * {{cite book |vauthors=Hershey W |year=1940 |title=The Book of Diamonds |publisher=Hearthside Press New York |url=http://www.farlang.com/diamonds/hershey-diamond-chapters/page_001 |isbn=978-1-4179-7715-4 |access-date=August 18, 2011 |archive-date=August 20, 2014 |archive-url=https://web.archive.org/web/20140820053011/http://www.farlang.com/diamonds/hershey-diamond-chapters/page_001 |url-status=live }} | |||
| ] | |||
| * {{cite book |vauthors=Koizumi S, Nebel CE, Nesladek M |year=2008 |title=Physics and Applications of CVD Diamond |publisher=Wiley VCH |isbn=978-3-527-40801-6 |url=https://books.google.com/books?id=pRFUZdHb688C |access-date=November 9, 2020 |archive-date=November 9, 2023 |archive-url=https://web.archive.org/web/20231109174432/https://books.google.com/books?id=pRFUZdHb688C |url-status=live }} | |||
| ] | |||
| * {{cite book |vauthors=Pan LS, Kani DR |year=1995 |title=Diamond: Electronic Properties and Applications |publisher=Kluwer Academic Publishers |url=https://books.google.com/books?id=ZtfFEoXkU8wC&pg=PP1 |isbn=978-0-7923-9524-9 }} | |||
| ] | |||
| * {{cite book | vauthors = Pagel-Theisen V |year=2001 |title=Diamond Grading ABC: the Manual |publisher=Rubin & Son |location=Antwerp |isbn=978-3-9800434-6-5}} | |||
| ] | |||
| * {{cite book | vauthors = Radovic RL, Walker RM, Thrower PA | author-link3 = Peter Thrower |year=1965 |title=Chemistry and physics of carbon: a series of advances |publisher=Marcel Dekker |location=New York |isbn=978-0-8247-0987-7}} | |||
| ] | |||
| * {{cite book |vauthors=Tolkowsky M |year=1919 |title=Diamond Design: A Study of the Reflection and Refraction of Light in a Diamond |publisher=E. & F.N. Spon |location=London |url=http://www.folds.net/diamond/index.html |access-date=March 13, 2007 |archive-date=March 12, 2023 |archive-url=https://web.archive.org/web/20230312204834/http://www.folds.net/diamond/index.html |url-status=live }} | |||
| ] | |||
| * {{cite book |vauthors=Wise RW |year=2016 |title=Secrets of the Gem Trade: The Connoisseur's Guide to Precious Gemstones |isbn=978-0-9728223-2-9 |publisher=Brunswick House Press |url=http://secretsofthegemtrade.com/ |edition=Second |access-date=May 11, 2021 |archive-date=August 19, 2019 |archive-url=https://web.archive.org/web/20190819182845/http://secretsofthegemtrade.com/ |url-status=live }} | |||
| ] | |||
| * {{cite book |vauthors=Zaitsev AM |year=2001 |title=Optical Properties of Diamond: A Data Handbook |publisher=Springer |url=https://books.google.com/books?id=msU4jkdCEhIC&pg=PP1 |isbn=978-3-540-66582-3 |access-date=November 9, 2020 |archive-date=November 9, 2023 |archive-url=https://web.archive.org/web/20231109174953/https://books.google.com/books?id=msU4jkdCEhIC&pg=PP1#v=onepage&q&f=false |url-status=live }} | |||
| ] | |||
| {{refend}} | |||
| ] | |||
| ] | |||
| == Further reading == | |||
| ] | |||
| * {{Cite magazine | vauthors = Epstein EJ |date=February 1982 |title=Have You Ever Tried to Sell a Diamond? |url=https://www.theatlantic.com/doc/198202/diamond |archive-url=https://web.archive.org/web/20060315041428/https://www.theatlantic.com/doc/198202/diamond |archive-date=March 15, 2006 |magazine=] |access-date=January 2, 2023}} | |||
| ] | |||
| * {{Cite web | vauthors = Tyson P |date=November 2000 |title=Diamonds in the Sky |url=https://www.pbs.org/wgbh/nova/diamond/sky.html |department=The Diamond Deception |work=] |publisher=] |access-date=January 2, 2023}} | |||
| ] | |||
| ] | |||
| == External links == | |||
| ] | |||
| {{Sister project links |wikt=diamond |commons=Diamond |b=no |n=no |q=Diamond |s=no |v=no}} | |||
| * | |||
| * {{cite web|url=https://www.gia.edu/doc/A-Contribution-to-Understanding-the-Effect-of-Blue-Fluorescence-on-the-Appearance-of-Diamonds |archive-url=https://web.archive.org/web/20170908111042/https://www.gia.edu/doc/A-Contribution-to-Understanding-the-Effect-of-Blue-Fluorescence-on-the-Appearance-of-Diamonds | title="A Contribution to the Understanding of Blue Fluorescence on the Appearance of Diamonds" |date=2007 |work=Gemological Institute of America (GIA) |archive-date=September 8, 2017 }} | |||
| {{Allotropes of carbon}} | |||
| {{Jewellery}} | |||
| {{Gemstones}} | |||
| {{Mohs}} | |||
| {{Authority control}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 18:34, 29 December 2024
Form of carbon This article is about the mineral. For the gemstone, see Diamond (gemstone). For other uses, see Diamond (disambiguation).
| Diamond | |
|---|---|
 A natural diamond crystal A natural diamond crystal | |
| General | |
| Category | Native minerals |
| Formula (repeating unit) | C |
| IMA symbol | Dia |
| Strunz classification | 1.CB.10a |
| Dana classification | 1.3.6.1 |
| Crystal system | Cubic |
| Crystal class | Hexoctahedral (m3m) H-M symbol: (4/m 3 2/m) |
| Space group | Fd3m (No. 227) |
| Structure | |
| Jmol (3D) | Interactive image |
| Identification | |
| Formula mass | 12.01 g/mol |
| Color | Typically yellow, brown, or gray to colorless. Less often blue, green, black, translucent white, pink, violet, orange, purple, and red. |
| Crystal habit | Octahedral |
| Twinning | Spinel law common (yielding "macle") |
| Cleavage | 111 (perfect in four directions) |
| Fracture | Irregular/Uneven |
| Mohs scale hardness | 10 (defining mineral) |
| Luster | Adamantine |
| Streak | Colorless |
| Diaphaneity | Transparent to subtransparent to translucent |
| Specific gravity | 3.52±0.01 |
| Density | 3.5–3.53 g/cm 3500–3530 kg/m |
| Polish luster | Adamantine |
| Optical properties | Isotropic |
| Refractive index | 2.418 (at 500 nm) |
| Birefringence | None |
| Pleochroism | None |
| Dispersion | 0.044 |
| Melting point | Pressure dependent |
| References | |

Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond as a form of carbon is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.
Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of defects or impurities (about one per million of lattice atoms) can color a diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a very high refractive index and a relatively high optical dispersion.
Most natural diamonds have ages between 1 billion and 3.5 billion years. Most were formed at depths between 150 and 250 kilometres (93 and 155 mi) in the Earth's mantle, although a few have come from as deep as 800 kilometres (500 mi). Under high pressure and temperature, carbon-containing fluids dissolved various minerals and replaced them with diamonds. Much more recently (hundreds to tens of million years ago), they were carried to the surface in volcanic eruptions and deposited in igneous rocks known as kimberlites and lamproites.
Synthetic diamonds can be grown from high-purity carbon under high pressures and temperatures or from hydrocarbon gases by chemical vapor deposition (CVD). Natural and synthetic diamonds are most commonly distinguished using optical techniques or thermal conductivity measurements.
Properties
Main article: Material properties of diamondDiamond is a solid form of pure carbon with its atoms arranged in a crystal. Solid carbon comes in different forms known as allotropes depending on the type of chemical bond. The two most common allotropes of pure carbon are diamond and graphite. In graphite, the bonds are sp orbital hybrids and the atoms form in planes, with each bound to three nearest neighbors, 120 degrees apart. In diamond, they are sp and the atoms form tetrahedra, with each bound to four nearest neighbors. Tetrahedra are rigid, the bonds are strong, and, of all known substances, diamond has the greatest number of atoms per unit volume, which is why it is both the hardest and the least compressible. It also has a high density, ranging from 3150 to 3530 kilograms per cubic metre (over three times the density of water) in natural diamonds and 3520 kg/m in pure diamond. In graphite, the bonds between nearest neighbors are even stronger, but the bonds between parallel adjacent planes are weak, so the planes easily slip past each other. Thus, graphite is much softer than diamond. However, the stronger bonds make graphite less flammable.
Diamonds have been adopted for many uses because of the material's exceptional physical characteristics. It has the highest thermal conductivity and the highest sound velocity. It has low adhesion and friction, and its coefficient of thermal expansion is extremely low. Its optical transparency extends from the far infrared to the deep ultraviolet and it has high optical dispersion. It also has high electrical resistance. It is chemically inert, not reacting with most corrosive substances, and has excellent biological compatibility.
Thermodynamics

The equilibrium pressure and temperature conditions for a transition between graphite and diamond are well established theoretically and experimentally. The equilibrium pressure varies linearly with temperature, between 1.7 GPa at 0 K and 12 GPa at 5000 K (the diamond/graphite/liquid triple point). However, the phases have a wide region about this line where they can coexist. At standard temperature and pressure, 20 °C (293 K) and 1 standard atmosphere (0.10 MPa), the stable phase of carbon is graphite, but diamond is metastable and its rate of conversion to graphite is negligible. However, at temperatures above about 4500 K, diamond rapidly converts to graphite. Rapid conversion of graphite to diamond requires pressures well above the equilibrium line: at 2000 K, a pressure of 35 GPa is needed.
Above the graphite–diamond–liquid carbon triple point, the melting point of diamond increases slowly with increasing pressure; but at pressures of hundreds of GPa, it decreases. At high pressures, silicon and germanium have a BC8 body-centered cubic crystal structure, and a similar structure is predicted for carbon at high pressures. At 0 K, the transition is predicted to occur at 1100 GPa.
Results published in an article in the scientific journal Nature Physics in 2010 suggest that, at ultra-high pressures and temperatures (about 10 million atmospheres or 1 TPa and 50,000 °C), diamond melts into a metallic fluid. The extreme conditions required for this to occur are present in the ice giants Neptune and Uranus. Both planets are made up of approximately 10 percent carbon and could hypothetically contain oceans of liquid carbon. Since large quantities of metallic fluid can affect the magnetic field, this could serve as an explanation as to why the geographic and magnetic poles of the two planets are unaligned.
Crystal structure
See also: Crystallographic defects in diamond
The most common crystal structure of diamond is called diamond cubic. It is formed of unit cells (see the figure) stacked together. Although there are 18 atoms in the figure, each corner atom is shared by eight unit cells and each atom in the center of a face is shared by two, so there are a total of eight atoms per unit cell. The length of each side of the unit cell is denoted by a and is 3.567 angstroms.
The nearest neighbor distance in the diamond lattice is 1.732a/4 where a is the lattice constant, usually given in Angstrøms as a = 3.567 Å, which is 0.3567 nm.
A diamond cubic lattice can be thought of as two interpenetrating face-centered cubic lattices with one displaced by 1⁄4 of the diagonal along a cubic cell, or as one lattice with two atoms associated with each lattice point. Viewed from a <1 1 1> crystallographic direction, it is formed of layers stacked in a repeating ABCABC ... pattern. Diamonds can also form an ABAB ... structure, which is known as hexagonal diamond or lonsdaleite, but this is far less common and is formed under different conditions from cubic carbon.
Crystal habit

Diamonds occur most often as euhedral or rounded octahedra and twinned octahedra known as macles. As diamond's crystal structure has a cubic arrangement of the atoms, they have many facets that belong to a cube, octahedron, rhombicosidodecahedron, tetrakis hexahedron, or disdyakis dodecahedron. The crystals can have rounded-off and unexpressive edges and can be elongated. Diamonds (especially those with rounded crystal faces) are commonly found coated in nyf, an opaque gum-like skin.
Some diamonds contain opaque fibers. They are referred to as opaque if the fibers grow from a clear substrate or fibrous if they occupy the entire crystal. Their colors range from yellow to green or gray, sometimes with cloud-like white to gray impurities. Their most common shape is cuboidal, but they can also form octahedra, dodecahedra, macles, or combined shapes. The structure is the result of numerous impurities with sizes between 1 and 5 microns. These diamonds probably formed in kimberlite magma and sampled the volatiles.
Diamonds can also form polycrystalline aggregates. There have been attempts to classify them into groups with names such as boart, ballas, stewartite, and framesite, but there is no widely accepted set of criteria. Carbonado, a type in which the diamond grains were sintered (fused without melting by the application of heat and pressure), is black in color and tougher than single crystal diamond. It has never been observed in a volcanic rock. There are many theories for its origin, including formation in a star, but no consensus.
Mechanical
Hardness

Diamond is the hardest material on the qualitative Mohs scale. To conduct the quantitative Vickers hardness test, samples of materials are struck with a pyramid of standardized dimensions using a known force – a diamond crystal is used for the pyramid to permit a wide range of materials to be tested. From the size of the resulting indentation, a Vickers hardness value for the material can be determined. Diamond's great hardness relative to other materials has been known since antiquity, and is the source of its name. This does not mean that it is infinitely hard, indestructible, or unscratchable. Indeed, diamonds can be scratched by other diamonds and worn down over time even by softer materials, such as vinyl phonograph records.
Diamond hardness depends on its purity, crystalline perfection, and orientation: hardness is higher for flawless, pure crystals oriented to the <111> direction (along the longest diagonal of the cubic diamond lattice). Therefore, whereas it might be possible to scratch some diamonds with other materials, such as boron nitride, the hardest diamonds can only be scratched by other diamonds and nanocrystalline diamond aggregates.
The hardness of diamond contributes to its suitability as a gemstone. Because it can only be scratched by other diamonds, it maintains its polish extremely well. Unlike many other gems, it is well-suited to daily wear because of its resistance to scratching—perhaps contributing to its popularity as the preferred gem in engagement or wedding rings, which are often worn every day.
The hardest natural diamonds mostly originate from the Copeton and Bingara fields located in the New England area in New South Wales, Australia. These diamonds are generally small, perfect to semiperfect octahedra, and are used to polish other diamonds. Their hardness is associated with the crystal growth form, which is single-stage crystal growth. Most other diamonds show more evidence of multiple growth stages, which produce inclusions, flaws, and defect planes in the crystal lattice, all of which affect their hardness. It is possible to treat regular diamonds under a combination of high pressure and high temperature to produce diamonds that are harder than the diamonds used in hardness gauges.
Diamonds cut glass, but this does not positively identify a diamond because other materials, such as quartz, also lie above glass on the Mohs scale and can also cut it. Diamonds can scratch other diamonds, but this can result in damage to one or both stones. Hardness tests are infrequently used in practical gemology because of their potentially destructive nature. The extreme hardness and high value of diamond means that gems are typically polished slowly, using painstaking traditional techniques and greater attention to detail than is the case with most other gemstones; these tend to result in extremely flat, highly polished facets with exceptionally sharp facet edges. Diamonds also possess an extremely high refractive index and fairly high dispersion. Taken together, these factors affect the overall appearance of a polished diamond and most diamantaires still rely upon skilled use of a loupe (magnifying glass) to identify diamonds "by eye".
Toughness
Somewhat related to hardness is another mechanical property toughness, which is a material's ability to resist breakage from forceful impact. The toughness of natural diamond has been measured as 50–65 MPa·m. This value is good compared to other ceramic materials, but poor compared to most engineering materials such as engineering alloys, which typically exhibit toughness over 80 MPa·m. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamond has a cleavage plane and is therefore more fragile in some orientations than others. Diamond cutters use this attribute to cleave some stones before faceting them. "Impact toughness" is one of the main indexes to measure the quality of synthetic industrial diamonds.
Yield strength
Diamond has compressive yield strength of 130–140 GPa. This exceptionally high value, along with the hardness and transparency of diamond, are the reasons that diamond anvil cells are the main tool for high pressure experiments. These anvils have reached pressures of 600 GPa. Much higher pressures may be possible with nanocrystalline diamonds.
Elasticity and tensile strength
Usually, attempting to deform bulk diamond crystal by tension or bending results in brittle fracture. However, when single crystalline diamond is in the form of micro/nanoscale wires or needles (~100–300 nanometers in diameter, micrometers long), they can be elastically stretched by as much as 9–10 percent tensile strain without failure, with a maximum local tensile stress of about 89–98 GPa, very close to the theoretical limit for this material.
Electrical conductivity
Other specialized applications also exist or are being developed, including use as semiconductors: some blue diamonds are natural semiconductors, in contrast to most diamonds, which are excellent electrical insulators. The conductivity and blue color originate from boron impurity. Boron substitutes for carbon atoms in the diamond lattice, donating a hole into the valence band.
Substantial conductivity is commonly observed in nominally undoped diamond grown by chemical vapor deposition. This conductivity is associated with hydrogen-related species adsorbed at the surface, and it can be removed by annealing or other surface treatments.
Thin needles of diamond can be made to vary their electronic band gap from the normal 5.6 eV to near zero by selective mechanical deformation.
High-purity diamond wafers 5 cm in diameter exhibit perfect resistance in one direction and perfect conductance in the other, creating the possibility of using them for quantum data storage. The material contains only 3 parts per million of nitrogen. The diamond was grown on a stepped substrate, which eliminated cracking.
Surface property
Diamonds are naturally lipophilic and hydrophobic, which means the diamonds' surface cannot be wet by water, but can be easily wet and stuck by oil. This property can be utilized to extract diamonds using oil when making synthetic diamonds. However, when diamond surfaces are chemically modified with certain ions, they are expected to become so hydrophilic that they can stabilize multiple layers of water ice at human body temperature.
The surface of diamonds is partially oxidized. The oxidized surface can be reduced by heat treatment under hydrogen flow. That is to say, this heat treatment partially removes oxygen-containing functional groups. But diamonds (spC) are unstable against high temperature (above about 400 °C (752 °F)) under atmospheric pressure. The structure gradually changes into spC above this temperature. Thus, diamonds should be reduced below this temperature.
Chemical stability
At room temperature, diamonds do not react with any chemical reagents including strong acids and bases.
In an atmosphere of pure oxygen, diamond has an ignition point that ranges from 690 °C (1,274 °F) to 840 °C (1,540 °F); smaller crystals tend to burn more easily. It increases in temperature from red to white heat and burns with a pale blue flame, and continues to burn after the source of heat is removed. By contrast, in air the combustion will cease as soon as the heat is removed because the oxygen is diluted with nitrogen. A clear, flawless, transparent diamond is completely converted to carbon dioxide; any impurities will be left as ash. Heat generated from cutting a diamond will not ignite the diamond, and neither will a cigarette lighter, but house fires and blow torches are hot enough. Jewelers must be careful when molding the metal in a diamond ring.
Diamond powder of an appropriate grain size (around 50 microns) burns with a shower of sparks after ignition from a flame. Consequently, pyrotechnic compositions based on synthetic diamond powder can be prepared. The resulting sparks are of the usual red-orange color, comparable to charcoal, but show a very linear trajectory which is explained by their high density. Diamond also reacts with fluorine gas above about 700 °C (1,292 °F).
Color
Main article: Diamond color

Diamond has a wide band gap of 5.5 eV corresponding to the deep ultraviolet wavelength of 225 nanometers. This means that pure diamond should transmit visible light and appear as a clear colorless crystal. Colors in diamond originate from lattice defects and impurities. The diamond crystal lattice is exceptionally strong, and only atoms of nitrogen, boron, and hydrogen can be introduced into diamond during the growth at significant concentrations (up to atomic percents). Transition metals nickel and cobalt, which are commonly used for growth of synthetic diamond by high-pressure high-temperature techniques, have been detected in diamond as individual atoms; the maximum concentration is 0.01% for nickel and even less for cobalt. Virtually any element can be introduced to diamond by ion implantation.
Nitrogen is by far the most common impurity found in gem diamonds and is responsible for the yellow and brown color in diamonds. Boron is responsible for the blue color. Color in diamond has two additional sources: irradiation (usually by alpha particles), that causes the color in green diamonds, and plastic deformation of the diamond crystal lattice. Plastic deformation is the cause of color in some brown and perhaps pink and red diamonds. In order of increasing rarity, yellow diamond is followed by brown, colorless, then by blue, green, black, pink, orange, purple, and red. "Black", or carbonado, diamonds are not truly black, but rather contain numerous dark inclusions that give the gems their dark appearance. Colored diamonds contain impurities or structural defects that cause the coloration, while pure or nearly pure diamonds are transparent and colorless. Most diamond impurities replace a carbon atom in the crystal lattice, known as a carbon flaw. The most common impurity, nitrogen, causes a slight to intense yellow coloration depending upon the type and concentration of nitrogen present. The Gemological Institute of America (GIA) classifies low saturation yellow and brown diamonds as diamonds in the normal color range, and applies a grading scale from "D" (colorless) to "Z" (light yellow). Yellow diamonds of high color saturation or a different color, such as pink or blue, are called fancy colored diamonds and fall under a different grading scale.
In 2008, the Wittelsbach Diamond, a 35.56-carat (7.112 g) blue diamond once belonging to the King of Spain, fetched over US$24 million at a Christie's auction. In May 2009, a 7.03-carat (1.406 g) blue diamond fetched the highest price per carat ever paid for a diamond when it was sold at auction for 10.5 million Swiss francs (6.97 million euros, or US$9.5 million at the time). That record was, however, beaten the same year: a 5-carat (1.0 g) vivid pink diamond was sold for US$10.8 million in Hong Kong on December 1, 2009.
Clarity
Clarity is one of the 4C's (color, clarity, cut and carat weight) that helps in identifying the quality of diamonds. The Gemological Institute of America (GIA) developed 11 clarity scales to decide the quality of a diamond for its sale value. The GIA clarity scale spans from Flawless (FL) to included (I) having internally flawless (IF), very, very slightly included (VVS), very slightly included (VS) and slightly included (SI) in between. Impurities in natural diamonds are due to the presence of natural minerals and oxides. The clarity scale grades the diamond based on the color, size, location of impurity and quantity of clarity visible under 10x magnification. Inclusions in diamond can be extracted by optical methods. The process is to take pre-enhancement images, identifying the inclusion removal part and finally removing the diamond facets and noises.
Fluorescence

Between 25% and 35% of natural diamonds exhibit some degree of fluorescence when examined under invisible long-wave ultraviolet light or higher energy radiation sources such as X-rays and lasers. Incandescent lighting will not cause a diamond to fluoresce. Diamonds can fluoresce in a variety of colors including blue (most common), orange, yellow, white, green and very rarely red and purple. Although the causes are not well understood, variations in the atomic structure, such as the number of nitrogen atoms present are thought to contribute to the phenomenon.
Thermal conductivity
Diamonds can be identified by their high thermal conductivity (900–2320 W·m·K). Their high refractive index is also indicative, but other materials have similar refractivity.
Geology
Diamonds are extremely rare, with concentrations of at most parts per billion in source rock. Before the 20th century, most diamonds were found in alluvial deposits. Loose diamonds are also found along existing and ancient shorelines, where they tend to accumulate because of their size and density. Rarely, they have been found in glacial till (notably in Wisconsin and Indiana), but these deposits are not of commercial quality. These types of deposit were derived from localized igneous intrusions through weathering and transport by wind or water.
Most diamonds come from the Earth's mantle, and most of this section discusses those diamonds. However, there are other sources. Some blocks of the crust, or terranes, have been buried deep enough as the crust thickened so they experienced ultra-high-pressure metamorphism. These have evenly distributed microdiamonds that show no sign of transport by magma. In addition, when meteorites strike the ground, the shock wave can produce high enough temperatures and pressures for microdiamonds and nanodiamonds to form. Impact-type microdiamonds can be used as an indicator of ancient impact craters. Popigai impact structure in Russia may have the world's largest diamond deposit, estimated at trillions of carats, and formed by an asteroid impact.
A common misconception is that diamonds form from highly compressed coal. Coal is formed from buried prehistoric plants, and most diamonds that have been dated are far older than the first land plants. It is possible that diamonds can form from coal in subduction zones, but diamonds formed in this way are rare, and the carbon source is more likely carbonate rocks and organic carbon in sediments, rather than coal.
Surface distribution
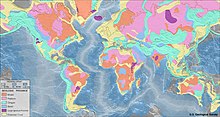
Diamonds are far from evenly distributed over the Earth. A rule of thumb known as Clifford's rule states that they are almost always found in kimberlites on the oldest part of cratons, the stable cores of continents with typical ages of 2.5 billion years or more. However, there are exceptions. The Argyle diamond mine in Australia, the largest producer of diamonds by weight in the world, is located in a mobile belt, also known as an orogenic belt, a weaker zone surrounding the central craton that has undergone compressional tectonics. Instead of kimberlite, the host rock is lamproite. Lamproites with diamonds that are not economically viable are also found in the United States, India, and Australia. In addition, diamonds in the Wawa belt of the Superior province in Canada and microdiamonds in the island arc of Japan are found in a type of rock called lamprophyre.
Kimberlites can be found in narrow (1 to 4 meters) dikes and sills, and in pipes with diameters that range from about 75 m to 1.5 km. Fresh rock is dark bluish green to greenish gray, but after exposure rapidly turns brown and crumbles. It is hybrid rock with a chaotic mixture of small minerals and rock fragments (clasts) up to the size of watermelons. They are a mixture of xenocrysts and xenoliths (minerals and rocks carried up from the lower crust and mantle), pieces of surface rock, altered minerals such as serpentine, and new minerals that crystallized during the eruption. The texture varies with depth. The composition forms a continuum with carbonatites, but the latter have too much oxygen for carbon to exist in a pure form. Instead, it is locked up in the mineral calcite (CaCO
3).
All three of the diamond-bearing rocks (kimberlite, lamproite and lamprophyre) lack certain minerals (melilite and kalsilite) that are incompatible with diamond formation. In kimberlite, olivine is large and conspicuous, while lamproite has Ti-phlogopite and lamprophyre has biotite and amphibole. They are all derived from magma types that erupt rapidly from small amounts of melt, are rich in volatiles and magnesium oxide, and are less oxidizing than more common mantle melts such as basalt. These characteristics allow the melts to carry diamonds to the surface before they dissolve.
Exploration

Kimberlite pipes can be difficult to find. They weather quickly (within a few years after exposure) and tend to have lower topographic relief than surrounding rock. If they are visible in outcrops, the diamonds are never visible because they are so rare. In any case, kimberlites are often covered with vegetation, sediments, soils, or lakes. In modern searches, geophysical methods such as aeromagnetic surveys, electrical resistivity, and gravimetry, help identify promising regions to explore. This is aided by isotopic dating and modeling of the geological history. Then surveyors must go to the area and collect samples, looking for kimberlite fragments or indicator minerals. The latter have compositions that reflect the conditions where diamonds form, such as extreme melt depletion or high pressures in eclogites. However, indicator minerals can be misleading; a better approach is geothermobarometry, where the compositions of minerals are analyzed as if they were in equilibrium with mantle minerals.
Finding kimberlites requires persistence, and only a small fraction contain diamonds that are commercially viable. The only major discoveries since about 1980 have been in Canada. Since existing mines have lifetimes of as little as 25 years, there could be a shortage of new natural diamonds in the future.
Ages
Diamonds are dated by analyzing inclusions using the decay of radioactive isotopes. Depending on the elemental abundances, one can look at the decay of rubidium to strontium, samarium to neodymium, uranium to lead, argon-40 to argon-39, or rhenium to osmium. Those found in kimberlites have ages ranging from 1 to 3.5 billion years, and there can be multiple ages in the same kimberlite, indicating multiple episodes of diamond formation. The kimberlites themselves are much younger. Most of them have ages between tens of millions and 300 million years old, although there are some older exceptions (Argyle, Premier and Wawa). Thus, the kimberlites formed independently of the diamonds and served only to transport them to the surface. Kimberlites are also much younger than the cratons they have erupted through. The reason for the lack of older kimberlites is unknown, but it suggests there was some change in mantle chemistry or tectonics. No kimberlite has erupted in human history.
Origin in mantle


Most gem-quality diamonds come from depths of 150–250 km in the lithosphere. Such depths occur below cratons in mantle keels, the thickest part of the lithosphere. These regions have high enough pressure and temperature to allow diamonds to form and they are not convecting, so diamonds can be stored for billions of years until a kimberlite eruption samples them.
Host rocks in a mantle keel include harzburgite and lherzolite, two type of peridotite. The most dominant rock type in the upper mantle, peridotite is an igneous rock consisting mostly of the minerals olivine and pyroxene; it is low in silica and high in magnesium. However, diamonds in peridotite rarely survive the trip to the surface. Another common source that does keep diamonds intact is eclogite, a metamorphic rock that typically forms from basalt as an oceanic plate plunges into the mantle at a subduction zone.
A smaller fraction of diamonds (about 150 have been studied) come from depths of 330–660 km, a region that includes the transition zone. They formed in eclogite but are distinguished from diamonds of shallower origin by inclusions of majorite (a form of garnet with excess silicon). A similar proportion of diamonds comes from the lower mantle at depths between 660 and 800 km.
Diamond is thermodynamically stable at high pressures and temperatures, with the phase transition from graphite occurring at greater temperatures as the pressure increases. Thus, underneath continents it becomes stable at temperatures of 950 degrees Celsius and pressures of 4.5 gigapascals, corresponding to depths of 150 kilometers or greater. In subduction zones, which are colder, it becomes stable at temperatures of 800 °C and pressures of 3.5 gigapascals. At depths greater than 240 km, iron–nickel metal phases are present and carbon is likely to be either dissolved in them or in the form of carbides. Thus, the deeper origin of some diamonds may reflect unusual growth environments.
In 2018 the first known natural samples of a phase of ice called Ice VII were found as inclusions in diamond samples. The inclusions formed at depths between 400 and 800 km, straddling the upper and lower mantle, and provide evidence for water-rich fluid at these depths.
Carbon sources
The mantle has roughly one billion gigatonnes of carbon (for comparison, the atmosphere-ocean system has about 44,000 gigatonnes). Carbon has two stable isotopes, C and C, in a ratio of approximately 99:1 by mass. This ratio has a wide range in meteorites, which implies that it also varied a lot in the early Earth. It can also be altered by surface processes like photosynthesis. The fraction is generally compared to a standard sample using a ratio δC expressed in parts per thousand. Common rocks from the mantle such as basalts, carbonatites, and kimberlites have ratios between −8 and −2. On the surface, organic sediments have an average of −25 while carbonates have an average of 0.
Populations of diamonds from different sources have distributions of δC that vary markedly. Peridotitic diamonds are mostly within the typical mantle range; eclogitic diamonds have values from −40 to +3, although the peak of the distribution is in the mantle range. This variability implies that they are not formed from carbon that is primordial (having resided in the mantle since the Earth formed). Instead, they are the result of tectonic processes, although (given the ages of diamonds) not necessarily the same tectonic processes that act in the present. Diamond-forming carbon originates in the top ≈700 kilometers (430 mi) of the upper mantle closest to the surface known as the asthenosphere.
Formation and growth

Diamonds in the mantle form through a metasomatic process where a C–O–H–N–S fluid or melt dissolves minerals in a rock and replaces them with new minerals. (The vague term C–O–H–N–S is commonly used because the exact composition is not known.) Diamonds form from this fluid either by reduction of oxidized carbon (e.g., CO2 or CO3) or oxidation of a reduced phase such as methane.
Using probes such as polarized light, photoluminescence, and cathodoluminescence, a series of growth zones can be identified in diamonds. The characteristic pattern in diamonds from the lithosphere involves a nearly concentric series of zones with very thin oscillations in luminescence and alternating episodes where the carbon is resorbed by the fluid and then grown again. Diamonds from below the lithosphere have a more irregular, almost polycrystalline texture, reflecting the higher temperatures and pressures as well as the transport of the diamonds by convection.
Transport to the surface

Geological evidence supports a model in which kimberlite magma rises at 4–20 meters per second, creating an upward path by hydraulic fracturing of the rock. As the pressure decreases, a vapor phase exsolves from the magma, and this helps to keep the magma fluid. At the surface, the initial eruption explodes out through fissures at high speeds (over 200 m/s (450 mph)). Then, at lower pressures, the rock is eroded, forming a pipe and producing fragmented rock (breccia). As the eruption wanes, there is pyroclastic phase and then metamorphism and hydration produces serpentinites.
Double diamonds
In rare cases, diamonds have been found that contain a cavity within which is a second diamond. The first double diamond, the Matryoshka, was found by Alrosa in Yakutia, Russia, in 2019. Another one was found in the Ellendale Diamond Field in Western Australia in 2021.
In space
Main article: Extraterrestrial diamondsAlthough diamonds on Earth are rare, they are very common in space. In meteorites, about three percent of the carbon is in the form of nanodiamonds, having diameters of a few nanometers. Sufficiently small diamonds can form in the cold of space because their lower surface energy makes them more stable than graphite. The isotopic signatures of some nanodiamonds indicate they were formed outside the Solar System in stars.
High pressure experiments predict that large quantities of diamonds condense from methane into a "diamond rain" on the ice giant planets Uranus and Neptune. Some extrasolar planets may be almost entirely composed of diamond.
Diamonds may exist in carbon-rich stars, particularly white dwarfs. One theory for the origin of carbonado, the toughest form of diamond, is that it originated in a white dwarf or supernova. Diamonds formed in stars may have been the first minerals.
Industry
See also: Diamonds as an investment, List of countries by diamond production, and Clean Diamond Trade Act
The most familiar uses of diamonds today are as gemstones used for adornment, and as industrial abrasives for cutting hard materials. The markets for gem-grade and industrial-grade diamonds value diamonds differently.
Gem-grade diamonds
Main article: Diamond (gemstone)The dispersion of white light into spectral colors is the primary gemological characteristic of gem diamonds. In the 20th century, experts in gemology developed methods of grading diamonds and other gemstones based on the characteristics most important to their value as a gem. Four characteristics, known informally as the four Cs, are now commonly used as the basic descriptors of diamonds: these are its mass in carats (a carat being equal to 0.2 grams), cut (quality of the cut is graded according to proportions, symmetry and polish), color (how close to white or colorless; for fancy diamonds how intense is its hue), and clarity (how free is it from inclusions). A large, flawless diamond is known as a paragon.
A large trade in gem-grade diamonds exists. Although most gem-grade diamonds are sold newly polished, there is a well-established market for resale of polished diamonds (e.g. pawnbroking, auctions, second-hand jewelry stores, diamantaires, bourses, etc.). One hallmark of the trade in gem-quality diamonds is its remarkable concentration: wholesale trade and diamond cutting is limited to just a few locations; in 2003, 92% of the world's diamonds were cut and polished in Surat, India. Other important centers of diamond cutting and trading are the Antwerp diamond district in Belgium, where the International Gemological Institute is based, London, the Diamond District in New York City, the Diamond Exchange District in Tel Aviv and Amsterdam. One contributory factor is the geological nature of diamond deposits: several large primary kimberlite-pipe mines each account for significant portions of market share (such as the Jwaneng mine in Botswana, which is a single large-pit mine that can produce between 12,500,000 and 15,000,000 carats (2,500 and 3,000 kg) of diamonds per year). Secondary alluvial diamond deposits, on the other hand, tend to be fragmented amongst many different operators because they can be dispersed over many hundreds of square kilometers (e.g., alluvial deposits in Brazil).
The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers, the most important being Antwerp, where 80% of all rough diamonds, 50% of all cut diamonds and more than 50% of all rough, cut and industrial diamonds combined are handled. This makes Antwerp a de facto "world diamond capital". The city of Antwerp also hosts the Antwerpsche Diamantkring, created in 1929 to become the first and biggest diamond bourse dedicated to rough diamonds. Another important diamond center is New York City, where almost 80% of the world's diamonds are sold, including auction sales.
The De Beers company, as the world's largest diamond mining company, holds a dominant position in the industry, and has done so since soon after its founding in 1888 by the British businessman Cecil Rhodes. De Beers is currently the world's largest operator of diamond production facilities (mines) and distribution channels for gem-quality diamonds. The Diamond Trading Company (DTC) is a subsidiary of De Beers and markets rough diamonds from De Beers-operated mines. De Beers and its subsidiaries own mines that produce some 40% of annual world diamond production. For most of the 20th century over 80% of the world's rough diamonds passed through De Beers, but by 2001–2009 the figure had decreased to around 45%, and by 2013 the company's market share had further decreased to around 38% in value terms and even less by volume. De Beers sold off the vast majority of its diamond stockpile in the late 1990s – early 2000s and the remainder largely represents working stock (diamonds that are being sorted before sale). This was well documented in the press but remains little known to the general public.
As a part of reducing its influence, De Beers withdrew from purchasing diamonds on the open market in 1999 and ceased, at the end of 2008, purchasing Russian diamonds mined by the largest Russian diamond company Alrosa. As of January 2011, De Beers states that it only sells diamonds from the following four countries: Botswana, Namibia, South Africa and Canada. Alrosa had to suspend their sales in October 2008 due to the global energy crisis, but the company reported that it had resumed selling rough diamonds on the open market by October 2009. Apart from Alrosa, other important diamond mining companies include BHP, which is the world's largest mining company; Rio Tinto, the owner of the Argyle (100%), Diavik (60%), and Murowa (78%) diamond mines; and Petra Diamonds, the owner of several major diamond mines in Africa.

Further down the supply chain, members of The World Federation of Diamond Bourses (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent diamond bourses in major cutting centers such as Tel Aviv, Antwerp, Johannesburg and other cities across the US, Europe and Asia. In 2000, the WFDB and The International Diamond Manufacturers Association established the World Diamond Council to prevent the trading of diamonds used to fund war and inhumane acts. WFDB's additional activities include sponsoring the World Diamond Congress every two years, as well as the establishment of the International Diamond Council (IDC) to oversee diamond grading.
Once purchased by Sightholders (which is a trademark term referring to the companies that have a three-year supply contract with DTC), diamonds are cut and polished in preparation for sale as gemstones ('industrial' stones are regarded as a by-product of the gemstone market; they are used for abrasives). The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide. Traditional diamond cutting centers are Antwerp, Amsterdam, Johannesburg, New York City, and Tel Aviv. Recently, diamond cutting centers have been established in China, India, Thailand, Namibia and Botswana. Cutting centers with lower cost of labor, notably Surat in Gujarat, India, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems in greater quantities than was previously economically feasible.
Diamonds prepared as gemstones are sold on diamond exchanges called bourses. There are 28 registered diamond bourses in the world. Bourses are the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or sold unset ("loose"). According to the Rio Tinto, in 2002 the diamonds produced and released to the market were valued at US$9 billion as rough diamonds, US$14 billion after being cut and polished, US$28 billion in wholesale diamond jewelry, and US$57 billion in retail sales.
Cutting
Main articles: Diamond cutting and Diamond cut
Mined rough diamonds are converted into gems through a multi-step process called "cutting". Diamonds are extremely hard, but also brittle and can be split up by a single blow. Therefore, diamond cutting is traditionally considered as a delicate procedure requiring skills, scientific knowledge, tools and experience. Its final goal is to produce a faceted jewel where the specific angles between the facets would optimize the diamond luster, that is dispersion of white light, whereas the number and area of facets would determine the weight of the final product. The weight reduction upon cutting is significant and can be of the order of 50%. Several possible shapes are considered, but the final decision is often determined not only by scientific, but also practical considerations. For example, the diamond might be intended for display or for wear, in a ring or a necklace, singled or surrounded by other gems of certain color and shape. Some of them may be considered as classical, such as round, pear, marquise, oval, hearts and arrows diamonds, etc. Some of them are special, produced by certain companies, for example, Phoenix, Cushion, Sole Mio diamonds, etc.
The most time-consuming part of the cutting is the preliminary analysis of the rough stone. It needs to address a large number of issues, bears much responsibility, and therefore can last years in case of unique diamonds. The following issues are considered:
- The hardness of diamond and its ability to cleave strongly depend on the crystal orientation. Therefore, the crystallographic structure of the diamond to be cut is analyzed using X-ray diffraction to choose the optimal cutting directions.
- Most diamonds contain visible non-diamond inclusions and crystal flaws. The cutter has to decide which flaws are to be removed by the cutting and which could be kept.
- Splitting a diamond with a hammer is difficult, a well-calculated, angled blow can cut the diamond, piece-by-piece, but it can also ruin the diamond itself. Alternatively, it can be cut with a diamond saw, which is a more reliable method.
After initial cutting, the diamond is shaped in numerous stages of polishing. Unlike cutting, which is a responsible but quick operation, polishing removes material by gradual erosion and is extremely time-consuming. The associated technique is well developed; it is considered as a routine and can be performed by technicians. After polishing, the diamond is reexamined for possible flaws, either remaining or induced by the process. Those flaws are concealed through various diamond enhancement techniques, such as repolishing, crack filling, or clever arrangement of the stone in the jewelry. Remaining non-diamond inclusions are removed through laser drilling and filling of the voids produced.
Marketing

Marketing has significantly affected the image of diamond as a valuable commodity.
N. W. Ayer & Son, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and the firm created new markets in countries where no diamond tradition had existed before. N. W. Ayer's marketing included product placement, advertising focused on the diamond product itself rather than the De Beers brand, and associations with celebrities and royalty. Without advertising the De Beers brand, De Beers was advertising its competitors' diamond products as well, but this was not a concern as De Beers dominated the diamond market throughout the 20th century. De Beers' market share dipped temporarily to second place in the global market below Alrosa in the aftermath of the global economic crisis of 2008, down to less than 29% in terms of carats mined, rather than sold. The campaign lasted for decades but was effectively discontinued by early 2011. De Beers still advertises diamonds, but the advertising now mostly promotes its own brands, or licensed product lines, rather than completely "generic" diamond products. The campaign was perhaps best captured by the slogan "a diamond is forever". This slogan is now being used by De Beers Diamond Jewelers, a jewelry firm which is a 50/50% joint venture between the De Beers mining company and LVMH, the luxury goods conglomerate.
Brown-colored diamonds constituted a significant part of the diamond production, and were predominantly used for industrial purposes. They were seen as worthless for jewelry (not even being assessed on the diamond color scale). After the development of Argyle diamond mine in Australia in 1986, and marketing, brown diamonds have become acceptable gems. The change was mostly due to the numbers: the Argyle mine, with its 35,000,000 carats (7,000 kg) of diamonds per year, makes about one-third of global production of natural diamonds; 80% of Argyle diamonds are brown.
Industrial-grade diamonds



Industrial diamonds are valued mostly for their hardness and thermal conductivity, making many of the gemological characteristics of diamonds, such as the 4 Cs, irrelevant for most applications. Eighty percent of mined diamonds (equal to about 135,000,000 carats (27,000 kg) annually) are unsuitable for use as gemstones and are used industrially. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; in 2014, 4,500,000,000 carats (900,000 kg) of synthetic diamonds were produced, 90% of which were produced in China. Approximately 90% of diamond grinding grit is currently of synthetic origin.
The boundary between gem-quality diamonds and industrial diamonds is poorly defined and partly depends on market conditions (for example, if demand for polished diamonds is high, some lower-grade stones will be polished into low-quality or small gemstones rather than being sold for industrial use). Within the category of industrial diamonds, there is a sub-category comprising the lowest-quality, mostly opaque stones, which are known as bort.
Industrial use of diamonds has historically been associated with their hardness, which makes diamond the ideal material for cutting and grinding tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial applications of this property include diamond-tipped drill bits and saws, and the use of diamond powder as an abrasive. Less expensive industrial-grade diamonds (bort) with more flaws and poorer color than gems, are used for such purposes. Diamond is not suitable for machining ferrous alloys at high speeds, as carbon is soluble in iron at the high temperatures created by high-speed machining, leading to greatly increased wear on diamond tools compared to alternatives.
Specialized applications include use in laboratories as containment for high-pressure experiments (see diamond anvil cell), high-performance bearings, and limited use in specialized windows. With the continuing advances being made in the production of synthetic diamonds, future applications are becoming feasible. The high thermal conductivity of diamond makes it suitable as a heat sink for integrated circuits in electronics.
Mining
See also: List of diamond mines and Exploration diamond drillingApproximately 130,000,000 carats (26,000 kg) of diamonds are mined annually, with a total value of nearly US$9 billion, and about 100,000 kg (220,000 lb) are synthesized annually.
Roughly 49% of diamonds originate from Central and Southern Africa, although significant sources of the mineral have been discovered in Canada, India, Russia, Brazil, and Australia. They are mined from kimberlite and lamproite volcanic pipes, which can bring diamond crystals, originating from deep within the Earth where high pressures and temperatures enable them to form, to the surface. The mining and distribution of natural diamonds are subjects of frequent controversy such as concerns over the sale of blood diamonds or conflict diamonds by African paramilitary groups. The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world.
Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care is required not to destroy larger diamonds, and then sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of X-ray fluorescence, after which the final sorting steps are done by hand. Before the use of X-rays became commonplace, the separation was done with grease belts; diamonds have a stronger tendency to stick to grease than the other minerals in the ore.


Historically, diamonds were found only in alluvial deposits in Guntur and Krishna district of the Krishna River delta in Southern India. India led the world in diamond production from the time of their discovery in approximately the 9th century BC to the mid-18th century AD, but the commercial potential of these sources had been exhausted by the late 18th century and at that time India was eclipsed by Brazil where the first non-Indian diamonds were found in 1725. Currently, one of the most prominent Indian mines is located at Panna.
Diamond extraction from primary deposits (kimberlites and lamproites) started in the 1870s after the discovery of the Diamond Fields in South Africa. Production has increased over time and now an accumulated total of 4,500,000,000 carats (900,000 kg) have been mined since that date. Twenty percent of that amount has been mined in the last five years, and during the last 10 years, nine new mines have started production; four more are waiting to be opened soon. Most of these mines are located in Canada, Zimbabwe, Angola, and one in Russia.
In the U.S., diamonds have been found in Arkansas, Colorado, New Mexico, Wyoming, and Montana. In 2004, the discovery of a microscopic diamond in the U.S. led to the January 2008 bulk-sampling of kimberlite pipes in a remote part of Montana. The Crater of Diamonds State Park in Arkansas is open to the public, and is the only mine in the world where members of the public can dig for diamonds.
Today, most commercially viable diamond deposits are in Russia (mostly in Sakha Republic, for example Mir pipe and Udachnaya pipe), Botswana, Australia (Northern and Western Australia) and the Democratic Republic of the Congo. In 2005, Russia produced almost one-fifth of the global diamond output, according to the British Geological Survey. Australia boasts the richest diamantiferous pipe, with production from the Argyle diamond mine reaching peak levels of 42 metric tons per year in the 1990s. There are also commercial deposits being actively mined in the Northwest Territories of Canada and Brazil. Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes.
Political issues
Main articles: Kimberley Process, Blood diamond, and Child labour in the diamond industryIn some of the more politically unstable central African and west African countries, revolutionary groups have taken control of diamond mines, using proceeds from diamond sales to finance their operations. Diamonds sold through this process are known as conflict diamonds or blood diamonds.
In response to public concerns that their diamond purchases were contributing to war and human rights abuses in central and western Africa, the United Nations, the diamond industry and diamond-trading nations introduced the Kimberley Process in 2002. The Kimberley Process aims to ensure that conflict diamonds do not become intermixed with the diamonds not controlled by such rebel groups. This is done by requiring diamond-producing countries to provide proof that the money they make from selling the diamonds is not used to fund criminal or revolutionary activities. Although the Kimberley Process has been moderately successful in limiting the number of conflict diamonds entering the market, some still find their way in. According to the International Diamond Manufacturers Association, conflict diamonds constitute 2–3% of all diamonds traded. Two major flaws still hinder the effectiveness of the Kimberley Process: (1) the relative ease of smuggling diamonds across African borders, and (2) the violent nature of diamond mining in nations that are not in a technical state of war and whose diamonds are therefore considered "clean".
The Canadian Government has set up a body known as the Canadian Diamond Code of Conduct to help authenticate Canadian diamonds. This is a stringent tracking system of diamonds and helps protect the "conflict free" label of Canadian diamonds.
Mineral resource exploitation in general causes irreversible environmental damage, which must be weighed against the socio-economic benefits to a country.
Synthetics, simulants, and enhancements
Synthetics
Main article: Synthetic diamondSynthetic diamonds are diamonds manufactured in a laboratory, as opposed to diamonds mined from the Earth. The gemological and industrial uses of diamond have created a large demand for rough stones. This demand has been satisfied in large part by synthetic diamonds, which have been manufactured by various processes for more than half a century. However, in recent years it has become possible to produce gem-quality synthetic diamonds of significant size. It is possible to make colorless synthetic gemstones that, on a molecular level, are identical to natural stones and so visually similar that only a gemologist with special equipment can tell the difference.
The majority of commercially available synthetic diamonds are yellow and are produced by so-called high-pressure high-temperature (HPHT) processes. The yellow color is caused by nitrogen impurities. Other colors may also be reproduced such as blue, green or pink, which are a result of the addition of boron or from irradiation after synthesis.
Another popular method of growing synthetic diamond is chemical vapor deposition (CVD). The growth occurs under low pressure (below atmospheric pressure). It involves feeding a mixture of gases (typically 1 to 99 methane to hydrogen) into a chamber and splitting them into chemically active radicals in a plasma ignited by microwaves, hot filament, arc discharge, welding torch, or laser. This method is mostly used for coatings, but can also produce single crystals several millimeters in size (see picture).
As of 2010, nearly all 5,000 million carats (1,000 tonnes) of synthetic diamonds produced per year are for industrial use. Around 50% of the 133 million carats of natural diamonds mined per year end up in industrial use. Mining companies' expenses average 40 to 60 US dollars per carat for natural colorless diamonds, while synthetic manufacturers' expenses average $2,500 per carat for synthetic, gem-quality colorless diamonds. However, a purchaser is more likely to encounter a synthetic when looking for a fancy-colored diamond because only 0.01% of natural diamonds are fancy-colored, while most synthetic diamonds are colored in some way.
-
Synthetic diamonds of various colors grown by the high-pressure high-temperature technique
-
 Colorless gem cut from diamond grown by chemical vapor deposition
Colorless gem cut from diamond grown by chemical vapor deposition
Simulants
Main article: Diamond simulant
A diamond simulant is a non-diamond material that is used to simulate the appearance of a diamond, and may be referred to as diamante. Cubic zirconia is the most common. The gemstone moissanite (silicon carbide) can be treated as a diamond simulant, though more costly to produce than cubic zirconia. Both are produced synthetically.
Enhancements
Main article: Diamond enhancementDiamond enhancements are specific treatments performed on natural or synthetic diamonds (usually those already cut and polished into a gem), which are designed to better the gemological characteristics of the stone in one or more ways. These include laser drilling to remove inclusions, application of sealants to fill cracks, treatments to improve a white diamond's color grade, and treatments to give fancy color to a white diamond.
Coatings are increasingly used to give a diamond simulant such as cubic zirconia a more "diamond-like" appearance. One such substance is diamond-like carbon—an amorphous carbonaceous material that has some physical properties similar to those of the diamond. Advertising suggests that such a coating would transfer some of these diamond-like properties to the coated stone, hence enhancing the diamond simulant. Techniques such as Raman spectroscopy should easily identify such a treatment.
Identification

Early diamond identification tests included a scratch test relying on the superior hardness of diamond. This test is destructive, as a diamond can scratch another diamond, and is rarely used nowadays. Instead, diamond identification relies on its superior thermal conductivity. Electronic thermal probes are widely used in the gemological centers to separate diamonds from their imitations. These probes consist of a pair of battery-powered thermistors mounted in a fine copper tip. One thermistor functions as a heating device while the other measures the temperature of the copper tip: if the stone being tested is a diamond, it will conduct the tip's thermal energy rapidly enough to produce a measurable temperature drop. This test takes about two to three seconds.
Whereas the thermal probe can separate diamonds from most of their simulants, distinguishing between various types of diamond, for example synthetic or natural, irradiated or non-irradiated, etc., requires more advanced, optical techniques. Those techniques are also used for some diamonds simulants, such as silicon carbide, which pass the thermal conductivity test. Optical techniques can distinguish between natural diamonds and synthetic diamonds. They can also identify the vast majority of treated natural diamonds. "Perfect" crystals (at the atomic lattice level) have never been found, so both natural and synthetic diamonds always possess characteristic imperfections, arising from the circumstances of their crystal growth, that allow them to be distinguished from each other.
Laboratories use techniques such as spectroscopy, microscopy, and luminescence under shortwave ultraviolet light to determine a diamond's origin. They also use specially made instruments to aid them in the identification process. Two screening instruments are the DiamondSure and the DiamondView, both produced by the DTC and marketed by the GIA.
Several methods for identifying synthetic diamonds can be performed, depending on the method of production and the color of the diamond. CVD diamonds can usually be identified by an orange fluorescence. D–J colored diamonds can be screened through the Swiss Gemmological Institute's Diamond Spotter. Stones in the D–Z color range can be examined through the DiamondSure UV/visible spectrometer, a tool developed by De Beers. Similarly, natural diamonds usually have minor imperfections and flaws, such as inclusions of foreign material, that are not seen in synthetic diamonds.
Screening devices based on diamond type detection can be used to make a distinction between diamonds that are certainly natural and diamonds that are potentially synthetic. Those potentially synthetic diamonds require more investigation in a specialized lab. Examples of commercial screening devices are D-Screen (WTOCD / HRD Antwerp), Alpha Diamond Analyzer (Bruker / HRD Antwerp), and D-Secure (DRC Techno).
Etymology, earliest use and composition discovery
The name diamond is derived from Ancient Greek: ἀδάμας (adámas), 'proper, unalterable, unbreakable, untamed', from ἀ- (a-), 'not' + Ancient Greek: δαμάω (damáō), 'to overpower, tame'. Diamonds are thought to have been first recognized and mined in India, where significant alluvial deposits of the stone could be found many centuries ago along the rivers Penner, Krishna, and Godavari. Diamonds have been known in India for at least 3,000 years but most likely 6,000 years.
Diamonds have been treasured as gemstones since their use as religious icons in ancient India. Their usage in engraving tools also dates to early human history. The popularity of diamonds has risen since the 19th century because of increased supply, improved cutting and polishing techniques, growth in the world economy, and innovative and successful advertising campaigns.
In 1772, the French scientist Antoine Lavoisier used a lens to concentrate the rays of the sun on a diamond in an atmosphere of oxygen, and showed that the only product of the combustion was carbon dioxide, proving that diamond is composed of carbon. Later, in 1797, the English chemist Smithson Tennant repeated and expanded that experiment. By demonstrating that burning diamond and graphite releases the same amount of gas, he established the chemical equivalence of these substances.
See also
Citations
- Warr LN (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. ISSN 0026-461X. S2CID 235729616.
- ^ "Diamond". Mindat. Archived from the original on May 6, 2009. Retrieved July 7, 2009.
- "Diamond". WebMineral. Archived from the original on January 7, 2019. Retrieved July 7, 2009.
- Delhaes P (2000). "Polymorphism of carbon". In Delhaes P (ed.). Graphite and precursors. Gordon & Breach. pp. 1–24. ISBN 978-90-5699-228-6.
- Pierson HO (2012). Handbook of carbon, graphite, diamond, and fullerenes: properties, processing, and applications. Noyes Publications. pp. 40–41. ISBN 978-0-8155-1739-9.
- Angus JC (1997). "Structure and thermochemistry of diamond". In Paoletti A, Tucciarone A (eds.). The physics of diamond. IOS Press. pp. 9–30. ISBN 978-1-61499-220-2.
- ^ Rock PA (1983). Chemical Thermodynamics. University Science Books. pp. 257–260. ISBN 978-1-891389-32-0.
- Gray T (October 8, 2009). "Gone in a Flash". Popular Science. Archived from the original on March 7, 2020. Retrieved October 31, 2018.
- Chen Y, Zhang L (2013). Polishing of diamond materials: mechanisms, modeling and implementation. Springer Science & Business Media. pp. 1–2. ISBN 978-1-84996-408-1.
- ^ Bundy P, Bassett WA, Weathers MS, Hemley RJ, Mao HK, Goncharov AF (1996). "The pressure-temperature phase and transformation diagram for carbon; updated through 1994". Carbon. 34 (2): 141–153. Bibcode:1996Carbo..34..141B. doi:10.1016/0008-6223(96)00170-4.
- Wang CX, Yang GW (2012). "Thermodynamic and kinetic approaches of diamond and related nanomaterials formed by laser ablation in liquid". In Yang G (ed.). Laser ablation in liquids: principles and applications in the preparation of nanomaterials. Pan Stanford. pp. 164–165. ISBN 978-981-4241-52-6.
- Wang X, Scandolo S, Car R (October 2005). "Carbon phase diagram from ab initio molecular dynamics". Physical Review Letters. 95 (18): 185701. Bibcode:2005PhRvL..95r5701W. doi:10.1103/PhysRevLett.95.185701. PMID 16383918.
- Correa AA, Bonev SA, Galli G (January 2006). "Carbon under extreme conditions: phase boundaries and electronic properties from first-principles theory". Proceedings of the National Academy of Sciences of the United States of America. 103 (5): 1204–1208. Bibcode:2006PNAS..103.1204C. doi:10.1073/pnas.0510489103. PMC 1345714. PMID 16432191.
- Bland E (January 15, 2010). "Diamond oceans possible on Uranus, Neptune". Discovery News. Archived from the original on March 11, 2012. Retrieved January 16, 2010.
- Silvera I (2010). "Diamond: Molten under pressure". Nature Physics. 6 (1): 9–10. Bibcode:2010NatPh...6....9S. doi:10.1038/nphys1491. S2CID 120836330. Archived from the original on July 30, 2022. Retrieved November 9, 2020.
- Rajendran V (2004). Materials science. Tata McGraw-Hill Pub. p. 2.16. ISBN 978-0-07-058369-6.
- ^ Ashcroft NW, Mermin ND (1976). Solid state physics. Holt, Rinehart and Winston. p. 76. ISBN 978-0-03-083993-1.
- Bandosz TJ, Biggs MJ, Gubbins KE, Hattori Y, Iiyama T, Kaneko T, Pikunic J, Thomson K (2003). "Molecular models of porous carbons". In Radovic LR (ed.). Chemistry and physics of carbon. Vol. 28. Marcel Dekker. pp. 46–47. ISBN 978-0-8247-0987-7.
- Webster R, Read PG (2000). Gems: Their sources, descriptions and identification (5th ed.). Great Britain: Butterworth-Heinemann. p. 17. ISBN 978-0-7506-1674-4.
- ^ Cartigny P, Palot M, Thomassot E, Harris JW (May 30, 2014). "Diamond Formation: A Stable Isotope Perspective". Annual Review of Earth and Planetary Sciences. 42 (1): 699–732. Bibcode:2014AREPS..42..699C. doi:10.1146/annurev-earth-042711-105259.
- Fukura S, Nakagawa T, Kagi H (November 2005). "High spatial resolution photoluminescence and Raman spectroscopic measurements of a natural polycrystalline diamond, carbonado". Diamond and Related Materials. 14 (11–12): 1950–1954. Bibcode:2005DRM....14.1950F. doi:10.1016/j.diamond.2005.08.046.
- Mohammad G, Siddiquei MM, Abu El-Asrar AM (2006). "Poly (ADP-ribose) polymerase mediates diabetes-induced retinal neuropathy". Mediators of Inflammation. 2013 (2): 510451. arXiv:physics/0608014. Bibcode:2006ApJ...653L.153G. doi:10.1086/510451. PMC 3857786. PMID 24347828. S2CID 59405368.
- "Diamonds from Outer Space: Geologists Discover Origin of Earth's Mysterious Black Diamonds". National Science Foundation. January 8, 2007. Archived from the original on December 9, 2007. Retrieved October 28, 2007.
- "Diamonds Are Indestructible, Right?". Dominion Jewelers. December 16, 2015. Archived from the original on September 26, 2020. Retrieved October 31, 2020.
- Seal M (November 25, 1958). "The abrasion of diamond". Proceedings of the Royal Society A. 248 (1254): 379–393. Bibcode:1958RSPSA.248..379S. doi:10.1098/rspa.1958.0250.
- Weiler HD (April 13, 2021) . "The wear and care of records and styli". Archived from the original on March 26, 2023. Retrieved August 25, 2024 – via Shure Applications Engineering.
- Neves AJ, Nazaré MH (2001). Properties, Growth and Applications of Diamond. Institution of Engineering and Technology. pp. 142–147. ISBN 978-0-85296-785-0. Archived from the original on February 19, 2023. Retrieved November 9, 2020.
- Boser U (2008). "Diamonds on Demand". Smithsonian. Vol. 39, no. 3. pp. 52–59. Archived from the original on March 2, 2012. Retrieved June 13, 2009.
- ^ Read PG (2005). Gemmology. Butterworth-Heinemann. pp. 165–166. ISBN 978-0-7506-6449-3. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- ^ Hazen RM (1999). The diamond makers. Cambridge University Press. pp. 7–10. ISBN 978-0-521-65474-6.
- O'Donoghue M (1997). Synthetic, Imitation and Treated Gemstones. Gulf Professional. pp. 34–37. ISBN 978-0-7506-3173-0.
- Lee J, Novikov NV (2005). Innovative superhard materials and sustainable coatings for advanced manufacturing. Springer. p. 102. ISBN 978-0-8493-3512-9.
- Marinescu ID, Tönshoff HK, Inasaki I (2000). Handbook of ceramic grinding and polishing. William Andrew. p. 21. ISBN 978-0-8155-1424-4.
- ^ Harlow GE (1998). The nature of diamonds. Cambridge University Press. pp. 223, 230–249. ISBN 978-0-521-62935-5.
- Eremets MI, Trojan IA, Gwaze P, Huth J, Boehler R, Blank VD (October 3, 2005). "The strength of diamond". Applied Physics Letters. 87 (14): 141902. Bibcode:2005ApPhL..87n1902E. doi:10.1063/1.2061853.
- ^ Dubrovinsky L, Dubrovinskaia N, Prakapenka VB, Abakumov AM (October 23, 2012). "Implementation of micro-ball nanodiamond anvils for high-pressure studies above 6 Mbar". Nature Communications. 3 (1): 1163. Bibcode:2012NatCo...3.1163D. doi:10.1038/ncomms2160. PMC 3493652. PMID 23093199.
- ^ Wogan T (November 2, 2012). "Improved diamond anvil cell allows higher pressures than ever before". Physics World. Nature Communications. Archived from the original on January 2, 2018. Retrieved July 1, 2022.
- Dang C, Chou JP, Dai B, Chou CT, Yang Y, Fan R, et al. (January 2021). "Achieving large uniform tensile elasticity in microfabricated diamond". Science. 371 (6524): 76–78. Bibcode:2021Sci...371...76D. doi:10.1126/science.abc4174. PMID 33384375. S2CID 229935085.
- Banerjee A, Bernoulli D, Zhang H, Yuen MF, Liu J, Dong J, et al. (April 2018). "Ultralarge elastic deformation of nanoscale diamond". Science. 360 (6386): 300–302. Bibcode:2018Sci...360..300B. doi:10.1126/science.aar4165. PMID 29674589. S2CID 5047604.
- LLorca J (April 2018). "On the quest for the strongest materials". Science. 360 (6386): 264–265. arXiv:2105.05099. Bibcode:2018Sci...360..264L. doi:10.1126/science.aat5211. PMID 29674578. S2CID 4986592.
- Collins AT (1993). "The Optical and Electronic Properties of Semiconducting Diamond". Philosophical Transactions of the Royal Society A. 342 (1664): 233–244. Bibcode:1993RSPTA.342..233C. doi:10.1098/rsta.1993.0017. S2CID 202574625.
- Landstrass MI, Ravi KV (1989). "Resistivity of chemical vapor deposited diamond films". Applied Physics Letters. 55 (10): 975–977. Bibcode:1989ApPhL..55..975L. doi:10.1063/1.101694.
- Zhang W, Ristein J, Ley L (October 2008). "Hydrogen-terminated diamond electrodes. II. Redox activity". Physical Review E. 78 (4 Pt 1): 041603. Bibcode:2008PhRvE..78d1603Z. doi:10.1103/PhysRevE.78.041603. PMID 18999435.
- Shi Z, Dao M, Tsymbalov E, Shapeev A, Li J, Suresh S (October 2020). "Metallization of diamond". Proceedings of the National Academy of Sciences of the United States of America. 117 (40): 24634–24639. Bibcode:2020PNAS..11724634S. doi:10.1073/pnas.2013565117. PMC 7547227. PMID 33020306.
- Irving M (April 28, 2022). "Two-inch diamond wafers could store a billion Blu-Ray's worth of data". New Atlas. Retrieved April 29, 2022.
- Wissner-Gross AD, Kaxiras E (August 2007). "Diamond stabilization of ice multilayers at human body temperature" (PDF). Physical Review E. 76 (2 Pt 1): 020501. Bibcode:2007PhRvE..76b0501W. doi:10.1103/physreve.76.020501. PMID 17929997. S2CID 44344503. Archived (PDF) from the original on July 24, 2011.
- Fujimoto A, Yamada Y, Koinuma M, Sato S (June 2016). "Origins of sp(3)C peaks in C1s X-ray Photoelectron Spectra of Carbon Materials". Analytical Chemistry. 88 (12): 6110–6114. doi:10.1021/acs.analchem.6b01327. PMID 27264720.
- Bauer M (2012). Precious Stones. Vol. 1. Dover Publications. pp. 115–117. ISBN 978-0-486-15125-0.
- "Diamond Care and Cleaning Guide". Gemological Institute of America. Archived from the original on August 1, 2019. Retrieved August 1, 2019.
- Jones C (August 27, 2016). "Diamonds are Flammable! How to Safeguard Your Jewelry". DMIA. Archived from the original on August 1, 2019. Retrieved August 1, 2019.
- Baird CS. "Can you light diamond on fire?". Science Questions with Surprising Answers. Archived from the original on August 1, 2019. Retrieved August 1, 2019.
- Lederle F, Koch J, Hübner EG (February 21, 2019). "Colored Sparks". European Journal of Inorganic Chemistry. 2019 (7): 928–937. doi:10.1002/ejic.201801300. S2CID 104449284.
- Collins AT, Kanda H, Isoya J, Ammerlaan CA, Van Wyk JA (1998). "Correlation between optical absorption and EPR in high-pressure diamond grown from a nickel solvent catalyst". Diamond and Related Materials. 7 (2–5): 333–338. Bibcode:1998DRM.....7..333C. doi:10.1016/S0925-9635(97)00270-7.
- Zaitsev AM (2000). "Vibronic spectra of impurity-related optical centers in diamond". Physical Review B. 61 (19): 12909–12922. Bibcode:2000PhRvB..6112909Z. doi:10.1103/PhysRevB.61.12909.
- Walker J (1979). "Optical absorption and luminescence in diamond" (PDF). Reports on Progress in Physics. 42 (10): 1605–1659. Bibcode:1979RPPh...42.1605W. CiteSeerX 10.1.1.467.443. doi:10.1088/0034-4885/42/10/001. S2CID 250857323. Archived (PDF) from the original on September 6, 2015.
- Hounsome LS, Jones R, Shaw MJ, Briddon PR, Öberg S, Briddon P, Öberg S (2006). "Origin of brown coloration in diamond". Physical Review B. 73 (12): 125203. Bibcode:2006PhRvB..73l5203H. doi:10.1103/PhysRevB.73.125203.
- Wise RW (2001). Secrets Of The Gem Trade, The Connoisseur's Guide To Precious Gemstones. Brunswick House Press. pp. 223–224. ISBN 978-0-9728223-8-1.
- Khan U (December 10, 2008). "Blue-grey diamond belonging to King of Spain has sold for record 16.3 GBP". The Daily Telegraph. London. Archived from the original on February 7, 2009. Retrieved March 31, 2010.
- Nebehay S (May 12, 2009). "Rare blue diamond sells for record $9.5 million". Reuters. Archived from the original on May 16, 2009. Retrieved May 13, 2009.
- Pomfret J (December 1, 2009). "Vivid pink diamond sells for record $10.8 million". Reuters. Archived from the original on December 2, 2020. Retrieved July 1, 2017.
- Cowing MD (2014). "Objective ciamond clarity grading" (PDF). Journal of Gemmology. 34 (4): 316–332. doi:10.15506/JoG.2014.34.4.316. Archived (PDF) from the original on April 18, 2021. Retrieved September 19, 2021.
- Wang W, Cai L (September 2019). "Inclusion extraction from diamond clarity images based on the analysis of diamond optical properties". Optics Express. 27 (19): 27242–27255. Bibcode:2019OExpr..2727242W. doi:10.1364/OE.27.027242. PMID 31674589. S2CID 203141270.
- "Fact Checking Diamond Fluorescence: 11 Myths Dispelled". GIA 4Cs. March 27, 2018. Archived from the original on March 24, 2022. Retrieved June 6, 2022.
- Wei L, Kuo PK, Thomas RL, Anthony TR, Banholzer WF (June 1993). "Thermal conductivity of isotopically modified single crystal diamond". Physical Review Letters. 70 (24): 3764–3767. Bibcode:1993PhRvL..70.3764W. doi:10.1103/PhysRevLett.70.3764. PMID 10053956.
- ^ Erlich EI, Hausel WD (2002). Diamond deposits: origin, exploration, and history of discovery. Littleton, CO: Society for Mining, Metallurgy, and Exploration. ISBN 978-0-87335-213-0.
- ^ Shirey SB, Shigley JE (December 1, 2013). "Recent Advances in Understanding the Geology of Diamonds". Gems & Gemology. 49 (4): 188–222. doi:10.5741/GEMS.49.4.188.
- Carlson RW (2005). The Mantle and Core. Elsevier. p. 248. ISBN 978-0-08-044848-0. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- Deutsch A, Masaitis VL, Langenhorst F, Grieve RA (2000). "Popigai, Siberia—well preserved giant impact structure, national treasury, and world's geological heritage". Episodes. 23 (1): 3–12. doi:10.18814/epiiugs/2000/v23i1/002.
- King H (2012). "How do diamonds form? They don't form from coal!". Geology and Earth Science News and Information. geology.com. Archived from the original on October 30, 2013. Retrieved June 29, 2012.
- Pak-Harvey A (October 31, 2013). "10 common scientific misconceptions". The Christian Science Monitor. Archived from the original on January 6, 2017. Retrieved August 30, 2017.
- Pohl WL (2011). Economic Geology: Principles and Practice. John Wiley & Sons. ISBN 978-1-4443-9486-3.
- Allaby M (2013). "mobile belt". A dictionary of geology and earth sciences (4th ed.). Oxford: Oxford University Press. ISBN 978-0-19-174433-4.
- Kjarsgaard BA (2007). "Kimberlite pipe models: significance for exploration" (PDF). In Milkereit B (ed.). Proceedings of Exploration 07: Fifth Decennial International Conference on Mineral Exploration. Decennial Mineral Exploration Conferences, 2007. pp. 667–677. Archived (PDF) from the original on December 24, 2012. Retrieved March 1, 2018.
- ^ Deep Carbon Observatory (2019). Deep Carbon Observatory: A Decade of Discovery. Washington, DC. doi:10.17863/CAM.44064. Archived from the original on December 17, 2019. Retrieved December 13, 2019.
{{cite book}}: CS1 maint: location missing publisher (link) - Cartier K (April 2, 2018). "Diamond Impurities Reveal Water Deep Within the Mantle". Eos. 99. doi:10.1029/2018EO095949.
- Perkins S (March 8, 2018). "Pockets of water may lie deep below Earth's surface". Science. Archived from the original on March 8, 2018. Retrieved June 30, 2022.
- Lee CA, Jiang H, Dasgupta R, Torres M (2019). "A Framework for Understanding Whole-Earth Carbon Cycling". In Orcutt BN, Daniel I, Dasgupta R (eds.). Deep carbon: past to present. Cambridge University Press. pp. 313–357. doi:10.1017/9781108677950.011. ISBN 978-1-108-67795-0. S2CID 210787128.
- Wei-Haas M (October 10, 2019). "Bizarre 'nesting doll' diamond found inside another diamond". National Geographic. Archived from the original on November 27, 2021. Retrieved November 27, 2021.
- Fowler C (November 26, 2021). "Rare 'double diamond' discovery comes as race to restart mothballed Ellendale mine heats up". Australian Broadcasting Corporation. Archived from the original on November 26, 2021. Retrieved November 27, 2021.
- Tielens AG (July 12, 2013). "The molecular universe". Reviews of Modern Physics. 85 (3): 1021–1081. Bibcode:2013RvMP...85.1021T. doi:10.1103/RevModPhys.85.1021.
- Kerr RA (October 1999). "Neptune may crush methane into diamonds". Science. 286 (5437): 25. doi:10.1126/science.286.5437.25a. PMID 10532884. S2CID 42814647.
- Scandolo S, Jeanloz R (November–December 2003). "The Centers of Planets: In laboratories and computers, shocked and squeezed matter turns metallic, coughs up diamonds and reveals Earth's white-hot center". American Scientist. 91 (6): 516–525. Bibcode:2003AmSci..91..516S. doi:10.1511/2003.38.905. JSTOR 27858301. S2CID 120975663.
- Kaplan S (August 25, 2017). "It rains solid diamonds on Uranus and Neptune". The Washington Post. Archived from the original on August 27, 2017. Retrieved October 16, 2017.
- Max Planck Institute for Radio Astronomy (August 25, 2011). "A planet made of diamond". Astronomy magazine. Archived from the original on May 14, 2023. Retrieved September 25, 2017.
- Heaney PJ, de Vicenzi EP (2005). "Strange Diamonds: the Mysterious Origins of Carbonado and Framesite". Elements. 1 (2): 85–89. Bibcode:2005Eleme...1...85H. doi:10.2113/gselements.1.2.85.
- Shumilova T, Tkachev S, Isaenko S, Shevchuk S, Rappenglück M, Kazakov V (April 2016). "A "diamond-like star" in the lab. Diamond-like glass". Carbon. 100: 703–709. Bibcode:2016Carbo.100..703S. doi:10.1016/j.carbon.2016.01.068.
- Wei-Haas M. "Life and Rocks May Have Co-Evolved on Earth". Smithsonian. Archived from the original on September 2, 2017. Retrieved September 26, 2017.
- Hesse RW (2007). Jewelrymaking through history. Greenwood Publishing Group. p. 42. ISBN 978-0-313-33507-5. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- Adiga A (April 12, 2004). "Uncommon Brilliance". Time. Archived from the original on March 10, 2007. Retrieved November 3, 2008.
- "Jwaneng". Debswana. Archived from the original on March 17, 2012. Retrieved March 9, 2012.
- ^ Tichotsky J (2000). Russia's Diamond Colony: The Republic of Sakha. Routledge. p. 254. ISBN 978-90-5702-420-7. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- "Jews Surrender Gem Trade to Indians". Spiegel Online. May 15, 2006. Archived from the original on November 26, 2010. Retrieved November 29, 2010.
- "The history of the Antwerp Diamond Center". Antwerp World Diamond Center. August 16, 2012. Archived from the original on February 22, 2013. Retrieved June 30, 2015.
- "Commission Decision of 25 July 2001 declaring a concentration to be compatible with the common market and the EEA Agreement". Case No COMP/M.2333 – De Beers/LVMH. EUR-Lex. 2003. Archived from the original on May 12, 2011. Retrieved February 6, 2009.
- "Business: Changing facets; Diamonds". The Economist. Vol. 382, no. 8517. 2007. p. 68. Archived from the original on May 12, 2011. Retrieved December 22, 2010.
- "Certainty in the Diamond Industry? Watch Out For Tipping Points – IDEX's Memo". idexonline.com. Archived from the original on January 9, 2015. Retrieved September 24, 2014.
- "The Elusive Sparcle". The Gem & Jewellery Export Promotion Council. Archived from the original on June 16, 2009. Retrieved April 26, 2009.
- Even-Zohar C (November 6, 2008). "Crisis Mitigation at De Beers". DIB online. Archived from the original on May 12, 2011. Retrieved April 26, 2009.
- Even-Zohar C (November 3, 1999). "De Beers to Halve Diamond Stockpile". National Jeweler. Archived from the original on July 5, 2009. Retrieved April 26, 2009.
- "Judgment of the Court of First Instance of 11 July 2007 – Alrosa v Commission". EUR-Lex. 2007. Archived from the original on December 1, 2017. Retrieved April 26, 2009.
- "Mining operations". The De Beers Group. 2007. Archived from the original on June 13, 2008. Retrieved January 4, 2011.
- "Media releases – Media Centre – Alrosa". Alrosa. December 22, 2009. Archived from the original on August 20, 2013. Retrieved January 4, 2011.
- "Another record profit for BHP". ABC News. August 22, 2007. Archived from the original on May 12, 2011. Retrieved August 23, 2007.
- "Our Companies". Rio Tinto web site. Rio Tinto. Archived from the original on May 11, 2013. Retrieved March 5, 2009.
- "Introduction | IDC". internationaldiamondcouncil.org. Archived from the original on October 18, 2022. Retrieved October 18, 2022.
- ^ Broadman HG, Isik G (2007). Africa's silk road. World Bank Publications. pp. 297–299. ISBN 978-0-8213-6835-0. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- "Bourse listing". World Federation of Diamond Bourses. Archived from the original on October 25, 2016. Retrieved February 12, 2012.
- "North America Diamond Sales Show No Sign of Slowing". A&W diamonds. Archived from the original on January 6, 2009. Retrieved May 5, 2009.
- ^ Pierson HO (1993). Handbook of carbon, graphite, diamond, and fullerenes: properties, processing, and applications. William Andrew. p. 280. ISBN 978-0-8155-1339-1.
- ^ James DS (1998). Antique jewellery: its manufacture, materials and design. Osprey Publishing. pp. 82–102. ISBN 978-0-7478-0385-0.
- "The Classical and Special Shapes of Diamonds". kristallsmolensk.com. Archived from the original on July 14, 2015. Retrieved July 14, 2015.
- Prelas MA, Popovici G, Bigelow LK (1998). Handbook of industrial diamonds and diamond films. CRC Press. pp. 984–992. ISBN 978-0-8247-9994-6. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- "Gem Cutting". Popular Mechanics. 74 (5): 760–764. 1940. ISSN 0032-4558. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- Rapaport M. "Keep the Diamond Dream Alive". Rapaport Magazine. Diamonds.net. Archived from the original on September 13, 2012. Retrieved September 9, 2012.
- ^ JCK Staff (January 26, 2011). "10 Things Rocking the Industry". JCK. Jckonline.com. Archived from the original on January 7, 2013. Retrieved September 9, 2012.
- ^ Epstein EJ (1982). "Have You Ever Tried To Sell a Diamond?". The Atlantic. Archived from the original on May 17, 2008. Retrieved May 5, 2009.
- Bates R (January 14, 2011). "Interview with Forevermark CEO". JCK. Jckonline.com. Archived from the original on November 28, 2012. Retrieved September 9, 2012.
- Harlow GE (1998). The nature of diamonds. Cambridge University Press. p. 34. ISBN 978-0-521-62935-5.
- Kogel JE (2006). Industrial minerals & rocks. Society for Mining, Metallurgy, and Exploration (U.S.). p. 416. ISBN 978-0-87335-233-8.
- "The Australian Diamond Industry". Archived from the original on July 16, 2009. Retrieved August 4, 2009.
- Erlich E, Hausel DW (2002). Diamond deposits: origin, exploration, and history of discovery. SME. p. 158. ISBN 978-0-87335-213-0.
- "Diamond: The mineral Diamond information and pictures". minerals.net. Archived from the original on October 23, 2014. Retrieved September 24, 2014.
- ^ "Industrial Diamonds Statistics and Information". United States Geological Survey. Archived from the original on May 6, 2009. Retrieved May 5, 2009.
- ^ Spear KE, Dismukes JP (1994). Synthetic Diamond: Emerging CVD Science and Technology. Wiley–IEEE. p. 628. ISBN 978-0-471-53589-8.
- Holtzapffel C (1856). Turning And Mechanical Manipulation. Holtzapffel & Co. pp. 176–178. ISBN 978-1-879335-39-4.
- Coelho RT, Yamada S, Aspinwall DK, Wise ML (1995). "The application of polycrystalline diamond (PCD) tool materials when drilling and reaming aluminum-based alloys including MMC". International Journal of Machine Tools and Manufacture. 35 (5): 761–774. doi:10.1016/0890-6955(95)93044-7.
- Sakamoto M, Endriz JG, Scifres DR (1992). "120 W CW output power from monolithic AlGaAs (800 nm) laser diode array mounted on diamond heatsink". Electronics Letters. 28 (2): 197–199. Bibcode:1992ElL....28..197S. doi:10.1049/el:19920123.
- ^ Yarnell A (2004). "The Many Facets of Man-Made Diamonds". Chemical and Engineering News. 82 (5): 26–31. doi:10.1021/cen-v082n005.p026. Archived from the original on October 28, 2008. Retrieved October 3, 2006.
- ^ "Conflict Diamonds". United Nations. March 21, 2001. Archived from the original on March 9, 2010. Retrieved May 5, 2009.
- Catelle WR (1911). The Diamond. John Lane Co. p. 159.
- ^ Hershey W (1940). The Book of Diamonds. New York: Hearthside Press. pp. 22–28. ISBN 978-1-4179-7715-4. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- Ball V (1881). "1". Diamonds, Gold and Coal of India. London: Trübner & Co. p. 1. Ball was a geologist in British service.
- "Biggest diamond found in Panna". Mail Today. July 1, 2010. Archived from the original on July 7, 2011.
- Shillington K (2005). Encyclopedia of African history. CRC Press. p. 767. ISBN 978-1-57958-453-5. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- ^ Janse AJ (2007). "Global Rough Diamond Production Since 1870". Gems & Gemology. 43 (2): 98–119. doi:10.5741/GEMS.43.2.98.
- ^ Lorenz V (2007). "Argyle in Western Australia: The world's richest diamantiferous pipe; its past and future". Gemmologie, Zeitschrift der Deutschen Gemmologischen Gesellschaft. 56 (1–2): 35–40.
- ^ Cooke S (October 17, 2004). "Microscopic diamond found in Montana". The Montana Standard. Archived from the original on January 21, 2005. Retrieved May 5, 2009.
- Marshall S, Shore J (2004). "The Diamond Life". Guerrilla News Network. Archived from the original on January 26, 2007. Retrieved March 21, 2007.
- Shigley JE, Chapman J, Ellison RK (2001). "Discovery and Mining of the Argyle Diamond Deposit, Australia" (PDF). Gems & Gemology. 37 (1): 26–41. doi:10.5741/GEMS.37.1.26. Archived from the original (PDF) on September 30, 2009. Retrieved February 20, 2010.
- ^ Basedau M, Mehler A (2005). Resource politics in Sub-Saharan Africa. GIGA-Hamburg. pp. 305–313. ISBN 978-3-928049-91-7. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- World Federation of Diamond Bourses (WFDB) and International Diamond Manufacturers Association: Joint Resolution of 19 July 2000. World Diamond Council. July 19, 2000. ISBN 978-90-04-13656-4. Archived from the original on November 9, 2023. Retrieved November 5, 2006.
- "Voluntary Code of Conduct For Authenticating Canadian Diamond Claims" (PDF). Canadian Diamond Code Committee. 2006. Archived from the original (PDF) on February 29, 2012. Retrieved October 30, 2007.
- Kjarsgaard BA, Levinson AA (2002). "Diamonds in Canada". Gems and Gemology. 38 (3): 208–238. doi:10.5741/GEMS.38.3.208.
- A meta-analysis of the environmental impact specific to diamond mining is in Oluleye G. Environmental Impacts of Mined Diamonds (PDF) (Report). Imperial College London Consultants. Archived (PDF) from the original on December 3, 2021. Retrieved July 1, 2022.
- ^ "The Global Diamond Industry: Lifting the Veil of Mystery" (PDF). Bain & Company. Archived (PDF) from the original on January 31, 2012. Retrieved January 14, 2012.
- Shigley JE, Abbaschian R (2002). "Gemesis Laboratory Created Diamonds". Gems & Gemology. 38 (4): 301–309. doi:10.5741/GEMS.38.4.301.
- Shigley JE, Shen AH, Breeding CM, McClure SF, Shigley JE (2004). "Lab Grown Colored Diamonds from Chatham Created Gems". Gems & Gemology. 40 (2): 128–145. doi:10.5741/GEMS.40.2.128.
- Werner M, Locher R (1998). "Growth and application of undoped and doped diamond films". Reports on Progress in Physics. 61 (12): 1665–1710. Bibcode:1998RPPh...61.1665W. doi:10.1088/0034-4885/61/12/002. S2CID 250878100.
- Pisani B (August 27, 2012). "The Business of Diamonds, From Mining to Retail". CNBC. Archived from the original on July 7, 2017. Retrieved September 9, 2017.
- Kogel JE (2006). Industrial Minerals & Rocks. SME. pp. 426–430. ISBN 978-0-87335-233-8. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- O'Donoghue M, Joyner L (2003). Identification of gemstones. Great Britain: Butterworth-Heinemann. pp. 12–19. ISBN 978-0-7506-5512-5.
- Barnard AS (2000). The diamond formula. Butterworth-Heinemann. p. 115. ISBN 978-0-7506-4244-6. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- Shigley JE (2007). "Observations on new coated gemstones". Gemmologie: Zeitschrift der Deutschen Gemmologischen Gesellschaft. 56 (1–2): 53–56.
- US 4488821, Wenckus JF, "Method and means of rapidly distinguishing a simulated diamond from natural diamond", published December 18, 1984, assigned to Ceres Electronics Corporation ; U.S. patent 4,488,821
- ^ Edwards HG, Chalmers GM (2005). Raman spectroscopy in archaeology and art history. Royal Society of Chemistry. pp. 387–394. ISBN 978-0-85404-522-8. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- ^ Welbourn C (2006). "Identification of Synthetic Diamonds: Present Status and Future Developments, Proceedings of the 4th International Gemological Symposium". Gems and Gemology. 42 (3): 34–35.
- Donahue PJ (April 19, 2004). "DTC Appoints GIA Distributor of DiamondSure and DiamondView". Professional Jeweler Magazine. Archived from the original on March 6, 2012. Retrieved March 2, 2009.
- "SSEF diamond spotter and SSEF illuminator". SSEF Swiss Gemmological Institute. Archived from the original on June 27, 2009. Retrieved May 5, 2009.
- Liddell HG, Scott R. "Adamas". A Greek-English Lexicon. Perseus Project. Archived from the original on November 9, 2023. Retrieved February 20, 2021.
- Pliny the Elder (2004). Natural History: A Selection. Penguin Books. p. 371. ISBN 978-0-14-044413-1.
- "Chinese made first use of diamond". BBC News. May 17, 2005. Archived from the original on March 20, 2007. Retrieved March 21, 2007.
- See:
- Lavoisier A (October 15, 2007) , "Premier mémoire sur la destruction du diamant par le feu" [First memoir on the destruction of diamond by fire], Histoire de l'Académie royale des sciences, avec les Mémoires de Mathématique & de Physique, tirés des registres de cette Académie [History of the Royal Academy of Sciences, with the Memoirs of Mathematics & Physics, drawn from the records of this academy]] (in French), Gallica: Académie des sciences, pp. 564–591, ISSN 1967-4783, ark:/12148/bpt6k35711, archived from the original on May 9, 2022, retrieved July 1, 2022
- Lavoisier A (October 15, 2007) , "Second mémoire sur la destruction du diamant par le feu" [Second memoir on the destruction of diamond by fire], Histoire de l'Académie royale des sciences, avec les Mémoires de Mathématique & de Physique, tirés des registres de cette Académie (in French), Gallica: Académie des sciences, pp. 591–616, ISSN 1967-4783, ark:/12148/bpt6k35711, archived from the original on July 10, 2022, retrieved July 1, 2022
- Smithson T (1797) . "On the nature of the diamond". Philosophical Transactions of the Royal Society of London. 87: 123–127. doi:10.1098/rstl.1797.0005. S2CID 186213726. Archived from the original on February 19, 2023. Retrieved July 1, 2022.
General and cited references
- Even-Zohar C (2007). From Mine to Mistress: Corporate Strategies and Government Policies in the International Diamond Industry (2nd ed.). Mining Journal Press. Archived from the original on March 10, 2020. Retrieved April 18, 2007.
- Davies G (1994). Properties and growth of diamond. INSPEC. ISBN 978-0-85296-875-8.
- O'Donoghue M (2006). Gems. Elsevier. ISBN 978-0-7506-5856-0.
- O'Donoghue M, Joyner L (2003). Identification of gemstones. Great Britain: Butterworth-Heinemann. ISBN 978-0-7506-5512-5.
- Feldman A, Robins LH (1991). Applications of Diamond Films and Related Materials. Elsevier. ISBN 978-1-48329124-6.
- Field JE (1979). The Properties of Diamond. London: Academic Press. ISBN 978-0-12-255350-9.
- Field JE (1992). The Properties of Natural and Synthetic Diamond. London: Academic Press. ISBN 978-0-12-255352-3.
- Hershey W (1940). The Book of Diamonds. Hearthside Press New York. ISBN 978-1-4179-7715-4. Archived from the original on August 20, 2014. Retrieved August 18, 2011.
- Koizumi S, Nebel CE, Nesladek M (2008). Physics and Applications of CVD Diamond. Wiley VCH. ISBN 978-3-527-40801-6. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
- Pan LS, Kani DR (1995). Diamond: Electronic Properties and Applications. Kluwer Academic Publishers. ISBN 978-0-7923-9524-9.
- Pagel-Theisen V (2001). Diamond Grading ABC: the Manual. Antwerp: Rubin & Son. ISBN 978-3-9800434-6-5.
- Radovic RL, Walker RM, Thrower PA (1965). Chemistry and physics of carbon: a series of advances. New York: Marcel Dekker. ISBN 978-0-8247-0987-7.
- Tolkowsky M (1919). Diamond Design: A Study of the Reflection and Refraction of Light in a Diamond. London: E. & F.N. Spon. Archived from the original on March 12, 2023. Retrieved March 13, 2007.
- Wise RW (2016). Secrets of the Gem Trade: The Connoisseur's Guide to Precious Gemstones (Second ed.). Brunswick House Press. ISBN 978-0-9728223-2-9. Archived from the original on August 19, 2019. Retrieved May 11, 2021.
- Zaitsev AM (2001). Optical Properties of Diamond: A Data Handbook. Springer. ISBN 978-3-540-66582-3. Archived from the original on November 9, 2023. Retrieved November 9, 2020.
Further reading
- Epstein EJ (February 1982). "Have You Ever Tried to Sell a Diamond?". The Atlantic Monthly. Archived from the original on March 15, 2006. Retrieved January 2, 2023.
- Tyson P (November 2000). "Diamonds in the Sky". The Diamond Deception. Nova. PBS. Retrieved January 2, 2023.
External links
- Properties of diamond: Ioffe database
- ""A Contribution to the Understanding of Blue Fluorescence on the Appearance of Diamonds"". Gemological Institute of America (GIA). 2007. Archived from the original on September 8, 2017.
| Allotropes of carbon | |
|---|---|
| sp forms | |
| sp forms | |
| sp forms | |
| mixed sp/sp forms | |
| other forms | |
| hypothetical forms | |
| related | |
| Jewellery | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forms | |||||||||||||
| Making |
| ||||||||||||
| Materials |
| ||||||||||||
| Terms |
| ||||||||||||
| |||||||||||||
| Gemstones | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gemmological classifications by E. Ya. Kievlenko (1980), updated | |||||||||
| Jewelry stones |
| ||||||||
| Jewelry-Industrial stones |
| ||||||||
| Industrial stones |
| ||||||||
| Related | |||||||||
| Mohs scale of mineral hardness | |
|---|---|