| Revision as of 19:15, 3 May 2022 editJF726 (talk | contribs)56 edits →Global warming potential← Previous edit | Latest revision as of 02:30, 9 December 2024 edit undoThoughtWarden (talk | contribs)Extended confirmed users900 edits Open access status updates in citations with OAbot #oabotTag: OAbot [2.1] | ||
| (515 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Gas in an atmosphere |
{{Short description|Gas in an atmosphere with certain absorption characteristics}} | ||
| {{Pp-move}} | |||
| {{About|properties of greenhouse gases|the sources and quantities of greenhouse gases emitted|Greenhouse gas emissions}} | |||
| {{Pp- |
{{Pp-pc}} | ||
| {{Pp-move-indef}} | |||
| {{Use dmy dates|date=June 2019}} | {{Use dmy dates|date=June 2019}} | ||
| {{about|the physical properties of greenhouse gases|how human activities are adding to greenhouse gases|Greenhouse gas emissions}} | |||
| ] of solar radiation on the Earth's surface caused by emission of greenhouse gases.]] | |||
| ] (warming influence) of different contributors to climate change thru 2019, as reported in the ].]] | |||
| ] that results when sunlight heats the Earth's surface. Three important greenhouse gases are shown symbolically in this image: ], ], and ].]] | |||
| A '''greenhouse gas''' ('''GHG''' or '''GhG''') is a ] that ] and ] ] within the ] range, causing the ].<ref name="IPCC AR4-SYR">{{cite web|url=http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr_appendix.pdf|title=IPCC AR4 SYR Appendix Glossary|access-date=14 December 2008|url-status=dead|archive-url=https://web.archive.org/web/20181117121314/http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr_appendix.pdf|archive-date=2018-11-17}}</ref> The primary greenhouse gases in ] are ] ({{H2O}}), ] ({{CO2}}), ] ({{CH4}}), ] ({{N2O}}), and ] ({{O3}}). Without greenhouse gases, the average temperature of ] would be about {{convert|-18|°C|°F}},<ref>{{Cite web|url=http://www.giss.nasa.gov/research/briefs/ma_01/|archive-url=https://web.archive.org/web/20050112211604/http://www.giss.nasa.gov/research/briefs/ma_01/|url-status=dead|archive-date=2005-01-12|title=NASA GISS: Science Briefs: Greenhouse Gases: Refining the Role of Carbon Dioxide|website=www.giss.nasa.gov|access-date=2016-04-26}}</ref> rather than the present average of {{convert|15|°C|°F}}.<ref>{{cite journal|year=2003|title=Modern global climate change|journal=Science|volume=302|issue=5651|pages=1719–23|bibcode=2003Sci...302.1719K|doi=10.1126/science.1090228|pmid=14657489|vauthors=Karl TR, Trenberth KE|s2cid=45484084|url=https://zenodo.org/record/1230878|access-date=26 July 2019|archive-date=22 April 2021|archive-url=https://web.archive.org/web/20210422194919/https://zenodo.org/record/1230878|url-status=live}}</ref><ref>{{cite book|url=http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter1.pdf|title=Historical overview of climate change science.|author1=Le Treut H.|author2=Somerville R.|author3=Cubasch U.|author4=Ding Y.|author5=Mauritzen C.|author5-link=Cecilie Mauritzen|author6=Mokssit A.|author7=Peterson T.|author8=Prather M.|access-date=14 December 2008|archive-date=26 November 2018|archive-url=https://web.archive.org/web/20181126204443/http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter1.pdf|url-status=live}} in {{harvp|IPCC AR4 WG1|2007}}</ref><ref name="h2o">{{cite web|url=http://nasascience.nasa.gov/earth-science/oceanography/ocean-earth-system/ocean-water-cycle|title=NASA Science Mission Directorate article on the water cycle|publisher=Nasascience.nasa.gov|access-date=2010-10-16|url-status=dead|archive-url=https://web.archive.org/web/20090117143544/http://nasascience.nasa.gov/earth-science/oceanography/ocean-earth-system/ocean-water-cycle|archive-date=17 January 2009}}</ref> The atmospheres of ], ] and ] also contain greenhouse gases. | |||
| ] of global warming that has happened so far. Future ] for long lived drivers like carbon dioxide emissions is not represented. Whiskers on each bar show the possible ]. ]] | |||
| '''Greenhouse gases''' ('''GHGs''') are the gases in the ] that raise the surface temperature of ]s such as the Earth. What distinguishes them from other gases is that they ] the ] that a ], resulting in the ].<ref name="AR6WG1annexVII">{{cite book |last1=Matthews |first1=J.B.R. |url=https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_AnnexVII.pdf |title=Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change |last2=Möller |first2=V. |last3=van Diemenn |first3=R. |last4=Fuglesvedt |first4=J.R. |date=2021-08-09 |publisher=] / ] |editor-last1=Masson-Delmotte |editor-first1=Valérie |editor-link1=Valérie Masson-Delmotte |pages=2215–2256 |chapter=Annex VII: Glossary |doi=10.1017/9781009157896.022 |isbn=9781009157896 |display-authors=etal |editor-last2=Zhai |editor-first2=Panmao |editor-link2=Panmao Zhai |editor-last3=Pirani |editor-first3=Anna |editor-last4=Connors |editor-first4=Sarah L. |editor-last5=Péan |editor-first5=Clotilde |display-editors=etal |doi-access=free}}</ref> The Earth is warmed by sunlight, causing its surface to ], which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of ] would be about {{convert|-18|°C|°F}},<ref name="NASACO2" /> rather than the present average of {{convert|15|°C|°F}}.<ref name="Trenberth2003" /><ref name=":0">Le Treut, H., R. Somerville, U. Cubasch, Y. Ding, C. Mauritzen, A. Mokssit, T. Peterson and M. Prather, 2007: "". In: "". . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.</ref> | |||
| The five most abundant greenhouse gases in Earth's atmosphere, listed in decreasing order of average global ], are:<ref>{{cite web |date=2016-08-01 |title=Atmospheric Concentration of Greenhouse Gases |url=https://www.epa.gov/sites/default/files/2016-08/documents/print_ghg-concentrations-2016.pdf |url-status=live |archive-url=https://web.archive.org/web/20211019134514/https://www.epa.gov/sites/default/files/2016-08/documents/print_ghg-concentrations-2016.pdf |archive-date=19 October 2021 |access-date=6 September 2021 |publisher=]}}</ref><ref>{{cite web |author=<!--Not stated--> |date=<!--Not stated--> |title=Inside the Earth's invisible blanket. |url=http://sequestration.org/science/greenhousegases.html |url-status=dead |archive-url=https://web.archive.org/web/20200728231450/http://sequestration.org/science/greenhousegases.html |archive-date=28 July 2020 |access-date=March 5, 2021 |website=sequestration.org |publisher=<!--Not stated--> |quote=}}</ref> ], ], ], ], ]. Other greenhouse gases of concern include ]s (CFCs and ]), ] (HFCs), ], ], and ]. Water vapor causes about half of the greenhouse effect, acting in response to other gases as a ].<ref name=":3">{{cite web |author=Gavin Schmidt |date=2010-10-01 |title=Taking the Measure of the Greenhouse Effect |url=https://www.giss.nasa.gov/research/briefs/2010_schmidt_05/ |publisher=NASA Goddard Institute for Space Studies – Science Briefs}}</ref> | |||
| Human activities since the beginning of the ] (around 1750) have increased the ] by almost 50%, from 280 ] in 1750 to 419 ppm in 2021.<ref>{{Cite web|last=Calma|first=Justine|date=2021-06-07|title=CO2 levels are at an all-time high — again|url=https://www.theverge.com/2021/6/7/22522736/carbon-dioxide-co2-record-climate-change|access-date=2021-06-17|website=The Verge|language=en|archive-date=19 June 2021|archive-url=https://web.archive.org/web/20210619120954/https://www.theverge.com/2021/6/7/22522736/carbon-dioxide-co2-record-climate-change|url-status=live}}</ref> The last time the atmospheric concentration of carbon dioxide was this high was over 3 million years ago.<ref>{{Cite web|url=https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide|title=Climate Change: Atmospheric Carbon Dioxide {{!}} NOAA Climate.gov|website=www.climate.gov|access-date=2020-03-02|archive-date=24 June 2013|archive-url=https://web.archive.org/web/20130624204311/https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide|url-status=live}}</ref> This increase has occurred despite the absorption of more than half of the emissions by various natural ] in the ].<ref name="cdiac">{{cite web|url=http://cdiac.ornl.gov/pns/faq.html|title=Frequently asked global change questions|publisher=]|access-date=23 February 2010|archive-date=17 August 2011|archive-url=https://web.archive.org/web/20110817044713/http://cdiac.ornl.gov/pns/faq.html|url-status=live}}</ref><ref>{{cite web|url=http://www.esrl.noaa.gov/gmd/ccgg/trends/|title=Trends in carbon dioxide|date=14 January 2008|publisher=Esrl.noaa.gov|author=ESRL Web Team|access-date=2011-09-11|archive-date=25 December 2018|archive-url=https://web.archive.org/web/20181225142754/https://www.esrl.noaa.gov/gmd/ccgg/trends/|url-status=live}}</ref> | |||
| Human activities since the beginning of the ] (around 1750) have increased ],<ref name="NOAA2022">{{cite web |date=3 June 2022 |title=Carbon dioxide now more than 50% higher than pre-industrial levels |url=https://www.noaa.gov/news-release/carbon-dioxide-now-more-than-50-higher-than-pre-industrial-levels |access-date=30 August 2022 |publisher=National Oceanic and Atmospheric Administration |language=en}}</ref> and methane levels by 150%.<ref>{{cite web |title=Understanding methane emissions |url=https://www.iea.org/reports/global-methane-tracker-2023/understanding-methane-emissions |publisher=International Energy Agency |quote=The concentration of methane in the atmosphere is currently over two-and-a-half times greater than its pre-industrial levels}}</ref> Carbon dioxide emissions are causing about three-quarters of ], while ] cause most of the rest.<ref>{{cite web |title=Global Greenhouse Gas Emissions Data |date=12 January 2016 |publisher=United States Environmental Protection Agency |url=https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data}}</ref> The vast majority of ] by humans come from the burning of ]s,<ref name="EPA_GHGdata">{{cite web |date=12 January 2016 |title=Global Greenhouse Gas Emissions Data |url=https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data |url-status=live |archive-url=https://web.archive.org/web/20191205123907/https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data |archive-date=5 December 2019 |access-date=30 December 2019 |publisher=] |quote=The burning of coal, natural gas, and oil for electricity and heat is the largest single source of global greenhouse gas emissions.}}</ref> with remaining contributions from ] and ].<ref name=":1">Canadell, J.G., P.M.S. Monteiro, M.H. Costa, L. Cotrim da Cunha, P.M. Cox, A.V. Eliseev, S. Henson, M. Ishii, S. Jaccard, C. Koven, A. Lohila, P.K. Patra, S. Piao, J. Rogelj, S. Syampungani, S. Zaehle, and K. Zickfeld, 2021: . In . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 673–816, doi:10.1017/9781009157896.007.</ref>{{rp|687}} ] originate from agriculture, fossil fuel production, waste, and other sources.<ref name=":4">{{cite web |title=Global Methane Tracker 2023 |date=21 February 2023 |url=https://www.iea.org/reports/global-methane-tracker-2023 |publisher=International Energy Agency}}</ref> The ] takes thousands of years to fully absorb {{CO2}} from the atmosphere,<ref>{{cite web |title=Climate Change Indicators: Greenhouse Gases |date=16 December 2015 |publisher=United States Environmental Protection Agency |url=https://www.epa.gov/climate-indicators/greenhouse-gases |quote=Carbon dioxide's lifetime cannot be represented with a single value because the gas is not destroyed over time, but instead moves among different parts of the ocean–atmosphere–land system. Some of the excess carbon dioxide is absorbed quickly (for example, by the ocean surface), but some will remain in the atmosphere for thousands of years, due in part to the very slow process by which carbon is transferred to ocean sediments.}}</ref> while methane lasts in the atmosphere for an average of only 12 years.<ref>{{cite web |title=Understanding methane emissions |publisher=International Energy Agency |url=https://www.iea.org/reports/global-methane-tracker-2023/understanding-methane-emissions}}</ref> | |||
| At current ] rates, temperatures could increase by 2 ] (3.6 ]), which the ]' ] (IPCC) says is the upper limit to avoid "dangerous" levels, by 2050.<ref>{{Cite web|date=2020-12-04|title=Analysis: When might the world exceed 1.5C and 2C of global warming?|url=https://www.carbonbrief.org/analysis-when-might-the-world-exceed-1-5c-and-2c-of-global-warming|access-date=2021-06-17|website=Carbon Brief|language=en|archive-date=6 June 2021|archive-url=https://web.archive.org/web/20210606135004/https://www.carbonbrief.org/analysis-when-might-the-world-exceed-1-5c-and-2c-of-global-warming|url-status=live}}</ref> The vast majority of ] carbon dioxide emissions come from ] of ]s, principally ], ] (including ]) and ], with additional contributions from ] and other changes in land use.<ref name="EPA_GHGdata">{{cite web|title=Global Greenhouse Gas Emissions Data|date=12 January 2016|url=https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data|access-date=30 December 2019|publisher=]|quote=The burning of coal, natural gas, and oil for electricity and heat is the largest single source of global greenhouse gas emissions.|archive-date=5 December 2019|archive-url=https://web.archive.org/web/20191205123907/https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data|url-status=live}}</ref><ref>{{cite web|title=AR4 SYR Synthesis Report Summary for Policymakers – 2 Causes of change|url=https://www.ipcc.ch/publications_and_data/ar4/syr/en/spms2.html|url-status=dead|archive-url=https://web.archive.org/web/20180228235005/http://www.ipcc.ch/publications_and_data/ar4/syr/en/spms2.html|archive-date=28 February 2018|access-date=9 October 2015|work=ipcc.ch}}</ref> | |||
| ] happen between the atmosphere, ]s, the ocean, and ]s. These flows have been fairly balanced over the past 1 million years,<ref>{{cite web |title=Climate Change Indicators: Atmospheric Concentrations of Greenhouse Gases |url=https://www.epa.gov/climate-indicators/climate-change-indicators-atmospheric-concentrations-greenhouse-gases |website=EPA.gov |date=27 June 2016 |publisher=U.S. Environmental Protection Agency |access-date=20 June 2024}}</ref> although greenhouse gas levels have varied widely in ]. Carbon dioxide levels are now higher than they have been for 3 million years.<ref>{{Cite web |last1=Lindsey |first1=Rebecca |title=Climate Change: Atmospheric Carbon Dioxide |url=https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide |url-status=live |archive-url=https://web.archive.org/web/20130624204311/https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide |archive-date=24 June 2013 |access-date=2020-03-02 |website=climate.gov}}</ref> If current emission rates continue then global warming will surpass {{convert|2.0|C-change}} sometime between 2040 and 2070. This is a level which the ] (IPCC) says is "dangerous".<ref name=":5">{{Cite web|date=2020-12-04|title=Analysis: When might the world exceed 1.5C and 2C of global warming?|url=https://www.carbonbrief.org/analysis-when-might-the-world-exceed-1-5c-and-2c-of-global-warming|access-date=2021-06-17|website=Carbon Brief|language=en|archive-date=6 June 2021|archive-url=https://web.archive.org/web/20210606135004/https://www.carbonbrief.org/analysis-when-might-the-world-exceed-1-5c-and-2c-of-global-warming|url-status=live}}</ref> | |||
| {{TOC limit|3}} | {{TOC limit|3}} | ||
| == Properties and mechanisms == | |||
| ==Gases in Earth's atmosphere== | |||
| ]s of ]. The largest absorption band of carbon dioxide is not far from the maximum in the ] from ground, and it partly closes the window of transparency of water—explaining carbon dioxide's major heat-trapping effect.]] | |||
| {{Main|Greenhouse effect|Atmosphere of Earth}} | |||
| Greenhouse gases are ] active, meaning that they absorb and emit ] in the same long wavelength range as what is emitted by the Earth's surface, clouds and atmosphere.<ref name="AR6_WGI_AnnexVII">IPCC, 2021: . In . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 2215–2256, ].</ref>{{rp|2233}} | |||
| 99% of the Earth's dry atmosphere (excluding ]) is made up of ] ({{chem|N|2}}) (78%) and ] ({{chem|O|2}}) (21%). Because their ]s contain ], they have no asymmetry in the ],<ref name="Archer2011Ch4" /> and so are ] by infrared thermal radiation,<ref>{{cite journal |last1=Wei |first1=Peng-Sheng |last2=Hsieh |first2=Yin-Chih |last3=Chiu |first3=Hsuan-Han |last4=Yen |first4=Da-Lun |last5=Lee |first5=Chieh |last6=Tsai |first6=Yi-Cheng |last7=Ting |first7=Te-Chuan |date=6 October 2018 |title=Absorption coefficient of carbon dioxide across atmospheric troposphere layer |journal=] |volume=4 |issue=10 |pages=e00785 |doi=10.1016/j.heliyon.2018.e00785 |doi-access=free |pmid=30302408 |pmc=6174548 |bibcode=2018Heliy...400785W |issn = 2405-8440 }}</ref> with only an extremely minor effect from ].<ref>{{Cite journal |last1=Höpfner |first1=M. |last2=Milz |first2=M. |last3=Buehler |first3=S. |last4=Orphall |first4=J. |last5=Stiller |first5=G. |date=24 May 2012 |title=The natural greenhouse effect of atmospheric oxygen (O<sub>2</sub>) and nitrogen (N<sub>2</sub>) |journal=Geophysical Research Letters |language=en |volume=39 |issue=L10706 |doi=10.1029/2012GL051409 |bibcode=2012GeoRL..3910706H |s2cid=128823108 |issn=1944-8007}}</ref><ref>{{cite web |title=Which Gases Are Greenhouse Gases? |url=https://www.acs.org/content/acs/en/climatescience/greenhousegases/whichgases.html |access-date=2021-05-31 |publisher=American Chemical Society}}</ref><ref>{{Cite journal |last1=Höpfner |first1=M. |last2=Milz |first2=M. |last3=Buehler |first3=S. |last4=Orphall |first4=J. |last5=Stiller |first5=G. |date=24 May 2012 |title=The natural greenhouse effect of atmospheric oxygen (O<sub>2</sub>) and nitrogen (N<sub>2</sub>) |journal=Geophysical Research Letters |language=en |volume=39 |issue=L10706 |doi=10.1029/2012GL051409 |bibcode=2012GeoRL..3910706H |issn=1944-8007 |s2cid=128823108}}</ref> A further 0.9% of the atmosphere is made up by ] (Ar), which is ], and so completely transparent to thermal radiation. On the other hand, ] (0.04%), ], ] and even less abundant ]es account for less than 0.1% of Earth's atmosphere, but because their molecules contain atoms of different elements, there is an asymmetry in ] which allows ]s to interact with electromagnetic radiation. This makes them infrared active, and so their presence causes ].<ref name="Archer2011Ch4">{{cite book |last1=Archer |first1=David |url=http://forecast.uchicago.edu/chapter4.pdf |title=Global Warming: Understanding the Forecast, Chapter 4: Greenhouse Gases |date=2011 |publisher=Wiley |isbn=978-0470943410 |edition=2 |access-date=14 June 2023}}</ref> | |||
| ===Non-greenhouse gases=== | |||
| The major constituents of Earth's atmosphere, ] ({{chem|N|2}}) (78%), ] ({{chem|O|2}}) (21%), and ] (Ar) (0.9%), are not greenhouse gases because ] such as {{chem|N|2}} and {{chem|O|2}} have no net change in the ] when they vibrate, and ] gases such as Ar do not have vibrational modes. Hence they are ] by ]. Some molecules containing just two atoms of different elements, such as ] (CO) and ] (HCl), do absorb infrared radiation, but these molecules are short-lived in the atmosphere owing to their ] or ]. Therefore, they do not contribute significantly to the greenhouse effect and often are omitted when discussing greenhouse gases. | |||
| === |
===Radiative forcing=== | ||
| {{Main|Radiative forcing}} | |||
| ]s of ]. The largest absorption band of ] is not far from the maximum in the ] from ground, and it partly closes the window of transparency of water; hence its major effect.]] | |||
| ] ]s of primary greenhouse gases. Water vapor absorbs over a broad range of wavelengths. Earth emits thermal radiation particularly strongly in the vicinity of the carbon dioxide 15-micron absorption band. The relative importance of water vapor decreases with increasing altitude.]] | |||
| {{see also|IPCC list of greenhouse gases|Carbon dioxide in Earth's atmosphere}} | |||
| Greenhouse gases are those that absorb and emit ].<ref name="IPCC AR4-SYR" /> Carbon dioxide (0.04%), nitrous oxide, methane, and ozone are trace gases that account for almost 0.1% of Earth's atmosphere and have an appreciable greenhouse effect. | |||
| Earth absorbs some of the radiant energy received from the sun, reflects some of it as light and reflects or radiates the rest back to space as ]. A planet's surface temperature depends on this balance between incoming and outgoing energy. When ] is shifted, its surface becomes warmer or cooler, leading to a variety of changes in global climate.<ref name="epa ggas">{{cite web |year=2016 |title=Climate Change Indicators in the United States – Greenhouse Gases |url=https://www.epa.gov/climate-indicators/greenhouse-gases |url-status=live |archive-url=https://web.archive.org/web/20160827230238/https://www.epa.gov/climate-indicators/greenhouse-gases |archive-date=27 August 2016 |access-date=5 September 2020 |publisher=U.S. Environmental Protection Agency (EPA)}}.</ref> ''Radiative forcing'' is a metric calculated in watts per square meter, which characterizes the impact of an external change in a factor that influences climate. It is calculated as the difference in top-of-atmosphere (TOA) energy balance immediately caused by such an external change.<!--,without taking the eventual adjustment processes in the troposphere or surface time to respond to reduce the imbalance.{{explain|January 2024}}--> A positive forcing, such as from increased concentrations of greenhouse gases, means more energy arriving than leaving at the top-of-atmosphere, which causes additional warming, while negative forcing, like from ]s forming in the atmosphere from ], leads to cooling.<ref name="AR6_WGI_AnnexVII" />{{rp|2245}}<ref name="epa cforce">{{cite web |year=2016 |title=Climate Change Indicators in the United States – Climate Forcing |url=https://www.epa.gov/climate-indicators/climate-change-indicators-climate-forcing |url-status=live |archive-url=https://web.archive.org/web/20160827223551/https://www.epa.gov/climate-indicators/climate-change-indicators-climate-forcing |archive-date=27 August 2016 |access-date=5 September 2020 |publisher=U.S. Environmental Protection Agency (EPA)}} {{Webarchive|url=https://web.archive.org/web/20200921073951/https://www.epa.gov/sites/production/files/2016-08/documents/print_climate-forcing-2016.pdf|date=21 September 2020}}</ref> | |||
| The most abundant greenhouse gases in Earth's atmosphere, listed in decreasing order of average global ], are:<ref>{{cite web |url=https://www.epa.gov/sites/default/files/2016-08/documents/print_ghg-concentrations-2016.pdf |title=Atmospheric Concentration of Greenhouse Gases |publisher=] |date=2016-08-01 |access-date=6 September 2021 |archive-date=19 October 2021 |archive-url=https://web.archive.org/web/20211019134514/https://www.epa.gov/sites/default/files/2016-08/documents/print_ghg-concentrations-2016.pdf |url-status=live }}</ref><ref>{{cite web |url=http://sequestration.org/science/greenhousegases.html |title=Inside the Earth's invisible blanket. |author=<!--Not stated--> |date=<!--Not stated--> |website=sequestration.org |publisher=<!--Not stated--> |access-date=March 5, 2021 |quote= |archive-date=28 July 2020 |archive-url=https://web.archive.org/web/20200728231450/http://sequestration.org/science/greenhousegases.html |url-status=dead }}</ref> | |||
| Within the lower atmosphere, greenhouse gases exchange thermal radiation with the surface and limit radiative heat flow away from it, which reduces the overall rate of upward radiative heat transfer.<ref name="Wallace2006">{{cite book |last1=Wallace |first1=J. M. |last2=Hobbs |first2=P. V. |title=Atmospheric Science |date=2006 |publisher=Academic Press |isbn=978-0-12-732951-2 |edition=2}}</ref>{{rp|139}}<ref name="Manabe1964">{{cite journal |last1=Manabe |first1=S. |last2=Strickler |first2=R. F. |title=Thermal Equilibrium of the Atmosphere with a Convective Adjustment |journal=J. Atmos. Sci. |date=1964 |volume=21 |issue=4 |pages=361–385 |doi=10.1175/1520-0469(1964)021<0361:TEOTAW>2.0.CO;2|bibcode=1964JAtS...21..361M |doi-access=free }}</ref> The increased concentration of greenhouse gases is also cooling the upper atmosphere, as it is much thinner than the lower layers, and any heat re-emitted from greenhouse gases is more likely to travel further to space than to interact with the fewer gas molecules in the upper layers. The upper atmosphere is also shrinking as the result.<ref>{{Cite web |last=Hatfield |first=Miles |date=30 June 2021 |title=NASA Satellites See Upper Atmosphere Cooling and Contracting Due to Climate Change |url=https://www.nasa.gov/general/nasa-satellites-see-upper-atmosphere-cooling-and-contracting-due-to-climate-change/ |publisher=] }}</ref> | |||
| * ] ({{chem|H|2|O}}) | |||
| <!-- Naming these effects contributes to a full understanding of the role of greenhouse gases. However, these effects are of secondary importance when it comes to understanding global warming. It is important to focus on top-of-atmosphere energy balance to correctly reason about global warming. It has been argued that the ''surface budget fallacy'', in which focus on the surface energy budget leads to faulty reasoning, constitutes a common fallacy when thinking about the greenhouse effect and global warming.<ref name="PierrehumbertTextbook">{{cite book |last1=Pierrehumbert |first1=Raymond T. |title=Principles of Planetary Climate |date=2010 |publisher=Cambridge University Press |isbn=978-0-521-86556-2}}</ref>{{rp|413}} | |||
| * ] ({{chem|CO|2}}) | |||
| * ] ({{chem|CH|4|}}) | |||
| * ] ({{chem|N|2|O}}) | |||
| * ] ({{chem|O|3|}}) | |||
| * ]s (CFCs and ]) | |||
| * ] (HFCs) | |||
| * ] (], ], etc.), ], and ] | |||
| ==== Chemical process contributions to radiative forcing ==== | |||
| Atmospheric concentrations are determined by the balance between sources (emissions of the gas from human activities and natural systems) and sinks (the removal of the gas from the atmosphere by conversion to a different chemical compound or absorption by bodies of water).<ref name="IPCC_WG1_AR4_Ch7">{{cite news |title= FAQ 7.1 |page= 14}} in {{harvp|IPCC AR4 WG1|2007}}</ref> The proportion of an emission remaining in the atmosphere after a specified time is the "]" (AF). The ''annual airborne fraction'' is the ratio of the atmospheric increase in a given year to that year's total emissions. As of 2006 the annual airborne fraction for CO<sub>2</sub> was about 0.45. The annual airborne fraction increased at a rate of 0.25 ± 0.21% per year over the period 1959–2006.<ref name=Canadell2007>{{cite journal|author = Canadell, J.G. |author2=Le Quere, C. |author3=Raupach, M.R. |author4=Field, C.B. |author5=Buitenhuis, E.T. |author6=Ciais, P. |author7=Conway, T.J. |author8=Gillett, N.P. |author9=Houghton, R.A. |author10=Marland, G. | year = 2007| title = Contributions to accelerating atmospheric CO<sub>2</sub> growth from economic activity, carbon intensity, and efficiency of natural sinks| journal = Proc. Natl. Acad. Sci. USA| volume = 104| issue = 47| pages = 18866–70 | pmid = 17962418| doi = 10.1073/pnas.0702737104| pmc = 2141868| bibcode = 2007PNAS..10418866C |doi-access=free }}</ref> | |||
| ] | |||
| Some gases contribute indirectly to altering the TOA radiative balance through participation in chemical processes within the atmosphere.{{citation needed|date=July 2023}} | |||
| ===Indirect radiative effects=== | |||
| ] | |||
| Oxidation of CO to {{CO2}} directly produces an unambiguous increase in ] although the reason is subtle. The peak of the thermal IR emission from Earth's surface is very close to a strong vibrational absorption band of {{CO2}} (] 15 microns, or ] 667 cm<sup>−1</sup>). On the other hand, the single CO vibrational band only absorbs IR at much shorter wavelengths (4.7 microns, or 2145 cm<sup>−1</sup>), where the emission of radiant energy from Earth's surface is at least a factor of ten lower. Oxidation of methane to {{CO2}}, which requires reactions with the OH radical, produces an instantaneous reduction in radiative absorption and emission since {{CO2}} is a weaker greenhouse gas than methane. However, the oxidations of CO and {{chem|CH|4}} are entwined since both consume OH radicals. In any case, the calculation of the total radiative effect includes both direct and indirect forcing. | Oxidation of CO to {{CO2}} directly produces an unambiguous increase in ] although the reason is subtle. The peak of the thermal IR emission from Earth's surface is very close to a strong vibrational absorption band of {{CO2}} (] 15 microns, or ] 667 cm<sup>−1</sup>). On the other hand, the single CO vibrational band only absorbs IR at much shorter wavelengths (4.7 microns, or 2145 cm<sup>−1</sup>), where the emission of radiant energy from Earth's surface is at least a factor of ten lower. Oxidation of methane to {{CO2}}, which requires reactions with the OH radical, produces an instantaneous reduction in radiative absorption and emission since {{CO2}} is a weaker greenhouse gas than methane. However, the oxidations of CO and {{chem|CH|4}} are entwined since both consume OH radicals. In any case, the calculation of the total radiative effect includes both direct and indirect forcing.{{citation needed|date=July 2023}} | ||
| A second type of indirect effect happens when chemical reactions in the atmosphere involving these gases change the concentrations of greenhouse gases. For example, the destruction of ]s (NMVOCs) in the atmosphere can produce ozone. The size of the indirect effect can depend strongly on where and when the gas is emitted.<ref name="forsteretal">{{cite book |url=https://www.ipcc.ch/report/ar4/wg1/changes-in-atmospheric-constituents-and-radiative-forcing/ |contribution=2.10.3 Indirect GWPs |title=Changes in Atmospheric Constituents and in Radiative Forcing |series=Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change |publisher=Cambridge University Press |year=2007 |author=Forster, P. |access-date=2012-12-02 |display-authors=etal |archive-date=9 February 2019 |archive-url=https://web.archive.org/web/20190209125821/https://www.ipcc.ch/report/ar4/wg1/changes-in-atmospheric-constituents-and-radiative-forcing/ |url-status=live }}</ref> | A second type of indirect effect happens when chemical reactions in the atmosphere involving these gases change the concentrations of greenhouse gases. For example, the destruction of ]s (NMVOCs) in the atmosphere can produce ozone. The size of the indirect effect can depend strongly on where and when the gas is emitted.<ref name="forsteretal">{{cite book |url=https://www.ipcc.ch/report/ar4/wg1/changes-in-atmospheric-constituents-and-radiative-forcing/ |contribution=2.10.3 Indirect GWPs |title=Changes in Atmospheric Constituents and in Radiative Forcing |series=Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change |publisher=Cambridge University Press |year=2007 |author=Forster, P. |access-date=2012-12-02 |display-authors=etal |archive-date=9 February 2019 |archive-url=https://web.archive.org/web/20190209125821/https://www.ipcc.ch/report/ar4/wg1/changes-in-atmospheric-constituents-and-radiative-forcing/ |url-status=live }}</ref> | ||
| NMVOCs include a large variety of chemically different compounds, such as ], ], ], ], ] and ].<ref>{{Citation|last1=Nesaratnam|first1=Suresh T.|title=Section 2: Meteorology and Air Pollutants|date=2014|url=http://dx.doi.org/10.1002/9781118863886.ch2|work=Air Quality Management|pages=15–98|place=Chichester, UK|publisher=John Wiley & Sons, Ltd|access-date=|last2=Taherzadeh|first2=Shahram|last3=Barratt|first3=Rod|doi=10.1002/9781118863886.ch2 |isbn=9781118863886 |url-access=subscription}}</ref> Essentially, NMVOCs are identical to ]s (VOCs), but with methane excluded.<ref>{{Cite web|title=System of Registries |publisher=US EPA|url=https://sor.epa.gov/sor_internet/registry/termreg/searchandretrieve/glossariesandkeywordlists/search.do?details=&vocabName=Glossary%20Climate%20Change%20Terms&filterTerm=nmvoc&checkedAcronym=false&checkedTerm=false&hasDefinitions=false&filterTerm=nmvoc&filterMatchCriteria=Contains|access-date=|website=sor.epa.gov }}</ref> Methane is excluded in ] contexts because it is not toxic. It is however a very potent greenhouse gas, with low reactivity and thus a long lifetime in the atmosphere.<ref>{{Cite book|url=http://doi.wiley.com/10.1002/9780470988657|title=Volatile Organic Compounds in the Atmosphere|date=2007|publisher=Blackwell Publishing Ltd|isbn=978-0-470-98865-7|editor-last=Koppmann|editor-first=Ralf|location=Oxford, UK |doi=10.1002/9780470988657}}</ref> An important subset of NMVOCs are the non-methane hydrocarbons (NMHCs). The same process that converts NMVOCs to carbon dioxide can also lead to the formation of tropospheric ozone. ]s have an indirect effect because they destroy stratospheric ozone. Finally, ] can lead to ozone production and {{chem|CH|4}} increases as well as producing stratospheric water vapor.<ref name="forsteretal" /><ref>{{Cite web|url=https://www.rechargenews.com/energy-transition/hydrogen-twice-as-powerful-a-greenhouse-gas-as-previously-thought-uk-government-study/2-1-1200115|title=Hydrogen 'twice as powerful a greenhouse gas as previously thought': UK government study|date=8 April 2022|website=Recharge | Latest renewable energy news}}</ref> --> | |||
| Methane has indirect effects in addition to forming {{CO2}}. The main chemical that reacts with methane in the atmosphere is the ] (OH), thus more methane means that the concentration of OH goes down. Effectively, methane increases its own atmospheric lifetime and therefore its overall radiative effect. The oxidation of methane can produce both ozone and water; and is a major source of water vapor in the normally dry ]. CO and NMVOCs produce {{CO2}} when they are oxidized. They remove OH from the atmosphere, and this leads to higher concentrations of methane. The surprising effect of this is that the global warming potential of CO is three times that of {{CO2}}.<ref name=maccartyetal>{{cite web|last=MacCarty|first=N.|title=Laboratory Comparison of the Global-Warming Potential of Six Categories of Biomass Cooking Stoves|url=http://www.scscertified.com/lcs/docs/Global_warming_full_9-6-07.pdf|publisher=Approvecho Research Center|url-status=dead|archive-url=https://web.archive.org/web/20131111144703/http://www.scscertified.com/lcs/docs/Global_warming_full_9-6-07.pdf|archive-date=11 November 2013}}</ref> The same process that converts NMVOCs to carbon dioxide can also lead to the formation of tropospheric ozone. ]s have an indirect effect because they destroy stratospheric ozone. Finally, ] can lead to ozone production and {{chem|CH|4}} increases as well as producing stratospheric water vapor.<ref name="forsteretal" /> | |||
| == |
== Contributions of specific gases to the greenhouse effect == | ||
| {{Main|Greenhouse effect}}Anthropogenic changes to the natural greenhouse effect are sometimes referred to as the ''enhanced greenhouse effect''.<ref name="AR6_WGI_AnnexVII" />{{rp|2223}} | |||
| The major non-gas contributor to Earth's greenhouse effect, ], also absorb and emit infrared radiation and thus have an effect on greenhouse gas radiative properties. Clouds are water droplets or ]s suspended in the atmosphere.<ref name="kiehl197">{{cite journal| title=Earth's annual global mean energy budget| first=J.T.| last=Kiehl|author2=Kevin E. Trenberth|journal=Bulletin of the American Meteorological Society| pages=197–208| volume=78| issue=2| year=1997| doi=10.1175/1520-0477(1997)078<0197:EAGMEB>2.0.CO;2 | bibcode=1997BAMS...78..197K}}</ref><ref name="realclimate.org">{{cite web| url=http://www.realclimate.org/index.php?p=142| date=6 April 2005| title=Water vapour: feedback or forcing?| publisher=RealClimate| access-date=1 May 2006| archive-date=24 June 2007| archive-url=https://web.archive.org/web/20070624015308/http://www.realclimate.org/index.php?p=142| url-status=live}}</ref> | |||
| This table shows the most important contributions to the overall greenhouse effect, without which the average temperature of ] would be about {{convert|-18|°C|°F}},<ref name="NASACO2">{{Cite web |title= Science Briefs: Greenhouse Gases: Refining the Role of Carbon Dioxide |url=http://www.giss.nasa.gov/research/briefs/ma_01/ |url-status=dead |archive-url=https://web.archive.org/web/20050112211604/http://www.giss.nasa.gov/research/briefs/ma_01/ |archive-date=2005-01-12 |access-date=2016-04-26 |website=NASA GISS |author1=Qiancheng Ma |date=March 1998 }}</ref> instead of around {{convert|15|°C|°F}}.<ref name="Trenberth2003">{{cite journal |vauthors=Karl TR, Trenberth KE |year=2003 |title=Modern global climate change |url=https://zenodo.org/record/1230878 |via=Zenodo |s2cid-access=free |url-status=live |journal=Science |volume=302 |issue=5651 |pages=1719–23 |bibcode=2003Sci...302.1719K |doi=10.1126/science.1090228 |pmid=14657489 |s2cid=45484084 |archive-url=https://web.archive.org/web/20210422194919/https://zenodo.org/record/1230878 |archive-date=22 April 2021 |access-date=26 July 2019}}</ref> This table also specifies ''tropospheric'' ], because this gas has a cooling effect in the ], but a warming influence comparable to ] and ] in the ].<ref>{{cite web |date=2016-08-01 |title=Atmospheric Concentration of Greenhouse Gases |url=https://www.epa.gov/sites/default/files/2016-08/documents/print_ghg-concentrations-2016.pdf |publisher=]}}</ref> | |||
| ===Role of water vapor=== | |||
| ] | |||
| ] accounts for the largest percentage of the greenhouse effect, between 36% and 66% for clear sky conditions and between 66% and 85% when including clouds.<ref name="realclimate.org" /> Water vapor concentrations fluctuate regionally, but human activity does not directly affect water vapor concentrations except at local scales, such as near irrigated fields. Indirectly, human activity that increases global temperatures will increase water vapor concentrations, a process known as water vapor feedback.<ref name="Held&Soden2000">{{Cite journal|last1=Held|first1=Isaac M.|last2=Soden|first2=Brian J.|date=November 2000|title=Water vapor feedback and global warming|journal=]|language=en|volume=25|issue=1|pages=441–475|citeseerx=10.1.1.22.9397|doi=10.1146/annurev.energy.25.1.441|issn=1056-3466|doi-access=free}}</ref> The atmospheric concentration of vapor is highly variable and depends largely on temperature, from less than 0.01% in extremely cold regions up to 3% by mass in saturated air at about 32 °C.<ref>{{cite book|author=Evans, Kimberly Masters|url=https://archive.org/details/environment00kimm_0|title=The environment: a revolution in attitudes|publisher=Thomson Gale|year=2005|isbn=978-0787690823|location=Detroit|chapter=The greenhouse effect and climate change|chapter-url={{google books |plainurl=y |id=DdtzAAAACAAJ}}|url-access=registration}}</ref> (See ].) | |||
| The average residence time of a water molecule in the atmosphere is only about nine days, compared to years or centuries for other greenhouse gases such as {{chem|CH|4}} and {{CO2}}.<ref>{{Cite web|date=15 April 2012|title=Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2010|url=https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2010|access-date=2019-12-30|publisher=U.S. Environmental Protection Agency|page=1.4|language=en|archive-date=30 December 2019|archive-url=https://web.archive.org/web/20191230161419/https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2010|url-status=live}}</ref> Water vapor responds to and amplifies effects of the other greenhouse gases. The ] establishes that more water vapor will be present per unit volume at elevated temperatures. This and other basic principles indicate that warming associated with increased concentrations of the other greenhouse gases also will increase the concentration of water vapor (assuming that the ] remains approximately constant; modeling and observational studies find that this is indeed so). Because water vapor is a greenhouse gas, this results in further warming and so is a "]" that amplifies the original warming. Eventually other earth processes{{which|date=October 2020}} offset these positive feedbacks, stabilising the global temperature at a new equilibrium and preventing the loss of Earth's water through a Venus-like ].<ref name="Held&Soden2000" /> | |||
| ==Impacts on the overall greenhouse effect== | |||
| {{Main|Greenhouse effect}} | |||
| {{Webarchive|url=https://web.archive.org/web/20120604034848/http://pubs.giss.nasa.gov/abs/sc05400j.html |date=4 June 2012 }}</ref> analysed how individual components of the atmosphere contribute to the total greenhouse effect. They estimated that water vapor accounts for about 50% of Earth's greenhouse effect, with clouds contributing 25%, carbon dioxide 20%, and the minor greenhouse gases and ]s accounting for the remaining 5%. In the study, the reference model atmosphere is for 1980 conditions. Image credit: ].<ref>{{citation | author=Lacis, A. | date=October 2010 | title=NASA GISS: CO<sub>2</sub>: The Thermostat that Controls Earth's Temperature | url=http://www.giss.nasa.gov/research/briefs/lacis_01/ | archive-url=https://web.archive.org/web/20101020041139/http://www.giss.nasa.gov/research/briefs/lacis_01/ | url-status=dead | archive-date=2010-10-20 | publisher=NASA GISS | location=New York}}</ref>]] | |||
| The contribution of each gas to the greenhouse effect is determined by the characteristics of that gas, its abundance, and any indirect effects it may cause. For example, the direct radiative effect of a mass of methane is about 84 times stronger than the same mass of carbon dioxide over a 20-year time frame<ref name=TableOfWarmingPotentials5/> but it is present in much smaller concentrations so that its total direct radiative effect has so far been smaller, in part due to its shorter atmospheric lifetime in the absence of additional ]. On the other hand, in addition to its direct radiative impact, methane has a large, indirect radiative effect because it contributes to ozone formation. Shindell et al. (2005)<ref>{{cite journal |doi=10.1029/2004GL021900 |url=http://www.nasa.gov/vision/earth/lookingatearth/methane.html |title=An emissions-based view of climate forcing by methane and tropospheric ozone |year=2005 |last1=Shindell |first1=Drew T. |journal=Geophysical Research Letters |volume=32 |page=L04803 |bibcode=2005GeoRL..32.4803S |issue=4 |access-date=3 September 2005 |archive-date=11 September 2005 |archive-url=https://web.archive.org/web/20050911070033/http://www.nasa.gov/vision/earth/lookingatearth/methane.html |url-status=live }}</ref> argues that the contribution to climate change from methane is at least double previous estimates as a result of this effect.<ref>{{cite web |url=http://www.nasa.gov/vision/earth/lookingatearth/methane.html |title=Methane's Impacts on Climate Change May Be Twice Previous Estimates |publisher=Nasa.gov |date=30 November 2007 |access-date=2010-10-16 |archive-date=11 September 2005 |archive-url=https://web.archive.org/web/20050911070033/http://www.nasa.gov/vision/earth/lookingatearth/methane.html |url-status=live }}</ref> | |||
| When ranked by their direct contribution to the greenhouse effect, the most important are:<ref name="kiehl197"/>{{Failed verification|date=April 2016}} | |||
| {| class="wikitable" style="text-align:center" id-"GHG-ranking" | {| class="wikitable" style="text-align:center" id-"GHG-ranking" | ||
| |+ Percent contribution to total greenhouse effect | |||
| ! !! colspan="2" | K&T (1997)<ref name="kiehl197">{{cite journal |last=Kiehl |first=J.T. |author2=Kevin E. Trenberth |year=1997 |title=Earth's annual global mean energy budget |journal=Bulletin of the American Meteorological Society |volume=78 |issue=2 |pages=197–208 |bibcode=1997BAMS...78..197K |doi=10.1175/1520-0477(1997)078<0197:EAGMEB>2.0.CO;2 |doi-access=free|url=http://www.geo.utexas.edu/courses/387h/PAPERS/kiehl.pdf }}</ref> !! colspan="2" | Schmidt (2010)<ref name="Schmidt2010paper">{{citation |author1=Schmidt, G.A. |title=The attribution of the present-day total greenhouse effect |url=http://pubs.giss.nasa.gov/docs/2010/2010_Schmidt_etal_1.pdf |work=J. Geophys. Res. |volume=115 |issue=D20 |pages=D20106 |df=dmy-all |year=2010 |archive-url=https://web.archive.org/web/20111022111918/http://pubs.giss.nasa.gov/docs/2010/2010_Schmidt_etal_1.pdf |url-status=dead |bibcode=2010JGRD..11520106S |doi=10.1029/2010JD014287 |archive-date=22 October 2011 |author2=R. Ruedy |author3=R.L. Miller |author4=A.A. Lacis |author-link1=Gavin Schmidt |doi-access=free}}, D20106. {{Webarchive|url=https://web.archive.org/web/20120604034848/http://pubs.giss.nasa.gov/abs/sc05400j.html|date=4 June 2012}}</ref> | |||
| |- | |||
| ! Contributor !! Clear Sky !! With Clouds !! Clear Sky !! With Clouds | |||
| |- | |||
| | Water vapor || 60 || 41 || 67 || 50 | |||
| |- | |- | ||
| | Clouds || || 31 || || 25 | |||
| ! Compound<br /> | |||
| ! style="text-align:left;"| Formula <br /> | |||
| ! Concentration in <br />]<ref>{{cite web | |||
| | title=Climate Change Indicators: Atmospheric Concentrations of Greenhouse Gases | |||
| | publisher=United States Environmental Protection Agency | |||
| | work=Climate Change Indicators | |||
| | access-date=2017-01-20 | |||
| | url=http://www3.epa.gov/climatechange/science/indicators/ghg/ghg-concentrations.html | |||
| | date=27 June 2016 | |||
| | archive-date=30 April 2016 | |||
| | archive-url=https://web.archive.org/web/20160430023622/https://www3.epa.gov/climatechange/science/indicators/ghg/ghg-concentrations.html | |||
| | url-status=live | |||
| }}</ref> (ppm) | |||
| ! Contribution <br /> (%) | |||
| |- | |- | ||
| | {{CO2|link=yes}} || 26 || 18 || 24 || 19 | |||
| | ] and clouds || {{chem|H|2|O}} || 10–50,000<sup>(A)</sup> || '''36–72%''' | |||
| |- | |- | ||
| | Tropospheric ] (O<sub>3</sub>) || 8 || || || | |||
| | ]|| {{CO2}} || ~400 || '''9–26%''' | |||
| |- | |- | ||
| | {{N2O|link=yes}} + {{CH4|link=yes}} || 6 || || || | |||
| | ]|| {{chem|CH|4}} || ~1.8 || '''4–9%''' | |||
| |- | |- | ||
| | Other || || 9 || 9 || 7 | |||
| | ]|| {{chem|O|3}} || 2–8<sup>(B)</sup> || '''3–7%''' | |||
| |- | |- | ||
| ! colspan= |
! colspan="5" style="font-size: 0.85em; padding: 5px 2px 5px 10px; text-align: left; font-weight: normal;" | | ||
| '''K&T (1997)''' used 353 ppm {{CO2}} and calculated 125 W/m{{sup|2}} total clear-sky greenhouse effect; relied on single atmospheric profile and cloud model. "With Clouds" percentages are from Schmidt (2010) interpretation of K&T (1997).<br /> | |||
| <sup>(A)</sup> Water vapor strongly varies locally<ref>Wallace, John M. and Peter V. Hobbs. Atmospheric Science; ''An Introductory Survey''. Elsevier. Second Edition, 2006. {{ISBN|978-0127329512}}. Chapter 1</ref><br /> | |||
| '''Schmidt (2010)''' used 1980 climatology with 339 ppm {{CO2}} and 155 W/m{{sup|2}} total greenhouse effect; accounted for temporal and 3-D spatial distribution of absorbers.<br /> | |||
| <sup>(B)</sup> The concentration in stratosphere. About 90% of the ozone in Earth's atmosphere is contained in the stratosphere. | |||
| |} | |} | ||
| === Special role of water vapor === | |||
| In addition to the main greenhouse gases listed above, other greenhouse gases include ], ]s and ]s (see ]). Some greenhouse gases are not often listed. For example, ] has a high ] (GWP) but is only present in very small quantities.<ref name="NF3">{{cite journal |last=Prather |first=Michael J. |author2=J Hsu |title={{chem|NF|3}}, the greenhouse gas missing from Kyoto |journal=] |volume=35 |pages=L12810 |year=2008 |doi=10.1029/2008GL034542 |bibcode=2008GeoRL..3512810P |issue=12 |url=https://escholarship.org/uc/item/45q10130 |doi-access=free |access-date=23 September 2019 |archive-date=23 September 2019 |archive-url=https://web.archive.org/web/20190923234215/https://escholarship.org/uc/item/45q10130 |url-status=live }}</ref> | |||
| ] | |||
| Water vapor is the most important greenhouse gas overall, being responsible for 41–67% of the greenhouse effect,<ref name="kiehl197" /><ref name="Schmidt2010paper" /> but its global concentrations are not directly affected by human activity. While local water vapor concentrations can be affected by developments such as ], it has little impact on the global scale due to its short ] of about nine days.<ref>{{cite web |date=27 April 1995 |title=AGU Water Vapor in the Climate System |url=http://www.eso.org/gen-fac/pubs/astclim/espas/pwv/mockler.html |url-status=live |archive-url=https://web.archive.org/web/20121020163357/http://www.eso.org/gen-fac/pubs/astclim/espas/pwv/mockler.html |archive-date=20 October 2012 |access-date=2011-09-11 |publisher=Eso.org}}</ref> Indirectly, an increase in global temperatures cause will also increase water vapor concentrations and thus their warming effect, in a process known as water vapor feedback. It occurs because ] establishes that more water vapor will be present per unit volume at elevated temperatures.<ref name="Held&Soden2000">{{Cite journal |last1=Held |first1=Isaac M. |last2=Soden |first2=Brian J. |date=November 2000 |title=Water vapor feedback and global warming |journal=] |language=en |volume=25 |issue=1 |pages=441–475 |citeseerx=10.1.1.22.9397 |doi=10.1146/annurev.energy.25.1.441 |issn=1056-3466 |doi-access=free}}</ref> Thus, local atmospheric concentration of water vapor varies from less than 0.01% in extremely cold regions and up to 3% by mass in saturated air at about 32 °C.<ref>{{cite book |author=Evans, Kimberly Masters |url=https://archive.org/details/environment00kimm_0 |title=The environment: a revolution in attitudes |publisher=Thomson Gale |year=2005 |isbn=978-0787690823 |location=Detroit |chapter=The greenhouse effect and climate change |chapter-url={{google books |plainurl=y |id=DdtzAAAACAAJ}} |url-access=registration}}</ref> | |||
| ===Proportion of direct effects at a given moment=== | |||
| It is not possible to state that a certain gas causes an exact percentage of the greenhouse effect. This is because some of the gases absorb and emit radiation at the same frequencies as others, so that the total greenhouse effect is not simply the sum of the influence of each gas. The higher ends of the ranges quoted are for each gas alone; the lower ends account for overlaps with the other gases.<ref name="kiehl197"/><ref name="realclimate.org"/> In addition, some gases, such as methane, are known to have large indirect effects that are still being quantified.<ref>{{cite journal|last=Isaksen|first=Ivar S.A.|author2=Michael Gauss|author3=Gunnar Myhre|author4=Katey M. Walter Anthony|author5=Carolyn Ruppel|title=Strong atmospheric chemistry feedback to climate warming from Arctic methane emissions|journal=Global Biogeochemical Cycles|date=20 April 2011|volume=25|doi=10.1029/2010GB003845|url=http://www.atmos.washington.edu/academics/classes/2011Q2/558/IsaksenGB2011.pdf|access-date=29 July 2011|bibcode=2011GBioC..25.2002I|issue=2|pages=n/a|hdl=1912/4553|archive-url=https://web.archive.org/web/20160304031242/http://www.atmos.washington.edu/academics/classes/2011Q2/558/IsaksenGB2011.pdf|archive-date=4 March 2016|url-status=dead}}</ref> | |||
| ===Global warming potential (GWP) and {{CO2}} equivalents=== | |||
| ===Atmospheric lifetime=== | |||
| {{excerpt|Global warming potential}} | |||
| Aside from ], which has a ] of about nine days,<ref>{{cite web |url=http://www.eso.org/gen-fac/pubs/astclim/espas/pwv/mockler.html |title=AGU Water Vapor in the Climate System |publisher=Eso.org |date=27 April 1995 |access-date=2011-09-11 |archive-date=15 February 2013 |archive-url=https://www.webcitation.org/6ERWeyWkC?url=http://www.eso.org/gen-fac/pubs/astclim/espas/pwv/mockler.html |url-status=live }}</ref> major greenhouse gases are well mixed and take many years to leave the atmosphere.<ref name="betts">{{cite book|url=http://www.grida.no/publications/other/ipcc%5Ftar/?src=/climate/ipcc_tar/wg1/218.htm|contribution=6.3 Well-mixed Greenhouse Gases|title=Chapter 6 Radiative Forcing of Climate Change|series=Working Group I: The Scientific Basis IPCC Third Assessment Report – Climate Change 2001|publisher=UNEP/GRID-Arendal – Publications|year=2001|author=Betts|access-date=2010-10-16|url-status=dead|archive-url=https://web.archive.org/web/20110629043240/http://www.grida.no/publications/other/ipcc_tar/?src=%2Fclimate%2Fipcc_tar%2Fwg1%2F218.htm|archive-date=29 June 2011}}</ref> Although it is not easy to know with precision how long it takes greenhouse gases to leave the atmosphere, there are estimates for the principal greenhouse gases. | |||
| Jacob (1999)<ref name=JacobDJ1999/> defines the lifetime <math>\tau</math> of an atmospheric ] X in a one-] as the average time that a molecule of X remains in the box. Mathematically <math>\tau</math> can | |||
| be defined as the ratio of the mass <math>m</math> (in kg) of X in the box to its removal rate, which is the sum of the flow of X out of the box | |||
| (<math>F_{out}</math>), | |||
| chemical loss of X | |||
| (<math>L</math>), | |||
| and ] of X | |||
| (<math>D</math>) | |||
| (all in kg/s): | |||
| <math>\tau = \frac{m}{F_{out}+L+D}</math>.<ref name=JacobDJ1999>{{cite book |last= Jacob |first= Daniel |title= Introduction to atmospheric chemistry |publisher= ] |year= 1999 |url= http://www-as.harvard.edu/people/faculty/djj/book/ |isbn= 978-0691001852 |pages= 25–26 |url-status=dead |archive-url= https://web.archive.org/web/20110902182732/http://www-as.harvard.edu/people/faculty/djj/book/ |archive-date= 2 September 2011 |df= dmy-all }}</ref> | |||
| If input of this gas into the box ceased, then after time <math>\tau</math>, its concentration would decrease by about 63%. | |||
| == List of all greenhouse gases == | |||
| The atmospheric lifetime of a species therefore measures the time required to restore equilibrium following a sudden increase or decrease in its concentration in the atmosphere. Individual atoms or molecules may be lost or deposited to sinks such as the soil, the oceans and other waters, or vegetation and other biological systems, reducing the excess to background concentrations. The average time taken to achieve this is the ]. | |||
| ] Global Monitoring Laboratory/Earth System Research Laboratories}}</ref>]] | |||
| The contribution of each gas to the enhanced greenhouse effect is determined by the characteristics of that gas, its abundance, and any indirect effects it may cause. For example, the direct radiative effect of a mass of methane is about 84 times stronger than the same mass of carbon dioxide over a 20-year time frame.<ref name="TableOfWarmingPotentials5">{{cite book |title=Intergovernmental Panel on Climate Change Fifth Assessment Report |page=731 |chapter=Appendix 8.A |access-date=6 November 2017 |chapter-url=http://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WG1AR5_Chapter08_FINAL.pdf |archive-url=https://web.archive.org/web/20171013100414/http://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WG1AR5_Chapter08_FINAL.pdf |archive-date=13 October 2017 |url-status=live}}</ref> Since the 1980s, greenhouse gas forcing contributions (relative to year 1750) are also estimated with high accuracy using IPCC-recommended expressions derived from ]s.<ref name="butmon2">{{Cite web |author=Butler J. and Montzka S. |year=2020 |title=The NOAA Annual Greenhouse Gas Index (AGGI) |url=https://www.esrl.noaa.gov/gmd/aggi/aggi.html |publisher=] Global Monitoring Laboratory/Earth System Research Laboratories}}</ref> | |||
| The concentration of a greenhouse gas is typically measured in ''parts per million'' (ppm) or ''parts per billion'' (ppb) by volume. A {{CO2}} concentration of 420 ppm means that 420 out of every million air molecules is a {{CO2}} molecule. The first 30 ppm increase in {{CO2}} concentrations took place in about 200 years, from the start of the ] to 1958; however the next 90 ppm increase took place within 56 years, from 1958 to 2014.<ref name="NOAA2022" /><ref name="Kibert2016">{{cite book |author=Charles J. Kibert |title=Sustainable Construction: Green Building Design and Delivery |publisher=Wiley |year=2016 |isbn=978-1119055327 |chapter=Background |chapter-url={{google books |plainurl=y |id=qv3iCwAAQBAJ|page=698}}}}</ref><ref>{{cite web |year=2005 |title=Full Mauna Loa CO<sub>2</sub> record |url=https://www.esrl.noaa.gov/gmd/ccgg/trends/full.html |url-status=live |archive-url=https://web.archive.org/web/20170428033710/https://www.esrl.noaa.gov/gmd/ccgg/trends/full.html |archive-date=28 April 2017 |access-date=6 May 2017 |publisher=Earth System Research Laboratories}}</ref> Similarly, the average annual increase in the 1960s was only 37% of what it was in 2000 through 2007.<ref>{{cite web |last=Tans |first=Pieter |date=3 May 2008 |title=Annual CO<sub>2</sub> mole fraction increase (ppm) for 1959–2007 |url=ftp://ftp.cmdl.noaa.gov/ccg/co2/trends/co2_gr_mlo.txt |publisher=National Oceanic and Atmospheric Administration Earth System Research Laboratories, Global Monitoring Division}} {{cite web |title=additional details |url=http://www.esrl.noaa.gov/gmd/ccgg/trends/ |url-status=live |archive-url=https://web.archive.org/web/20181225142754/https://www.esrl.noaa.gov/gmd/ccgg/trends/ |archive-date=25 December 2018 |access-date=15 May 2008}}; see also {{cite journal |last1=Masarie |first1=K.A. |last2=Tans |first2=P.P. |year=1995 |title=Extension and integration of atmospheric carbon dioxide data into a globally consistent measurement record |url=https://zenodo.org/record/1231364 |url-status=live |journal=J. Geophys. Res. |volume=100 |issue=D6 |pages=11593–610 |bibcode=1995JGR...10011593M |doi=10.1029/95JD00859 |archive-url=https://web.archive.org/web/20210308193900/https://zenodo.org/record/1231364 |archive-date=8 March 2021 |access-date=26 July 2019}}</ref> | |||
| ] has a variable atmospheric lifetime, and cannot be specified precisely.<ref>{{cite web |url= http://www.realclimate.org/index.php/archives/2005/03/how-long-will-global-warming-last |title= How long will global warming last? |date= 15 March 2005 |publisher= RealClimate |access-date= 2012-06-12 |archive-date= 4 March 2021 |archive-url= https://web.archive.org/web/20210304213944/http://www.realclimate.org/index.php/archives/2005/03/how-long-will-global-warming-last/ |url-status= live }}</ref><ref name="TableOfWarmingPotentials5" /> Although more than half of the CO<sub>2</sub> emitted is removed from the atmosphere within a century, some fraction (about 20%) of emitted CO<sub>2</sub> remains in the atmosphere for many thousands to hundreds of thousands of years.<ref>{{Harvnb|IPCC AR6 WG1 Ch1|2021|loc=Annex VII - Glossary}}</ref><ref>{{cite book |contribution= Frequently Asked Question 10.3: If emissions of greenhouse gases are reduced, how quickly do their concentrations in the atmosphere decrease? |title= Global Climate Projections |contribution-url= http://www.ipcc.ch/publications_and_data/ar4/wg1/en/faq-10-3.html |access-date= 2011-06-01 |display-editors= etal |archive-date= 24 December 2011 |archive-url= https://web.archive.org/web/20111224051815/http://www.ipcc.ch/publications_and_data/ar4/wg1/en/faq-10-3.html |url-status= dead }} in {{harvp|IPCC AR4 WG1|2007}}</ref><ref name="carbon_lifetime1">See also: {{Cite journal |url= http://geosci.uchicago.edu/~archer/reprints/archer.2005.fate_co2.pdf |first= David |last= Archer |title= Fate of fossil fuel CO<sub>2</sub> in geologic time |journal= ] |volume= 110 |issue= C9 |pages= C09S05.1–6 |year= 2005 |doi= 10.1029/2004JC002625 |access-date= 27 July 2007 |bibcode= 2005JGRC..11009S05A |doi-access= free |archive-date= 19 December 2005 |archive-url= https://web.archive.org/web/20051219075117/http://geosci.uchicago.edu/~archer/reprints/archer.2005.fate_co2.pdf |url-status= live }}</ref><ref name="carbon_lifetime2">See also: {{Cite journal |first1= Ken |last1= Caldeira |first2= Michael E. |last2= Wickett |url= http://www.ipsl.jussieu.fr/~jomce/acidification/paper/Caldeira_Wickett_2005_JGR.pdf |title= Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean |journal= ] |volume= 110 |issue= C9 |pages= C09S04.1–12 |year= 2005 |doi= 10.1029/2004JC002671 |access-date= 27 July 2007 |archive-url= https://web.archive.org/web/20070810202611/http://www.ipsl.jussieu.fr/~jomce/acidification/paper/Caldeira_Wickett_2005_JGR.pdf |archive-date= 10 August 2007 |bibcode= 2005JGRC..11009S04C|doi-access= free }}</ref> Similar issues apply to other greenhouse gases, many of which have longer mean lifetimes than CO<sub>2</sub>, e.g. ] has a mean atmospheric lifetime of 121 years.<ref name=TableOfWarmingPotentials5/> | |||
| Many observations are available online in a variety of ]. The table below shows the most influential long-lived, well-mixed greenhouse gases, along with their tropospheric concentrations and direct ]s, as identified by the ] (IPCC).<ref name="ar5">{{cite book |url=https://www.ipcc.ch/report/ar5/wg1/ |title=AR5 Climate Change 2013: The Physical Science Basis |contribution=Chapter 8}}</ref> Abundances of these ]es are regularly measured by atmospheric scientists from samples collected throughout the world.<ref>{{cite web |title=Global Monitoring Laboratory |url=https://www.esrl.noaa.gov/gmd/ |access-date=2020-12-11 |publisher=NOAA Earth System Research Laboratories}}</ref><ref>{{cite web |title=World Data Centre for Greenhouse Gases |url=https://gaw.kishou.go.jp/ |access-date=2020-12-11 |publisher=World Meteorological Organization Global Atmosphere Watch Programme and Japan Meteorological Agency}}</ref><ref>{{cite web |title=Advanced Global Atmospheric Gas Experiment |url=https://agage.mit.edu/ |access-date=2020-12-11 |publisher=Massachusetts Institute of Technology}}</ref> It excludes water vapor because changes in its concentrations are calculated as a ] indirectly caused by changes in other greenhouse gases, as well as ozone, whose concentrations are only modified indirectly by various ] that cause ]. Some short-lived gases (e.g. ], ]) and ]s (e.g. ] or ]) are also excluded because of limited role and strong variation, along with minor refrigerants and other ] gases, which have been mass-produced in smaller quantities than those in the table.<ref name="ar5" />{{rp|731–738}} and Annex III of the 2021 IPCC WG1 Report<ref name="ar6">{{citation |title=Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change |date=2021-08-09 |editor=Dentener F. J. |url=https://www.ipcc.ch/report/ar6/wg1/#FullReport |section=Annex III: Tables of historical and projected well-mixed greenhouse gas mixing ratios and effective radiative forcing of all climate forcers |section-url=https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_AnnexIII.pdf |publisher=Cambridge University Press |editor2=B. Hall |editor3=C. Smith}}</ref>{{rp|4–9}} | |||
| ===Radiative forcing and annual greenhouse gas index=== | |||
| ] Global Monitoring Laboratory/Earth System Research Laboratories |author=Butler J. and Montzka S. |year=2020 |access-date=5 September 2020 |archive-date=22 September 2013 |archive-url=https://web.archive.org/web/20130922035917/https://www.esrl.noaa.gov/gmd/aggi/aggi.html |url-status=live }}</ref>]] | |||
| Earth absorbs some of the radiant energy received from the sun, reflects some of it as light and reflects or radiates the rest back to space as ]. A planet's surface temperature depends on this balance between incoming and outgoing energy. When ] is shifted, its surface becomes warmer or cooler, leading to a variety of changes in global climate.<ref name="epa ggas">{{cite web| year = 2016| publisher = U.S. Environmental Protection Agency (EPA)| title = Climate Change Indicators in the United States - Greenhouse Gases| url = https://www.epa.gov/climate-indicators/greenhouse-gases| access-date = 5 September 2020| archive-date = 27 August 2016| archive-url = https://web.archive.org/web/20160827230238/https://www.epa.gov/climate-indicators/greenhouse-gases| url-status = live}}.</ref> | |||
| {| class="wikitable sortable" style="text-align:center" | |||
| A number of natural and man-made mechanisms can affect the global energy balance and force changes in Earth's climate. Greenhouse gases are one such mechanism. Greenhouse gases absorb and emit some of the outgoing energy radiated from Earth's surface, causing that heat to be retained in the lower atmosphere.<ref name="epa ggas"/> As ], some greenhouse gases remain in the atmosphere for decades or even centuries, and therefore can affect Earth's energy balance over a long period. ] quantifies (in Watts per square meter) the effect of factors that influence Earth's energy balance; including changes in the concentrations of greenhouse gases. Positive radiative forcing leads to warming by increasing the net incoming energy, whereas negative radiative forcing leads to cooling.<ref name="epa cforce">{{cite web| year = 2016| publisher = U.S. Environmental Protection Agency (EPA)| title = Climate Change Indicators in the United States - Climate Forcing| url = https://www.epa.gov/climate-indicators/climate-change-indicators-climate-forcing| access-date = 5 September 2020| archive-date = 27 August 2016| archive-url = https://web.archive.org/web/20160827223551/https://www.epa.gov/climate-indicators/climate-change-indicators-climate-forcing| url-status = live}} {{Webarchive|url=https://web.archive.org/web/20200921073951/https://www.epa.gov/sites/production/files/2016-08/documents/print_climate-forcing-2016.pdf |date=21 September 2020 }}</ref> | |||
| |+IPCC list of greenhouse gases with lifetime, 100-year ], concentrations in the ] and radiative forcings. The abbreviations TAR, AR4, AR5 and AR6 refer to the different IPCC reports over the years. The baseline is pre-industrialization (year 1750). | |||
| ! rowspan="2" |Species | |||
| ! rowspan="2" |] | |||
| (years) | |||
| <ref name="ar5" />{{rp|731}} | |||
| ! rowspan="2" |100-yr | |||
| ] | |||
| <ref name="ar5" />{{rp|731}} | |||
| ! colspan="5" |Mole Fraction <sup>a</sup> + ] {{ref label|ERF|B|B}} | |||
| ! rowspan="2" |Concentrations | |||
| over time<ref name="hats">{{cite web |title=Long-term global trends of atmospheric trace gases |url=https://www.esrl.noaa.gov/gmd/hats/data.html |access-date=2021-02-11 |publisher=NOAA Earth System Research Laboratories}}</ref><ref name="agage">{{cite web |title=AGAGE Data and Figures |url=https://agage.mit.edu/data/agage-data |access-date=2021-02-11 |publisher=Massachusetts Institute of Technology}}</ref> | |||
| up to year 2022 | |||
| The Annual Greenhouse Gas Index (AGGI) is defined by atmospheric scientists at ] as the ratio of total direct radiative forcing due to long-lived and well-mixed greenhouse gases for any year for which adequate global measurements exist, to that present in year 1990.<ref name="butmon" /><ref>{{cite web |url=https://www.climate.gov/news-features/understanding-climate/climate-change-annual-greenhouse-gas-index |title=Climate change: annual greenhouse gas index |publisher=] Climate.gov science news & Information for a climate smart nation |author=LuAnn Dahlman |date=14 August 2020 |access-date=5 September 2020 |archive-date=16 August 2013 |archive-url=https://web.archive.org/web/20130816013542/https://www.climate.gov/news-features/understanding-climate/climate-change-annual-greenhouse-gas-index |url-status=live }}</ref> These radiative forcing levels are relative to those present in year 1750 (i.e. prior to the start of the ]). 1990 is chosen because it is the baseline year for the ], and is the publication year of the ]. As such, NOAA states that the AGGI "measures the commitment that (global) society has already made to living in a changing climate. It is based on the highest quality atmospheric observations from sites around the world. Its uncertainty is very low."<ref>{{Cite web |url=https://www.esrl.noaa.gov/gmd/aggi/ |title=The NOAA Annual Greenhouse Gas Index (AGGI) - An Introduction |publisher=] Global Monitoring Laboratory/Earth System Research Laboratories |access-date=5 September 2020 |archive-date=27 November 2020 |archive-url=https://web.archive.org/web/20201127013113/https://www.esrl.noaa.gov/gmd/aggi/ |url-status=live }}</ref> | |||
| ===Global warming potential=== | |||
| The ] (GWP) depends on both the efficiency of the molecule as a greenhouse gas and its atmospheric lifetime. GWP is measured relative to the same '''mass''' of {{CO2}} and evaluated for a specific timescale. Thus, if a gas has a high (positive) ] but also a short lifetime, it will have a large GWP on a 20-year scale but a small one on a 100-year scale. Conversely, if a molecule has a longer atmospheric lifetime than {{CO2}} its GWP will increase when the timescale is considered. Carbon dioxide is defined to have a GWP of 1 over all time periods. | |||
| ] has an atmospheric lifetime of 12 ± 2 years.<ref name="IPCC_AR6_Table7.15">{{Harvnb|IPCC AR6 WG1 Ch1|2021. Table 7.15.}}</ref> The ] lists the GWP as 83 over a time scale of 20 years, 30 over 100 years and 10 over 500 years.<ref name="IPCC_AR6_Table7.15">{{Harvnb|IPCC AR6 WG1 Ch1|2021. Table 7.15.}}</ref> A 2014 analysis, however, states that although methane's initial impact is about 100 times greater than that of {{CO2}}, because of the shorter atmospheric lifetime, after six or seven decades, the impact of the two gases is about equal, and from then on methane's relative role continues to decline.<ref>{{Cite news|url=http://newsoffice.mit.edu/2014/how-count-methane-emissions-0425|title=How to count methane emissions|work=MIT News|access-date=2018-08-20|first=David L.|last=Chandler|archive-date=16 January 2015|archive-url=https://web.archive.org/web/20150116035714/http://newsoffice.mit.edu/2014/how-count-methane-emissions-0425|url-status=live}} Referenced paper is {{cite journal |first1=Jessika |last1=Trancik |first2=Morgan |last2=Edwards |url=https://www.see.ed.ac.uk/~shs/Methane/Methane%20and%20time.pdf |title=Climate impacts of energy technologies depend on emissions timing |archive-url=https://web.archive.org/web/20150116044523/https://www.see.ed.ac.uk/~shs/Methane/Methane%20and%20time.pdf |archive-date=16 January 2015 |journal=Nature Climate Change |volume=4 |date=25 April 2014 |issue=5 |page=347 |doi=10.1038/nclimate2204 |bibcode=2014NatCC...4..347E |hdl=1721.1/96138 |access-date=15 January 2015 |hdl-access=free }}</ref> The decrease in GWP at longer times is because ] decomposes to water and {{CO2}} through chemical reactions in the atmosphere. | |||
| Examples of the atmospheric lifetime and ] relative to {{CO2}} for several greenhouse gases are given in the following table: | |||
| {| class="wikitable" style="text-align: right" | |||
| |+ Atmospheric lifetime and ] relative to {{CO2}} at different time horizon for various greenhouse gases | |||
| |- | |- | ||
| !Baseline | |||
| ! rowspan="2" style="text-align:left;" | Gas name | |||
| Year 1750 | |||
| ! rowspan="2" | Chemical <br /> formula !! rowspan="2" | Lifetime <br /> (years)<ref name="TableOfWarmingPotentials5">{{cite book |title=Intergovernmental Panel on Climate Change Fifth Assessment Report |chapter-url=http://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WG1AR5_Chapter08_FINAL.pdf |chapter=Appendix 8.A |page=731 |access-date=6 November 2017 |archive-date=13 October 2017 |archive-url=https://web.archive.org/web/20171013100414/http://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WG1AR5_Chapter08_FINAL.pdf |url-status=live }}</ref>||rowspan="2"| Radiative Efficiency <br /> (Wm{{sup|−2}}ppb{{sup|−1}}, molar basis)<ref name="TableOfWarmingPotentials5"/> | |||
| !]<ref name="tar">{{cite book |url=https://www.ipcc.ch/report/ar3/wg1/ |title=TAR Climate Change 2001: The Scientific Basis |page=358 |contribution=Chapter 6}}</ref> | |||
| ! colspan="3" | Global warming potential (GWP) for given time horizon | |||
| Year 1998 | |||
| !]<ref name="ar4">{{cite book |url=http://www.ipcc.ch/ipccreports/ar4-wg1.htm |title=AR4 Climate Change 2007: The Physical Science Basis |page=141 |contribution=Chapter 2}}</ref> | |||
| Year 2005 | |||
| !]<ref name="ar5" />{{rp|678}} | |||
| Year 2011 | |||
| !]<ref name="ar6" />{{rp|4–9}} | |||
| Year 2019 | |||
| |- | |- | ||
| |] | |||
| ! 20-yr<ref name=TableOfWarmingPotentials5/> !! 100-yr<ref name=TableOfWarmingPotentials5/>!! 500-yr<ref name="TableOfWarmingPotentials">{{cite book |title= IPCC Fourth Assessment Report |chapter-url= http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter2.pdf |chapter= Table 2.14 |page= 212 |access-date= 16 December 2008 |archive-date= 15 December 2007 |archive-url= https://web.archive.org/web/20071215200559/http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter2.pdf |url-status= live }}</ref> | |||
| |{{ref label|COL|A|A}} | |||
| |1 | |||
| |278 | |||
| |365 '''(1.46)''' | |||
| |379 '''(1.66)''' | |||
| |391 '''(1.82)''' | |||
| |410 '''(2.16)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | style="text-align:left;"| ] | |||
| |12.4 | |||
| | style="text-align:center;"| {{CO2}}|| <sup>(A)</sup>||{{val|1.37e-5}} ||1 || 1 || 1 | |||
| |28 | |||
| |700 | |||
| |1,745 '''(0.48)''' | |||
| |1,774 '''(0.48)''' | |||
| |1,801 '''(0.48)''' | |||
| |1866 '''(0.54)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | style="text-align:left;"| ] | |||
| |121 | |||
| | style="text-align:center;"| {{chem|CH|4}}|| 12 ||{{val|3.63e-4}} || 84 || 28 || 7.6 | |||
| |265 | |||
| |- id="N2O" | |||
| |270 | |||
| | style="text-align:left;"| ] | |||
| |314 '''(0.15)''' | |||
| | style="text-align:center;"| {{chem|N|2|O}} || 121 ||{{val|3e-3}} || 264 || 265 || 153 | |||
| |319 '''(0.16)''' | |||
| |324 '''(0.17)''' | |||
| |332 '''(0.21)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | style="text-align:left;"| ] | |||
| |45 | |||
| | style="text-align:center;"| {{chem|CCl|2|F|2}}|| 100 ||{{val|0.32}} || 10 800 || 10 200 || 5 200 | |||
| |4,660 | |||
| |0 | |||
| |268 '''(0.07)''' | |||
| |251 '''(0.063)''' | |||
| |238 '''(0.062)''' | |||
| |226 '''(0.066)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | style="text-align:left;"| ] | |||
| |100 | |||
| | style="text-align:center;"| {{chem|CHClF|2}}|| 12 ||{{val|0.21}}|| 5 280 || 1 760 || 549 | |||
| |10,200 | |||
| |0 | |||
| |533 '''(0.17)''' | |||
| |538 '''(0.17)''' | |||
| |528 '''(0.17)''' | |||
| |503 '''(0.18)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | style="text-align:left;"| ] | |||
| |640 | |||
| | style="text-align:center;"| {{chem|CF|4}}|| 50 000 ||{{val|0.09}}|| 4 880 || 6 630 || 11 200 | |||
| |13,900 | |||
| |0 | |||
| |4 '''(0.001)''' | |||
| | – | |||
| |2.7 '''(0.0007)''' | |||
| |3.28 '''(0.0009)''' | |||
| | | |||
| |- | |- | ||
| |] | |||
| | style="text-align:left;"| ] | |||
| |85 | |||
| | style="text-align:center;"| {{chem|C|2|F|6}}|| 10 000||{{val|0.25}}|| 8 210 || 11 100 || 18 200 | |||
| |6,490 | |||
| |0 | |||
| |84 '''(0.03)''' | |||
| |79 '''(0.024)''' | |||
| |74 '''(0.022)''' | |||
| |70 '''(0.021)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | style="text-align:left;"| ] | |||
| |190 | |||
| | style="text-align:center;"| {{chem|SF|6}}|| 3 200 ||{{val|0.57}}|| 17 500 || 23 500 || 32 600 | |||
| |7,710 | |||
| |0 | |||
| |15 '''(0.005)''' | |||
| | – | |||
| | – | |||
| |16 '''(0.005)''' | |||
| | | |||
| |- | |- | ||
| |] | |||
| | style="text-align:left;"| ] | |||
| |1,020 | |||
| | style="text-align:center;"| {{chem|NF|3}}|| 500 ||{{val|0.20}}|| 12 800 || 16 100 || 20 700 | |||
| |5,860 | |||
| |0 | |||
| |7 '''(0.001)''' | |||
| | – | |||
| |8.37 '''(0.0017)''' | |||
| |8.67 '''(0.0021)''' | |||
| | | |||
| |- | |- | ||
| |] | |||
| ! colspan="7" |<small><sup>(A)</sup> No single lifetime for atmospheric {{CO2}} can be given.</small> | |||
| |11.9 | |||
| |} | |||
| |5,280 | |||
| |0 | |||
| The use of ] (except some essential uses) has been phased out due to its ] properties.<ref>{{citation | |||
| |132 '''(0.03)''' | |||
| |url=http://www.norden.org/en/publications/publications/2003-516/ | |||
| |169 '''(0.033)''' | |||
| |title=Use of ozone depleting substances in laboratories | |||
| |213 '''(0.0447)''' | |||
| |first1=Miska | |||
| |247 '''(0.0528)''' | |||
| |last1=Vaara | |||
| |] | |||
| |year=2003 | |||
| |page=170 | |||
| |isbn=978-9289308847 | |||
| |publisher=TemaNord | |||
| |url-status=dead | |||
| |archive-url=https://web.archive.org/web/20110806001547/http://www.norden.org/en/publications/publications/2003-516/ | |||
| |archive-date=6 August 2011 | |||
| }}</ref> The phasing-out of less active ] will be completed in 2030.<ref>]</ref> | |||
| {{multiple image |caption_align=center |align=center |width= |direction=horizontal | |||
| |image1=M15-162b-EarthAtmosphere-CarbonDioxide-FutureRoleInGlobalWarming-Simulation-20151109.jpg | |||
| |caption1=] in ]'s ] if ''half'' of ]<ref name="NYT-20151110">{{cite news |last=St. Fleur |first=Nicholas |title=Atmospheric Greenhouse Gas Levels Hit Record, Report Says |url=https://www.nytimes.com/2015/11/11/science/atmospheric-greenhouse-gas-levels-hit-record-report-says.html |date=10 November 2015 |work=] |access-date=11 November 2015 |archive-date=22 April 2019 |archive-url=https://web.archive.org/web/20190422090414/https://www.nytimes.com/2015/11/11/science/atmospheric-greenhouse-gas-levels-hit-record-report-says.html |url-status=live }}</ref><ref name="AP-20151109">{{cite news |last=Ritter |first=Karl |title=UK: In 1st, global temps average could be 1 degree C higher |url=http://apnews.excite.com/article/20151109/climate_countdown-greenhouse_gases-d8a21f0397.html |date=9 November 2015 |work=] |access-date=11 November 2015 |archive-date=17 November 2015 |archive-url=https://web.archive.org/web/20151117021206/http://apnews.excite.com/article/20151109/climate_countdown-greenhouse_gases-d8a21f0397.html |url-status=live }}</ref> are ''not'' absorbed.<br />(] ]; 9 November 2015) | |||
| |width1=315 | |||
| }} | |||
| ==Natural and anthropogenic sources== | |||
| ] levels as measured in the atmosphere and reflected in ]s. Bottom: The amount of net carbon increase in the atmosphere, compared to carbon emissions from burning ].]] | |||
| <!-- File was deleted from Wikimedia Commons - 8 August 2020 ]. Changes are measured in ]s of carbon per year (GtC/y). Canadell et al. (2007) estimated the growth rate of global average atmospheric CO<sub>2</sub> for 2000–2006 as 1.93 parts-per-million per year (4.1 ]s of carbon per year).<ref> | |||
| {{citation | |||
| | author=Canadell, J.G. | |||
| | title=Contributions to Accelerating Atmospheric CO2 Growth from Economic Activity, Carbon Intensity, and Efficiency of Natural Sinks (Results and Discussion: Growth in Atmospheric CO<sub>2</sub>) | |||
| | date=20 November 2007 | |||
| | journal=Proceedings of the National Academy of Sciences of the United States of America | |||
| | volume=104 | |||
| | issue=47 | |||
| | pages=18866–70 | |||
| | doi=10.1073/pnas.0702737104 | |||
| | pmid=17962418 | |||
| | pmc=2141868| bibcode = 2007PNAS..10418866C |display-authors=etal}} | |||
| </ref>]] --> | |||
| Aside from purely human-produced synthetic halocarbons, most greenhouse gases have both natural and human-caused sources. During the pre-industrial ], concentrations of existing gases were roughly constant, because the large natural sources and sinks roughly balanced. In the industrial era, human activities have added greenhouse gases to the atmosphere, mainly through the burning of fossil fuels and clearing of forests.<ref>{{cite web |url= http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter1.pdf |title= Historical Overview of Climate Change Science – FAQ 1.3 Figure 1 |page= 116 |access-date= 25 April 2008 |archive-date= 26 November 2018 |archive-url= https://web.archive.org/web/20181126204443/http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter1.pdf |url-status= live }} in {{harvp|IPCC AR4 WG1|2007}}</ref><ref>{{cite web |url=https://ipcc.ch/pdf/special-reports/spm/sres-en.pdf |title=Chapter 3, IPCC Special Report on Emissions Scenarios, 2000 |publisher=Intergovernmental Panel on Climate Change |year=2000 |access-date=2010-10-16 |archive-date=20 August 2018 |archive-url=https://web.archive.org/web/20180820085208/http://www.ipcc.ch/pdf/special-reports/spm/sres-en.pdf |url-status=live }}</ref> | |||
| The 2021 ] noted that "From a physical science perspective, limiting human-induced global warming to a specific level requires limiting cumulative CO2 emissions, reaching at least net zero CO2 emissions, along with strong reductions in other greenhouse gas emissions. Strong, rapid and sustained reductions in CH4 emissions would also limit the warming effect resulting from declining aerosol pollution and would improve air quality."<ref>{{Cite web|title=Sixth Assessment Report|url=https://www.ipcc.ch/report/ar6/wg1/#SPM|access-date=2021-12-18|website=www.ipcc.ch|language=en}}</ref> | |||
| ''Abbreviations used in the two tables below: ppm = ]; ppb = parts-per-billion; ppt = parts-per-trillion; W/m<sup>2</sup> = ]s per ]'' | |||
| {| class="wikitable" | |||
| |+ Current greenhouse gas concentrations<ref name="blasing ghg concentrations">{{harvp|Blasing|2013}}</ref> | |||
| ! Gas | |||
| ! Pre-1750<br />]<br />concentration<ref name="blasing pre 1750 ghg conc"> | |||
| {{citation | url=http://www.grida.no/climate/ipcc_tar/wg1/130.htm | contribution=Table 4.1 | title=Atmospheric Chemistry and Greenhouse Gases | author=Ehhalt, D. | display-authors=etal | url-status=dead | archive-url=https://web.archive.org/web/20130103151645/http://www.grida.no/climate/ipcc_tar/wg1/130.htm | archive-date=3 January 2013 | df=dmy-all }}, in {{harvp|IPCC TAR WG1|2001|pp=244–45}}. Referred to by: {{harvp|Blasing|2013}}. Based on {{harvp|Blasing|2013}}: Pre-1750 concentrations of CH4,N2O and current concentrations of O3, are taken from Table 4.1 (a) of the IPCC Intergovernmental Panel on Climate Change, 2001. Following the convention of IPCC (2001), inferred global-scale trace-gas concentrations from prior to 1750 are assumed to be practically uninfluenced by human activities such as increasingly specialized ], ], and combustion of fossil fuels. Preindustrial concentrations of industrially manufactured compounds are given as zero. The short atmospheric lifetime of ozone (hours-days) together with the spatial variability of its sources precludes a globally or vertically homogeneous distribution, so that a fractional unit such as parts per billion would not apply over a range of altitudes or geographical locations. Therefore a different unit is used to integrate the varying concentrations of ozone in the vertical dimension over a unit area, and the results can then be averaged globally. This unit is called a ] (D.U.), after G.M.B. Dobson, one of the first investigators of atmospheric ozone. A Dobson unit is the amount of ozone in a column that, unmixed with the rest of the atmosphere, would be 10 micrometers thick at standard temperature and pressure. | |||
| </ref> | |||
| !Recent<br />]<br />concentration<ref>Because atmospheric concentrations of most gases tend to vary systematically over the course of a year, figures given represent averages over a 12-month period for all gases except ozone (O3), for which a current global value has been estimated (IPCC, 2001, Table 4.1a). {{CO2}} averages for year 2012 are taken from the National Oceanic and Atmospheric Administration, Earth System Research Laboratory, web site: www.esrl.noaa.gov/gmd/ccgg/trends maintained by Dr. Pieter Tans. For other chemical species, the values given are averages for 2011. These data are found on the CDIAC AGAGE web site: http://cdiac.ornl.gov/ndps/alegage.html {{Webarchive|url=https://web.archive.org/web/20130121031710/http://cdiac.ornl.gov/ndps/alegage.html |date=21 January 2013 }} or the AGAGE home page: http://agage.eas.gatech.edu {{Webarchive|url=https://web.archive.org/web/20150107053614/http://agage.eas.gatech.edu/ |date=7 January 2015 }}.</ref> | |||
| !Absolute increase<br />since 1750 | |||
| !Percentage<br />increase<br />since 1750 | |||
| !Increased<br />radiative forcing<br />(W/m<sup>2</sup>)<ref name="forster table of ghg conc"> | |||
| {{citation | url=http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-3.html | contribution=Table 2.1 | title=Changes in Atmospheric Constituents and in Radiative Forcing | author=Forster, P. | display-authors=etal | access-date=30 October 2012 | archive-url=https://web.archive.org/web/20121012045712/http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-3.html | archive-date=12 October 2012 | url-status=dead }}, in {{harvp|IPCC AR4 WG1|2007|p=141}}. Referred to by: {{harvp|Blasing|2013}}</ref> | |||
| |- | |- | ||
| |] | |||
| | ] ({{CO2}}) || 280 ]<ref> | |||
| |9.2 | |||
| {{cite book | |||
| |2,550 | |||
| |url=http://www.grida.no/climate/ipcc_tar/wg1/096.htm | |||
| |0 | |||
| |contribution=Executive summary | |||
| |10 '''(0.001)''' | |||
| |title=The Carbon Cycle and Atmospheric Carbon Dioxide | |||
| |18 '''(0.0025)''' | |||
| |author=Prentice, I.C. | |||
| |21.4 '''(0.0034)''' | |||
| |display-authors=etal | |||
| |24.4 '''(0.0039)''' | |||
| |url-status=dead | |||
| |] | |||
| |archive-url=https://web.archive.org/web/20091207172617/http://www.grida.no/climate/ipcc_tar/wg1/096.htm | |||
| |archive-date=7 December 2009 | |||
| }}, in {{harvp|IPCC TAR WG1|2001|p=185}}. Referred to by: {{harvp|Blasing|2013}} | |||
| </ref>|| 411 ppm<ref name=WMO_20201123>{{cite web |title=Carbon dioxide levels continue at record levels, despite COVID-19 lockdown |url=https://public.wmo.int/en/media/press-release/carbon-dioxide-levels-continue-record-levels-despite-covid-19-lockdown |website=WMO.int |publisher=World Meteorological Organization |archive-url=https://web.archive.org/web/20201201072451/https://public.wmo.int/en/media/press-release/carbon-dioxide-levels-continue-record-levels-despite-covid-19-lockdown |archive-date=1 December 2020 |date=23 November 2020 |url-status=live }}</ref> || 131 ppm || 47% || 2.05<ref>{{harvp|IPCC AR4 WG1|2007|p=140|ps=:"The simple formulae ... in Ramaswamy et al. (2001) are still valid. and give an RF of +3.7 W m–2 for a doubling in the CO2 mixing ratio. ... RF increases logarithmically with mixing ratio"}} Calculation: ln(new ppm/old ppm)/ln(2)*3.7</ref> | |||
| |- | |- | ||
| |] | |||
| | ] ({{chem|CH|4}}) || 700 ppb<ref>ppb = parts-per-billion</ref> || 1893 ppb /<ref name="blasing ghg conc notes" >The first value in a cell represents Mace Head, Ireland, a mid-latitude Northern-Hemisphere site, while the second value represents ], ], a mid-latitude Southern-Hemisphere site. "Current" values given for these gases are annual arithmetic averages based on monthly background concentrations for year 2011. The {{chem|SF|6}} values are from the AGAGE gas chromatography – mass spectrometer (gc-ms) Medusa measuring system.</ref><ref>{{cite web |title=Advanced Global Atmospheric Gases Experiment (AGAGE) |url=http://cdiac.ornl.gov/ftp/ale_gage_Agage/ |access-date=30 October 2012 |archive-date=21 January 2013 |archive-url=https://web.archive.org/web/20130121031611/http://cdiac.ornl.gov/ftp/ale_gage_Agage/ |url-status=live }} Data compiled from finer time scales in the {{cite web |title=ALE/GAGE/AGAGE database |url=http://cdiac.ornl.gov/ndps/alegage.html |author1=Prinn |author2=etc |year=2000 |access-date=30 October 2012 |archive-date=21 January 2013 |archive-url=https://web.archive.org/web/20130121031710/http://cdiac.ornl.gov/ndps/alegage.html |url-status=live }}</ref><br />1762 ppb<ref name="blasing ghg conc notes"/> || 1193 ppb /<br />1062 ppb || 170.4% /<br />151.7% || 0.49 | |||
| |17.2 | |||
| |5,020 | |||
| |0 | |||
| |11 '''(0.002)''' | |||
| |15 '''(0.0031)''' | |||
| |21.2 '''(0.0040)''' | |||
| |22.3 '''(0.0043)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ] ({{chem|N|2|O}}) || 270 ppb<ref name="forster table of ghg conc"/><ref>The pre-1750 value for {{chem|N|2|O}} is consistent with ice-core records from 10,000 BCE through 1750 CE: {{citation | chapter-url=http://www.ipcc.ch/publications_and_data/ar4/wg1/en/spmsspm-human-and.html | title=Figure SPM.1 | chapter=Summary for policymakers | publisher=IPCC | access-date=30 October 2012 | archive-date=2 November 2018 | archive-url=https://web.archive.org/web/20181102212113/http://www.ipcc.ch/publications_and_data/ar4/wg1/en/spmsspm-human-and.html | url-status=live }}, in {{harvp|IPCC AR4 WG1|2007|p=3}}. Referred to by: {{harvp|Blasing|2013}}</ref> || 326 ppb /<ref name="blasing ghg conc notes"/><br />324 ppb<ref name="blasing ghg conc notes"/> || 56 ppb /<br />54 ppb || 20.7% /<br />20.0% || 0.17 | |||
| |5 | |||
| |160 | |||
| |0 | |||
| |69 '''(0.004)''' | |||
| |19 '''(0.0011)''' | |||
| |6.32 '''(0.0004)''' | |||
| |1.6 '''(0.0001)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ]<br />] ({{chem|O|3}}) || 237 ppb<ref name="blasing pre 1750 ghg conc"/> || 337 ppb<ref name="blasing pre 1750 ghg conc"/> || 100 ppb || 42% || 0.4<ref>Changes in ] ozone have resulted in a decrease in radiative forcing of 0.05 W/m<sup>2</sup>: {{citation | url=http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-9-3.html | contribution=Table 2.12 | title=Changes in Atmospheric Constituents and in Radiative Forcing | author=Forster, P. | display-authors=etal | access-date=30 October 2012 | archive-url=https://web.archive.org/web/20130128114001/http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-9-3.html | archive-date=28 January 2013 | url-status=dead }}, in {{harvp|IPCC AR4 WG1|2007|p=204}}. Referred to by: {{harvp|Blasing|2013}}</ref> | |||
| | |
|26 | ||
| |1,730 | |||
| |0 | |||
| {| class="wikitable" | |||
| |102 '''(0.01)''' | |||
| |+ Relevant to radiative forcing and/or ]; all of the following have no natural sources and hence zero amounts pre-industrial<ref name="blasing ghg concentrations"/> | |||
| |93 '''(0.012)''' | |||
| ! Gas !! Recent<br />]<br />concentration !! Increased<br />radiative forcing<br />(W/m<sup>2</sup>) | |||
| |85.8 '''(0.0146)''' | |||
| |78 '''(0.0129)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ]<br />(trichlorofluoromethane)<br />({{chem|CCl|3|F}}) || 236 ppt /<br />234 ppt || 0.061 | |||
| |222 | |||
| |12,400 | |||
| |0 | |||
| |14 '''(0.002)''' | |||
| |18 '''(0.0033)''' | |||
| |24 '''(0.0043)''' | |||
| |32.4 '''(0.0062)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ] ({{chem|CCl|2|F|2}}) || 527 ppt /<br />527 ppt || 0.169 | |||
| |5.2 | |||
| |677 | |||
| |0 | |||
| | – | |||
| | – | |||
| |4.92 '''(0.0005)''' | |||
| |20 '''(0.0022)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ] ({{chem|Cl|2|FC-CClF|2}}) || 74 ppt /<br />74 ppt || 0.022 | |||
| |28.2 | |||
| |3,170 | |||
| |0 | |||
| | – | |||
| |3.7 '''(0.0009)''' | |||
| |9.58 '''(0.0022)''' | |||
| |29.4 '''(0.0069)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ] ({{chem|CHClF|2}}) || 231 ppt /<br />210 ppt || 0.046 | |||
| |13.4 | |||
| |1,300 | |||
| |0 | |||
| |7.5 '''(0.001)''' | |||
| |35 '''(0.0055)''' | |||
| |62.7 '''(0.0100)''' | |||
| |107.6 '''(0.018)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ] ({{chem|CH|3|CCl|2|F}}) || 24 ppt /<br />21 ppt || 0.0036 | |||
| |47.1 | |||
| |4,800 | |||
| |0 | |||
| | – | |||
| | – | |||
| |12.0 '''(0.0019)''' | |||
| |24 '''(0.0040)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ] ({{chem|CH|3|CClF|2}}) || 23 ppt /<br />21 ppt || 0.0042 | |||
| |1.5 | |||
| |138 | |||
| |0 | |||
| |0.5 '''(0.0000)''' | |||
| |3.9 '''(0.0004)''' | |||
| |6.4 '''(0.0006)''' | |||
| |7.1 '''(0.0007)''' | |||
| |] | |||
| |- | |- | ||
| |] (PFC-14) | |||
| | ] ({{chem|CBrClF|2}}) || 4.1 ppt /<br />4.0 ppt || 0.0012 | |||
| |50,000 | |||
| |6,630 | |||
| |40 | |||
| |80 '''(0.003)''' | |||
| |74 '''(0.0034)''' | |||
| |79 '''(0.0040)''' | |||
| |85.5 '''(0.0051)''' | |||
| |] | |||
| |- | |- | ||
| |] (PFC-116) | |||
| | ] ({{chem|CBrClF|3}}) || 3.3 ppt /<br />3.3 ppt || 0.001 | |||
| |10,000 | |||
| |11,100 | |||
| |0 | |||
| |3 '''(0.001)''' | |||
| |2.9 '''(0.0008)''' | |||
| |4.16 '''(0.0010)''' | |||
| |4.85 '''(0.0013)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ] ({{chem|CH|2|FCF|3}}) || 75 ppt /<br />64 ppt || 0.0108 | |||
| |3,200 | |||
| |23,500 | |||
| |0 | |||
| |4.2 '''(0.002)''' | |||
| |5.6 '''(0.0029)''' | |||
| |7.28 '''(0.0041)''' | |||
| |9.95 '''(0.0056)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ] ({{chem|CCl|4}}) || 85 ppt /<br />83 ppt || 0.0143 | |||
| |36 | |||
| |4,090 | |||
| |0 | |||
| | – | |||
| | – | |||
| |1.71 '''(0.0003)''' | |||
| |2.5 '''(0.0005)''' | |||
| |] | |||
| |- | |- | ||
| |] | |||
| | ] ({{chem|SF|6}}) || 7.79 ppt /<ref name="cdiac sf6">{{cite web |title={{chem|SF|6}} data from January 2004 |url=http://cdiac.ornl.gov/ftp/ale_gage_Agage/AGAGE/gc-ms-medusa/monthly |access-date=2 January 2013 |archive-date=21 January 2013 |archive-url=https://web.archive.org/web/20130121031702/http://cdiac.ornl.gov/ftp/ale_gage_Agage/AGAGE/gc-ms-medusa/monthly/ |url-status=live }} {{cite news|title=Data from 1995 through 2004|publisher= National Oceanic and Atmospheric Administration (NOAA), Halogenated and other Atmospheric Trace Species (HATS)}} {{cite web |title=Concentrations of {{chem|SF|6}} from 1970 through 1999, obtained from Antarctic firn (consolidated deep snow) air samples |url=http://cdiac.ornl.gov/trends/otheratg/sturges/sturges.html |last=Sturges |first=W.T. |display-authors=et al. |access-date=2 January 2013 |archive-date=21 January 2013 |archive-url=https://web.archive.org/web/20130121031758/http://cdiac.ornl.gov/trends/otheratg/sturges/sturges.html |url-status=live }}</ref><br />7.39 ppt<ref name="cdiac sf6"/> || 0.0043 | |||
| | |
|500 | ||
| |16,100 | |||
| | Other ]s || Varies by<br />substance || collectively<br />0.02 | |||
| | |
|0 | ||
| | – | |||
| | Halocarbons in total || || 0.3574 | |||
| | – | |||
| |0.9 '''(0.0002)''' | |||
| |2.05 '''(0.0004)''' | |||
| |] | |||
| |} | |} | ||
| <sup>a</sup> ]s: μmol/mol = ppm = parts per million (10<sup>6</sup>); nmol/mol = ppb = parts per billion (10<sup>9</sup>); pmol/mol = ppt = parts per trillion (10<sup>12</sup>). | |||
| ] | |||
| ]s provide evidence for greenhouse gas concentration variations over the past 800,000 years (see the ]). Both CO<sub>2</sub> and {{chem|CH|4}} vary between glacial and interglacial phases, and concentrations of these gases correlate strongly with temperature. Direct data does not exist for periods earlier than those represented in the ice core record, a record that indicates CO<sub>2</sub> ]s stayed within a range of 180 ppm to 280 ppm throughout the last 800,000 years, until the increase of the last 250 years. However, various proxies and modeling suggests larger variations in past epochs; 500 million years ago CO<sub>2</sub> levels were likely 10 times higher than now.<ref>]</ref> Indeed, higher CO<sub>2</sub> concentrations are thought to have prevailed throughout most of the ] ], with concentrations four to six times current concentrations during the Mesozoic era, and ten to fifteen times current concentrations during the early Palaeozoic era until the middle of the ] period, about 400 ].<ref name=Berner1994>{{cite journal |last=Berner |first=Robert A. |date=January 1994 |title=GEOCARB II: a revised model of atmospheric CO<sub>2</sub> over Phanerozoic time |url=http://www.neotrucks.com/pdf/01.1994.02berner.pdf |journal=American Journal of Science |volume=294 |issue=1 |pages=56–91 |doi=10.2475/ajs.294.1.56 |bibcode=1994AmJS..294...56B }}{{dead link|date=March 2018 |bot=InternetArchiveBot |fix-attempted=yes }}</ref><ref name=Royeretal2001>{{cite journal |last=Royer |first=D.L. |author2=R.A. Berner |author3=D.J. Beerling |author-link3 = David Beerling|year=2001 |title= Phanerozoic atmospheric CO<sub>2</sub> change: evaluating geochemical and paleobiological approaches |journal=Earth-Science Reviews |volume=54 |pages=349–92 |doi=10.1016/S0012-8252(00)00042-8 |bibcode=2001ESRv...54..349R |issue=4}}</ref><ref name="Berner&Kothavala2001">{{cite journal |last=Berner |first=Robert A. |author2=Kothavala, Zavareth |year=2001 |title=GEOCARB III: a revised model of atmospheric CO<sub>2</sub> over Phanerozoic time |url=http://www.geology.yale.edu/~ajs/2001/Feb/qn020100182.pdf |journal=American Journal of Science |volume=301 |issue=2 |pages=182–204 |doi=10.2475/ajs.301.2.182 |url-status=dead |archive-url=https://web.archive.org/web/20040806205206/http://www.geology.yale.edu/~ajs/2001/Feb/qn020100182.pdf |archive-date=6 August 2004 |bibcode=2001AmJS..301..182B |citeseerx=10.1.1.393.582 }}</ref> The spread of land plants is thought to have reduced CO<sub>2</sub> concentrations during the late Devonian, and plant activities as both sources and sinks of CO<sub>2</sub> have since been important in providing stabilising feedbacks.<ref name=Beerling2005>{{cite journal |last=Beerling |first=D.J. |author-link = David Beerling|author2=Berner, R.A. |year=2005 |title=Feedbacks and the co-evolution of plants and atmospheric CO<sub>2</sub> |journal=Proc. Natl. Acad. Sci. USA |volume=102 |pages=1302–05 |doi=10.1073/pnas.0408724102 |pmid=15668402 |issue=5 |pmc=547859|bibcode = 2005PNAS..102.1302B |doi-access=free }}</ref> | |||
| Earlier still, a 200-million year period of intermittent, widespread glaciation extending close to the equator (]) appears to have been ended suddenly, about 550 Ma, by a colossal volcanic outgassing that raised the {{CO2}} concentration of the atmosphere abruptly to 12%, about 350 times modern levels, causing extreme greenhouse conditions and carbonate deposition as ] at the rate of about 1 mm per day.<ref name=Hoffmannetal1998>{{cite journal |last=Hoffmann |first=PF |author2=AJ Kaufman |author3=GP Halverson |author4=DP Schrag |s2cid=13046760 |year=1998 |title=A neoproterozoic snowball earth |journal=Science |volume=281| issue=5381 |pages=1342–46 |doi=10.1126/science.281.5381.1342 |pmid=9721097 |bibcode=1998Sci...281.1342H}}</ref> This episode marked the close of the ] Eon, and was succeeded by the generally warmer conditions of the Phanerozoic, during which multicellular animal and plant life evolved. No volcanic carbon dioxide emission of comparable scale has occurred since. In the modern era, emissions to the atmosphere from volcanoes are approximately 0.645 billion tonnes of {{CO2}} per year, whereas humans contribute 29 billion tonnes of {{CO2}} each year.<ref>{{Cite news|url=https://www.forbes.com/sites/startswithabang/2017/06/06/how-much-co2-does-a-single-volcano-emit/|title=How Much CO2 Does A Single Volcano Emit?|last=Siegel|first=Ethan|work=Forbes|access-date=2018-09-06|language=en|archive-date=6 June 2017|archive-url=https://web.archive.org/web/20170606122158/https://www.forbes.com/sites/startswithabang/2017/06/06/how-much-co2-does-a-single-volcano-emit/|url-status=live}}</ref><ref name=Hoffmannetal1998 /><ref name=gerlach1991>{{cite journal |last=Gerlach |first=TM |year=1991 |title=Present-day CO<sub>2</sub> emissions from volcanoes |journal=Transactions of the American Geophysical Union |volume=72 |pages=249–55 |doi=10.1029/90EO10192 |bibcode=1991EOSTr..72..249. |issue=23}}</ref><ref>See also: {{cite web | |||
| |url=http://www.usgs.gov/newsroom/article.asp?ID=2827&from=rss_home | |||
| |title=U.S. Geological Survey | |||
| |date=14 June 2011 | |||
| |access-date=15 October 2012 | |||
| |archive-date=25 September 2012 | |||
| |archive-url=https://web.archive.org/web/20120925170105/http://www.usgs.gov/newsroom/article.asp?ID=2827&from=rss_home | |||
| |url-status=live | |||
| }}</ref> | |||
| {{note label|COL|A|A}} The IPCC states that ''"no single atmospheric lifetime can be given"'' for CO<sub>2</sub>.<ref name="ar5" />{{rp|731}} This is mostly due to the rapid growth and cumulative magnitude of the disturbances to Earth's ] by the geologic extraction and burning of fossil carbon.<ref name="Friedlingstein2020" /> As of year 2014, fossil CO<sub>2</sub> emitted as a theoretical 10 to 100 GtC pulse on top of the existing atmospheric concentration was expected to be 50% removed by land vegetation and ocean ] in less than about a century, as based on the projections of ] referenced in the AR5 assessment.<ref>{{cite book |title=Intergovernmental Panel on Climate Change Fifth Assessment Report – Supplemental Material |page=8SM-16 |chapter=Figure 8.SM.4 |chapter-url=https://www.ipcc.ch/site/assets/uploads/2018/07/WGI_AR5.Chap_.8_SM.pdf}}</ref> A substantial fraction (20–35%) was also projected to remain in the atmosphere for centuries to millennia, where fractional persistence increases with pulse size.<ref>{{cite journal |last=Archer |first=David |year=2009 |title=Atmospheric lifetime of fossil fuel carbon dioxide |url=https://orbi.uliege.be/handle/2268/12933 |journal=Annual Review of Earth and Planetary Sciences |volume=37 |issue=1 |pages=117–34 |bibcode=2009AREPS..37..117A |doi=10.1146/annurev.earth.031208.100206 |hdl=2268/12933}}</ref><ref>{{Cite journal |author=Joos, F. |author2=Roth, R. |author3=Fuglestvedt, J.D. |display-authors=etal |year=2013 |title=Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis |url=https://www.atmos-chem-phys.net/13/2793/2013/ |journal=Atmospheric Chemistry and Physics |volume=13 |issue=5 |pages=2793–2825 |doi=10.5194/acpd-12-19799-2012 |doi-access=free |hdl-access=free |hdl=20.500.11850/58316}}</ref> | |||
| ===Ice cores=== | |||
| {{note label|ERF|B|B}} Values are relative to year 1750. AR6 reports the ''effective radiative forcing'' which includes effects of rapid adjustments in the atmosphere and at the surface.<ref>{{Cite journal |author=Hansen, J. |author2=Sato, M. |author3=Ruedy, R. |display-authors=etal |year=2005 |title=Efficacy of Climate Forcings |journal=Journal of Geophysical Research: Atmospheres |volume=119 |issue=D18104 |doi=10.1029/2005JD005776 |doi-access=free|bibcode=2005JGRD..11018104H }}</ref> | |||
| ] | |||
| show that before industrial emissions started atmospheric CO<sub>2</sub> mole fractions were about 280 ] (ppm), and stayed between 260 and 280 during the preceding ten thousand years.<ref>{{cite journal|doi=10.1029/2001GB001417|title=High-resolution Holocene {{chem|N|2|O}} ice core record and its relationship with {{chem|CH|4}} and CO<sub>2</sub>|year=2002|last1=Flückiger|first1=Jacqueline|journal=Global Biogeochemical Cycles|volume=16|page=1010|bibcode=2002GBioC..16a..10F|doi-access=free}}</ref> Carbon dioxide mole fractions in the atmosphere have gone up by approximately 35 percent since the 1900s, rising from 280 parts per million by volume to 387 parts per million in 2009. One study using evidence from ] of fossilized leaves suggests greater variability, with carbon dioxide mole fractions above 300 ppm during the period seven to ten thousand years ago,<ref>{{cite journal |author1=Friederike Wagner |author2=Bent Aaby |author3=Henk Visscher |title=Rapid atmospheric CO<sub>2</sub> changes associated with the 8,200-years-B.P. cooling event |journal=Proc. Natl. Acad. Sci. USA |volume=99 |issue=19 |year=2002 |pages=12011–14 |doi=10.1073/pnas.182420699 |pmid=12202744 |pmc=129389|bibcode = 2002PNAS...9912011W |doi-access=free }}</ref> though others have argued that these findings more likely reflect calibration or contamination problems rather than actual CO<sub>2</sub> variability.<ref>{{cite journal |author1=Andreas Indermühle |author2=Bernhard Stauffer |author3=Thomas F. Stocker |title=Early Holocene Atmospheric CO<sub>2</sub> Concentrations |journal=Science |volume=286 |issue=5446 |year=1999 |page=1815 |doi=10.1126/science.286.5446.1815a|doi-access=free }} {{cite journal|title=Early Holocene atmospheric CO<sub>2</sub>concentrations|journal=Science|doi=10.1126/science.286.5446.1815a|volume=286|issue=5446|pages=1815a–15|year=1999|last1=IndermÜhle|first1=A|doi-access=free}}</ref><ref>{{cite journal|author1=H. J. Smith |author2=M. Wahlen |author3=D. Mastroianni | title=The CO<sub>2</sub> concentration of air trapped in GISP2 ice from the Last Glacial Maximum-Holocene transition| journal=Geophysical Research Letters| volume=24| issue=1| year=1997| pages=1–4| doi=10.1029/96GL03700| bibcode=1997GeoRL..24....1S}}</ref> Because of the way air is trapped in ice (pores in the ice close off slowly to form bubbles deep within the firn) and the time period represented in each ice sample analyzed, these figures represent averages of atmospheric concentrations of up to a few centuries rather than annual or decadal levels. | |||
| == Factors affecting concentrations == | |||
| ===Changes since the Industrial Revolution=== | |||
| Atmospheric concentrations are determined by the balance between sources (emissions of the gas from human activities and natural systems) and sinks (the removal of the gas from the atmosphere by conversion to a different chemical compound or absorption by bodies of water).<ref>Denman, K.L., G. Brasseur, A. Chidthaisong, P. Ciais, P.M. Cox, R.E. Dickinson, D. Hauglustaine, C. Heinze, E. Holland, D. Jacob, U. Lohmann, S Ramachandran, P.L. da Silva Dias, S.C. Wofsy and X. Zhang, 2007: . In: . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.</ref>{{rp|512}} | |||
| ] | |||
| ] | |||
| === Airborne fraction === | |||
| Since the beginning of the ], the concentrations of many of the greenhouse gases have increased. For example, the mole fraction of carbon dioxide has increased from 280 ppm to 415 ppm, or 120 ppm over modern pre-industrial levels. The first 30 ppm increase took place in about 200 years, from the start of the Industrial Revolution to 1958; however the next 90 ppm increase took place within 56 years, from 1958 to 2014.<ref name="Kibert2016">{{cite book|author=Charles J. Kibert|title=Sustainable Construction: Green Building Design and Delivery|chapter-url={{google books |plainurl=y |id=qv3iCwAAQBAJ|page=698}}|year=2016|publisher=Wiley|isbn=978-1119055327|chapter=Background}}</ref><ref>{{cite web|url=https://www.esrl.noaa.gov/gmd/ccgg/trends/full.html|title=Full Mauna Loa CO<sub>2</sub> record|year=2005|publisher=Earth System Research Laboratory|access-date=6 May 2017|archive-date=28 April 2017|archive-url=https://web.archive.org/web/20170428033710/https://www.esrl.noaa.gov/gmd/ccgg/trends/full.html|url-status=live}}</ref> | |||
| ]s, including plant growth, soil uptake, and ocean uptake (]).]] | |||
| The proportion of an emission remaining in the atmosphere after a specified time is the "]" (AF). The ''annual airborne fraction'' is the ratio of the atmospheric increase in a given year to that year's total emissions. The annual airborne fraction for {{CO2}} had been stable at 0.45 for the past six decades even as the emissions have been increasing. This means that the other 0.55 of emitted {{CO2}} is absorbed by the land and atmosphere carbon sinks within the first year of an emission.<ref name="Friedlingstein2020">{{Cite journal |last1=Friedlingstein |first1=Pierre |last2=O'Sullivan |first2=Michael |last3=Jones |first3=Matthew W. |last4=Andrew |first4=Robbie M. |last5=Hauck |first5=Judith |last6=Olsen |first6=Are |last7=Peters |first7=Glen P. |last8=Peters |first8=Wouter |last9=Pongratz |first9=Julia |last10=Sitch |first10=Stephen |last11=Le Quéré |first11=Corinne |last12=Canadell |first12=Josep G. |last13=Ciais |first13=Philippe |last14=Jackson |first14=Robert B. |last15=Alin |first15=Simone |date=2020 |title=Global Carbon Budget 2020 |journal=Earth System Science Data |language=en |volume=12 |issue=4 |pages=3269–3340 |doi=10.5194/essd-12-3269-2020 |bibcode=2020ESSD...12.3269F |issn=1866-3516 |doi-access=free |hdl=20.500.11850/458765 |hdl-access=free }}</ref> In the high-emission scenarios, the effectiveness of carbon sinks will be lower, increasing the atmospheric fraction of {{CO2}} even though the raw amount of emissions absorbed will be higher than in the present.<ref name="AR6WG1CH5">{{Cite book |last1=Canadell |first1=J. G. |last2=Monteiro |first2=P. M. S. |last3=Costa |first3=M. H. |last4=Cotrim da Cunha |first4=L. |last5=Ishii |first5=M. |last6=Jaccard |first6=S. |last7=Cox |first7=P. M. |last8=Eliseev |first8=A. V. |last9=Henson |first9=S. |last10=Koven |first10=C. |last11=Lohila |first11=A. |last12=Patra |first12=P. K. |last13=Piao |first13=S. |last14=Rogelj |first14=J. |last15=Syampungani |first15=S. |last16=Zaehle |first16=S. |last17=Zickfeld |first17=K. |year=2021 |title=IPCC Sixth Assessment Report: Working Group 1 |chapter=Global Carbon and Other Biogeochemical Cycles and Feedbacks |chapter-url=https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_Chapter_05.pdf}}</ref>{{rp|746}} | |||
| Recent data also shows that the concentration is increasing at a higher rate. In the 1960s, the average annual increase was only 37% of what it was in 2000 through 2007.<ref>{{cite web |first=Pieter |last=Tans |date=3 May 2008 |url=ftp://ftp.cmdl.noaa.gov/ccg/co2/trends/co2_gr_mlo.txt |title=Annual CO<sub>2</sub> mole fraction increase (ppm) for 1959–2007 |publisher=National Oceanic and Atmospheric Administration Earth System Research Laboratory, Global Monitoring Division }} {{cite web |url=http://www.esrl.noaa.gov/gmd/ccgg/trends/ |title=additional details |access-date=15 May 2008 |archive-date=25 December 2018 |archive-url=https://web.archive.org/web/20181225142754/https://www.esrl.noaa.gov/gmd/ccgg/trends/ |url-status=live }}; see also {{cite journal |first1=K.A. |last1=Masarie |first2=P.P. |last2=Tans |year=1995 |title=Extension and integration of atmospheric carbon dioxide data into a globally consistent measurement record |journal=J. Geophys. Res. |volume=100 |issue=D6 |pages=11593–610 |doi=10.1029/95JD00859 |bibcode=1995JGR...10011593M |url=https://zenodo.org/record/1231364 |access-date=26 July 2019 |archive-date=8 March 2021 |archive-url=https://web.archive.org/web/20210308193900/https://zenodo.org/record/1231364 |url-status=live }}</ref> | |||
| === Atmospheric lifetime === | |||
| Total cumulative emissions from 1870 to 2017 were 425±20 GtC (1539 Gt{{CO2}}) from ] and industry, and 180±60 GtC (660 Gt{{CO2}}) from ]. Land-use change, ], caused about 31% of cumulative emissions over 1870–2017, ] 32%, oil 25%, and gas 10%.<ref>{{Cite web|url=https://www.globalcarbonproject.org/carbonbudget/18/highlights.htm|title=Global Carbon Project (GCP)|website=www.globalcarbonproject.org|language=en|access-date=2019-05-19|archive-date=4 April 2019|archive-url=https://web.archive.org/web/20190404014758/https://www.globalcarbonproject.org/carbonbudget/18/highlights.htm|url-status=dead}}</ref> | |||
| ] | |||
| Major greenhouse gases are well mixed and take many years to leave the atmosphere.<ref name="betts">{{cite book |author=Betts |url=http://www.grida.no/publications/other/ipcc%5Ftar/?src=/climate/ipcc_tar/wg1/218.htm |title=Chapter 6 Radiative Forcing of Climate Change |publisher=UNEP/GRID-Arendal – Publications |year=2001 |series=Working Group I: The Scientific Basis IPCC Third Assessment Report – Climate Change 2001 |contribution=6.3 Well-mixed Greenhouse Gases |access-date=2010-10-16 |archive-url=https://web.archive.org/web/20110629043240/http://www.grida.no/publications/other/ipcc_tar/?src=%2Fclimate%2Fipcc_tar%2Fwg1%2F218.htm |archive-date=29 June 2011 |url-status=dead}}</ref> | |||
| Today,{{when|date=May 2019}} the stock of carbon in the atmosphere increases by more than 3 million tonnes per annum (0.04%) compared with the existing stock.{{clarify|date=April 2011|reason=in what year was this measurement made?}} This increase is the result of human activities by burning fossil fuels, deforestation and forest degradation in tropical and boreal regions.<ref name="Tarziu2011"> | |||
| {{cite journal| author1 = Dumitru-Romulus Târziu| author2 = Victor-Dan Păcurar| title = Pădurea, climatul și energia| language = ro| journal = ]| issn = 1583-7890| volume = 126| issue = 1| pages = 34–39| date = Jan 2011| url = http://www.revistapadurilor.ro/(16720)| archive-url = https://archive.today/20130416111853/http://www.revistapadurilor.ro/(16720)| url-status = dead| archive-date = 2013-04-16| id = 16720| access-date = 2012-06-11}}(webpage has a translation button) | |||
| </ref> | |||
| The atmospheric lifetime of a greenhouse gas refers to the time required to restore equilibrium following a sudden increase or decrease in its concentration in the atmosphere. Individual atoms or molecules may be lost or deposited to sinks such as the soil, the oceans and other waters, or vegetation and other biological systems, reducing the excess to background concentrations. The average time taken to achieve this is the ]. This can be represented through the following formula, where the lifetime <math>\tau</math> of an atmospheric ] X in a one-] is the average time that a molecule of X remains in the box.<ref name="JacobDJ1999">{{cite book |last=Jacob |first=Daniel |url=http://www-as.harvard.edu/people/faculty/djj/book/ |title=Introduction to atmospheric chemistry |publisher=] |year=1999 |isbn=978-0691001852 |pages=25–26 |archive-url=https://web.archive.org/web/20110902182732/http://www-as.harvard.edu/people/faculty/djj/book/ |archive-date=2 September 2011 |url-status=dead |df=dmy-all}}</ref> | |||
| The other greenhouse gases produced from human activity show similar increases in both amount and rate of increase. Many observations are available online in a variety of ]. | |||
| {{Clear}} | |||
| <math>\tau</math> can also be defined as the ratio of the mass <math>m</math> (in kg) of X in the box to its removal rate, which is the sum of the flow of X out of the box | |||
| {{anchor|Greenhouse gas#water vapor feedback|Greenhouse gas#water-vapor feedback}} | |||
| (<math>F_\text{out}</math>), | |||
| chemical loss of X | |||
| (<math>L</math>), | |||
| and ] of X | |||
| (<math>D</math>) | |||
| (all in kg/s): | |||
| :<math>\tau = \frac{m}{F_\text{out}+L+D}</math>.<ref name="JacobDJ1999" /> | |||
| If input of this gas into the box ceased, then after time <math>\tau</math>, its concentration would decrease by about 63%. | |||
| Changes to any of these variables can alter the atmospheric lifetime of a greenhouse gas. For instance, methane's atmospheric lifetime is estimated to have been lower in the 19th century than now, but to have been higher in the second half of the 20th century than after 2000.<ref name="Arora2018">{{Cite journal |last1=Arora |first1=Vivek K. |last2=Melton |first2=Joe R. |last3=Plummer |first3=David |date=1 August 2018 |title=An assessment of natural methane fluxes simulated by the CLASS-CTEM model |journal=Biogeosciences |volume=15 |issue=15 |pages=4683–4709 |doi=10.5194/bg-15-4683-2018 |doi-access=free |bibcode=2018BGeo...15.4683A |url=http://pdfs.semanticscholar.org/93b2/b104e0c79cc158781d3b28105e2a48d05389.pdf }}</ref> Carbon dioxide has an even more variable lifetime, which cannot be specified down to a single number.<ref>{{cite web |date=15 March 2005 |title=How long will global warming last? |url=http://www.realclimate.org/index.php/archives/2005/03/how-long-will-global-warming-last |url-status=live |archive-url=https://web.archive.org/web/20210304213944/http://www.realclimate.org/index.php/archives/2005/03/how-long-will-global-warming-last/ |archive-date=4 March 2021 |access-date=2012-06-12 |publisher=RealClimate}}</ref><ref name="TableOfWarmingPotentials5" /><ref name="AR6_WGI_AnnexVII" />{{rp|2237}} Scientists instead say that while the first 10% of carbon dioxide's airborne fraction (not counting the ~50% absorbed by land and ocean sinks within the emission's first year) is removed "quickly", the vast majority of the airborne fraction – 80% – lasts for "centuries to millennia". The remaining 10% stays for tens of thousands of years. In some models, this longest-lasting fraction is as large as 30%.<ref>{{cite web |date=17 January 2023 |title=How long will global warming last? |url= | |||
| === Anthropogenic greenhouse gas emissions === | |||
| https://climate.mit.edu/ask-mit/how-do-we-know-how-long-carbon-dioxide-remains-atmosphere |publisher=] Climate Portal }}</ref><ref>{{cite web |last=Atkinson |first=Kate |date=19 July 2023 |title=How long will global warming last? |url=https://www.aap.com.au/factcheck/carbon-atmospheric-residence-claim-is-full-of-gas/ |publisher=] }}</ref> | |||
| The way land is used affects climate change and the emission of greenhouse gases; the agriculture, land uses, and other land uses sector, on average, accounted for 13-21% of global total anthropogenic greenhouse gas (GHG) emissions in the period 2010-2019 (medium confidence). <ref>Agriculture, Forestry, and Other Land Uses Ch7 from "Climate Change 2022: Mitigation of Climate Change". ''www.ipcc.ch''. Retrieved 6 April 2022.</ref>] | |||
| ] | |||
| {{Excerpt|Greenhouse gas emissions|Sources|only=paragraph|this=This section is|paragraphs=1-2}} | |||
| === During geologic time scales === | |||
| ==Removal from the atmosphere== | |||
| {{excerpt|Carbon dioxide in Earth's atmosphere#Concentrations in the geologic past|paragraphs=1-2}} | |||
| ===Natural processes=== | |||
| Greenhouse gases can be ] by various processes, as a consequence of: | |||
| * a physical change (condensation and precipitation remove water vapor from the atmosphere). | |||
| * a chemical reaction within the atmosphere. For example, methane is ] by reaction with naturally occurring ], OH'''·''' and degraded to {{CO2}} and water vapor ({{CO2}} from the oxidation of methane is not included in the methane ]). Other chemical reactions include solution and solid phase chemistry occurring in atmospheric aerosols. | |||
| * a physical exchange between the atmosphere and the other components of the planet. An example is the mixing of atmospheric gases into the oceans. | |||
| * a chemical change at the interface between the atmosphere and the other components of the planet. This is the case for {{CO2}}, which is reduced by ] of plants, and which, after dissolving in the oceans, reacts to form ] and ] and ] ions (see ]). | |||
| * a ]. ] are dissociated by ] light releasing Cl'''·''' and F'''·''' as ]s in the ] with harmful effects on ] (halocarbons are generally too stable to disappear by chemical reaction in the atmosphere). | |||
| == Monitoring == | |||
| ===Negative emissions=== | |||
| {{Further|Greenhouse gas monitoring|Greenhouse gas inventory|Greenhouse gas emissions}} | |||
| {{main|Carbon dioxide removal}} | |||
| ] involves the direct ] of atmospheric concentrations and direct and indirect measurement of ]. Indirect methods calculate emissions of greenhouse gases based on related metrics such as fossil fuel extraction.<ref name="Friedlingstein2020" /> | |||
| A number of technologies remove greenhouse gases emissions from the atmosphere. Most widely analysed are those that remove carbon dioxide from the atmosphere, either to geologic formations such as ] and ],<ref name=RoyalSociety>{{cite web|work=The Royal Society |year=2009 |url=http://royalsociety.org/displaypagedoc.asp?id=35151 |title=Geoengineering the climate: science, governance and uncertainty |access-date=12 September 2009 |url-status=dead |archive-url=https://web.archive.org/web/20090907031520/http://royalsociety.org/displaypagedoc.asp?id=35151 |archive-date=7 September 2009 }}</ref> or to the soil as in the case with ].<ref name="RoyalSociety"/> The IPCC has pointed out that many long-term climate scenario models require large-scale man-made negative emissions to avoid serious climate change.<ref name=IPCC2007>{{citation|last1=Fischer|first1=B.S.|first2=N.|last2=Nakicenovic|first3=K.|last3=Alfsen|first4=J. Corfee|last4=Morlot|first5=F.|last5=de la Chesnaye|first6=J.-Ch.|last6=Hourcade|first7=K.|last7=Jiang|first8=M.|last8=Kainuma|first9=E.|last9=La Rovere|first10=A.|last10=Matysek|first11=A.|last11=Rana|first12=K.|last12=Riahi|first13=R.|last13=Richels|first14=S.|last14=Rose|first15=D.|last15=van Vuuren|first16=R.|last16=Warren|url=http://www.ipcc.ch/pdf/assessment-report/ar4/wg3/ar4-wg3-chapter3.pdf|title=Issues related to mitigation in the long term context|access-date=13 September 2009|archive-date=22 September 2018|archive-url=https://web.archive.org/web/20180922223618/http://www.ipcc.ch/pdf/assessment-report/ar4/wg3/ar4-wg3-chapter3.pdf|url-status=live}} in {{harvp|Rogner|Zhou|Bradley|Crabbé|2007}}</ref> | |||
| There are several different methods of measuring carbon dioxide concentrations in the atmosphere, including ] and ].<ref>{{Cite journal |last=Harris |first=Daniel C. |date=2010 |title=Charles David Keeling and the Story of Atmospheric CO2 Measurements |journal=Analytical Chemistry |language=en |volume=82 |issue=19 |pages=7865–7870 |doi=10.1021/ac1001492 |issn=0003-2700 |pmid=20536268}}</ref> Methane and nitrous oxide are measured by other instruments, such as the range-resolved infrared ] (DIAL).<ref>{{Cite journal |last1=Innocenti |first1=Fabrizio |last2=Robinson |first2=Rod |last3=Gardiner |first3=Tom |last4=Finlayson |first4=Andrew |last5=Connor |first5=Andy |date=2017 |title=Differential Absorption Lidar (DIAL) Measurements of Landfill Methane Emissions |journal=Remote Sensing |language=en |volume=9 |issue=9 |pages=953 |bibcode=2017RemS....9..953I |doi=10.3390/rs9090953 |doi-access=free|url=http://pdfs.semanticscholar.org/3683/aff466b2a818e872901f2df9c58d3cb8b20b.pdf }}</ref> ] such as by the ] and through networks of ]s such as the ].<ref name="Friedlingstein2020" /> | |||
| ==History of scientific research== | |||
| In the late 19th century, scientists experimentally discovered that {{chem|N|2}} and {{chem|O|2}} do not absorb infrared radiation (called, at that time, "dark radiation"), while water (both as true vapor and condensed in the form of microscopic droplets suspended in clouds) and {{CO2}} and other poly-atomic gaseous molecules do absorb infrared radiation.<ref name=CarbonicAcid>{{cite journal |last1=Arrhenius |first1=Svante |title=On the influence of carbonic acid in the air upon the temperature of the ground |journal=The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science |date=1896 |volume=41 |issue=251 |pages=237–276 |doi=10.1080/14786449608620846 |url=http://www.rsc.org/images/Arrhenius1896_tcm18-173546.pdf |access-date=1 December 2020 |archive-date=18 November 2020 |archive-url=https://web.archive.org/web/20201118065555/https://www.rsc.org/images/Arrhenius1896_tcm18-173546.pdf |url-status=live }}</ref><ref>{{cite journal|last1=Arrhenius |first1=Svante|title=On the Influence of Carbonic Acid in the Air Upon the Temperature of the Ground|journal=Publications of the Astronomical Society of the Pacific|year=1897|pages=14|volume=9|issue=54|bibcode = 1897PASP....9...14A |doi = 10.1086/121158 |doi-access=free}}</ref> In the early 20th century, researchers realized that greenhouse gases in the atmosphere made Earth's overall temperature higher than it would be without them. During the late 20th century, a ] evolved that increasing concentrations of greenhouse gases in the atmosphere cause a substantial rise in global temperatures and changes to other parts of the climate system,<ref>{{Cite journal | last1 = Cook | first1 = J. | last2 = Nuccitelli | first2 = D. | last3 = Green | first3 = S.A. | last4 = Richardson | first4 = M. | last5 = Winkler | first5 = B.R. | last6 = Painting | first6 = R. | last7 = Way | first7 = R. | last8 = Jacobs | first8 = P. | last9 = Skuce | first9 = A. | doi = 10.1088/1748-9326/8/2/024024 | title = Quantifying the consensus on anthropogenic global warming in the scientific literature | journal = Environmental Research Letters | volume = 8 | issue = 2 | page = 024024 | year = 2013 | bibcode = 2013ERL.....8b4024C | doi-access = free }}</ref> with ] for the ] and for ]. | |||
| The Annual Greenhouse Gas Index (AGGI) is defined by atmospheric scientists at ] as the ratio of total direct radiative forcing due to long-lived and well-mixed greenhouse gases for any year for which adequate global measurements exist, to that present in year 1990.<ref name="butmon" /><ref>{{cite web |author=LuAnn Dahlman |date=14 August 2020 |title=Climate change: annual greenhouse gas index |url=https://www.climate.gov/news-features/understanding-climate/climate-change-annual-greenhouse-gas-index |url-status=live |archive-url=https://web.archive.org/web/20130816013542/https://www.climate.gov/news-features/understanding-climate/climate-change-annual-greenhouse-gas-index |archive-date=16 August 2013 |access-date=5 September 2020 |publisher=NOAA Climate.gov science news & Information for a climate smart nation}}</ref> These radiative forcing levels are relative to those present in year 1750 (i.e. prior to the start of the ]). 1990 is chosen because it is the baseline year for the ], and is the publication year of the ]. As such, NOAA states that the AGGI "measures the commitment that (global) society has already made to living in a changing climate. It is based on the highest quality atmospheric observations from sites around the world. Its uncertainty is very low."<ref>{{Cite web |title=The NOAA Annual Greenhouse Gas Index (AGGI) – An Introduction |url=https://www.esrl.noaa.gov/gmd/aggi/ |url-status=live |archive-url=https://web.archive.org/web/20201127013113/https://www.esrl.noaa.gov/gmd/aggi/ |archive-date=27 November 2020 |access-date=5 September 2020 |publisher=] Global Monitoring Laboratory/Earth System Research Laboratories}}</ref> | |||
| ===Data networks=== | |||
| {{excerpt|Carbon dioxide in Earth's atmosphere#Data networks|paragraphs=1}} | |||
| == Types of sources == | |||
| === Natural sources === | |||
| {{Further|Carbon cycle}} | |||
| The natural flows of carbon between the atmosphere, ocean, ]s, and sediments are fairly balanced; so carbon levels would be roughly stable without human influence.<ref name="Prentice_etal_20012">{{cite book |last=Prentice |first=I.C. |title=Climate change 2001: the scientific basis: contribution of Working Group I to the Third Assessment Report of the Intergouvernmental Panel on Climate Change |year=2001 |editor1-last=Houghton |editor1-first=J.T. |chapter=The carbon cycle and atmospheric carbon dioxide |hdl=10067/381670151162165141}}</ref><ref name="U2">{{cite web |year=2009 |title=An Introduction to the Global Carbon Cycle |url=http://globecarboncycle.unh.edu/CarbonCycleBackground.pdf |url-status=live |archive-url=https://web.archive.org/web/20161008110835/http://globecarboncycle.unh.edu/CarbonCycleBackground.pdf |archive-date=8 October 2016 |access-date=6 February 2016 |publisher=University of New Hampshire |df=dmy-all}}</ref> Carbon dioxide is removed from the atmosphere primarily through ] and enters the terrestrial and oceanic biospheres. Carbon dioxide also dissolves directly from the atmosphere into bodies of water (ocean, lakes, etc.), as well as dissolving in precipitation as raindrops fall through the atmosphere. When dissolved in water, carbon dioxide reacts with water molecules and forms ], which contributes to ]. It can then be absorbed by rocks through ]. It also can acidify other surfaces it touches or be washed into the ocean.<ref name="Planet">{{Cite journal |title=Many Planets, One Earth // Section 4: Carbon Cycling and Earth's Climate |url=http://www.learner.org/courses/envsci/unit/text.php?unit=1&secNum=4 |url-status=live |journal=Many Planets, One Earth |volume=4 |archive-url=https://web.archive.org/web/20120417175417/http://www.learner.org/courses/envsci/unit/text.php?unit=1&secNum=4 |archive-date=17 April 2012 |access-date=2012-06-24 |df=dmy-all}}</ref>{{excerpt|Atmospheric carbon cycle|paragraphs=1}} | |||
| === Human-made sources === | |||
| ] | |||
| {{Main|Greenhouse gas emissions}} | |||
| The vast majority of carbon dioxide emissions by humans come from the burning of ]s. Additional contributions come from cement manufacturing, ] production, and changes in ] like ].<ref name=":1" />{{rp|687}}<ref name="EPA_GHGdata" /><ref name=":2">{{cite web |title=AR4 SYR Synthesis Report Summary for Policymakers – 2 Causes of change |url=https://www.ipcc.ch/publications_and_data/ar4/syr/en/spms2.html |url-status=dead |archive-url=https://web.archive.org/web/20180228235005/http://www.ipcc.ch/publications_and_data/ar4/syr/en/spms2.html |archive-date=28 February 2018 |access-date=9 October 2015 |work=ipcc.ch}}</ref> ] originate ], fossil fuel production, waste, and other sources.<ref name=":4" /> | |||
| If current ] continue then temperature rises will surpass {{convert|2.0|C-change}} sometime between 2040 and 2070, which is the level the United Nations' ] (IPCC) says is "dangerous".<ref name=":5" /> | |||
| Most greenhouse gases have both natural and human-caused sources. An exception are purely human-produced synthetic halocarbons which have no natural sources. During the pre-industrial ], concentrations of existing gases were roughly constant, because the large natural sources and sinks roughly balanced. In the industrial era, human activities have added greenhouse gases to the atmosphere, mainly through the burning of ] and clearing of forests.<ref>{{cite web |year=2000 |title=Chapter 3, IPCC Special Report on Emissions Scenarios, 2000 |url=https://ipcc.ch/pdf/special-reports/spm/sres-en.pdf |url-status=live |archive-url=https://web.archive.org/web/20180820085208/http://www.ipcc.ch/pdf/special-reports/spm/sres-en.pdf |archive-date=20 August 2018 |access-date=2010-10-16 |publisher=Intergovernmental Panel on Climate Change}}</ref><ref name=":0" />{{rp|115}}{{Excerpt|Greenhouse gas emissions|Overview of main sources|only=paragraph|this=This section is|paragraphs=1-2}} | |||
| == Reducing human-caused greenhouse gases == | |||
| {{main|Climate change mitigation}} | |||
| === Needed emissions cuts === | |||
| {{Excerpt|Climate change mitigation|Needed emissions cuts}} | |||
| === Removal from the atmosphere through negative emissions === | |||
| {{main|Carbon dioxide removal|Net zero emissions|Carbon sink}} | |||
| Several technologies remove greenhouse gas emissions from the atmosphere. Most widely analyzed are those that remove carbon dioxide from the atmosphere, either to geologic formations such as ] and ],<ref name="RoyalSociety">{{cite web |year=2009 |title=Geoengineering the climate: science, governance and uncertainty |url=http://royalsociety.org/displaypagedoc.asp?id=35151 |url-status=dead |archive-url=https://web.archive.org/web/20090907031520/http://royalsociety.org/displaypagedoc.asp?id=35151 |archive-date=7 September 2009 |access-date=12 September 2009 |work=The Royal Society}}</ref> or to the soil as in the case with ].<ref name="RoyalSociety" /> Many long-term climate scenario models require large-scale human-made negative emissions to avoid serious climate change.<ref>Fisher, B.S., N. Nakicenovic, K. Alfsen, J. Corfee Morlot, F. de la Chesnaye, J.-Ch. Hourcade, K. Jiang, M. Kainuma, E. La Rovere, A. Matysek, A. Rana, K. Riahi, R. Richels, S. Rose, D. van Vuuren, R. Warren, 2007: , In , Cambridge University Press, Cambridge,</ref> | |||
| Negative emissions approaches are also being studied for atmospheric methane, called ].<ref>{{Cite journal |last1=Jackson |first1=Robert B. |last2=Abernethy |first2=Sam |last3=Canadell |first3=Josep G. |last4=Cargnello |first4=Matteo |last5=Davis |first5=Steven J. |last6=Féron |first6=Sarah |last7=Fuss |first7=Sabine |last8=Heyer |first8=Alexander J. |last9=Hong |first9=Chaopeng |last10=Jones |first10=Chris D. |last11=Damon Matthews |first11=H. |last12=O'Connor |first12=Fiona M. |last13=Pisciotta |first13=Maxwell |last14=Rhoda |first14=Hannah M. |last15=de Richter |first15=Renaud |date=2021-11-15 |title=Atmospheric methane removal: a research agenda |journal=Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences |language=en |volume=379 |issue=2210 |pages=20200454 |bibcode=2021RSPTA.37900454J |doi=10.1098/rsta.2020.0454 |issn=1364-503X |pmc=8473948 |pmid=34565221}}</ref> | |||
| == History of discovery == | |||
| {{Further|History of climate change science|Greenhouse effect#History}} | |||
| ], March 1912, p. 341.</ref>]] | |||
| In the late 19th century, scientists experimentally discovered that {{chem|N|2}} and {{chem|O|2}} do not absorb infrared radiation (called, at that time, "dark radiation"), while water (both as true vapor and condensed in the form of microscopic droplets suspended in clouds) and {{CO2}} and other poly-atomic gaseous molecules do absorb infrared radiation.<ref name="CarbonicAcid">{{cite journal |last1=Arrhenius |first1=Svante |date=1896 |title=On the influence of carbonic acid in the air upon the temperature of the ground |url=http://www.rsc.org/images/Arrhenius1896_tcm18-173546.pdf |url-status=live |journal=The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science |volume=41 |issue=251 |pages=237–276 |doi=10.1080/14786449608620846 |archive-url=https://web.archive.org/web/20201118065555/https://www.rsc.org/images/Arrhenius1896_tcm18-173546.pdf |archive-date=18 November 2020 |access-date=1 December 2020}}</ref><ref>{{cite journal |last1=Arrhenius |first1=Svante |year=1897 |title=On the Influence of Carbonic Acid in the Air Upon the Temperature of the Ground |journal=Publications of the Astronomical Society of the Pacific |volume=9 |issue=54 |pages=14 |bibcode=1897PASP....9...14A |doi=10.1086/121158 |doi-access=free}}</ref> In the early 20th century, researchers realized that greenhouse gases in the atmosphere made Earth's overall temperature higher than it would be without them. The term ''greenhouse'' was first applied to this phenomenon by ] in 1901.<ref>{{cite web |last1=Easterbrook |first1=Steve |date=18 August 2015 |title=Who first coined the term "Greenhouse Effect"? |url=http://www.easterbrook.ca/steve/2015/08/who-first-coined-the-term-greenhouse-effect/ |url-status=live |archive-url=https://web.archive.org/web/20151113131713/http://www.easterbrook.ca/steve/2015/08/who-first-coined-the-term-greenhouse-effect/ |archive-date=13 November 2015 |access-date=11 November 2015 |website=Serendipity}}</ref><ref>{{cite journal |author=Ekholm N |year=1901 |title=On The Variations Of The Climate Of The Geological And Historical Past And Their Causes |journal=Quarterly Journal of the Royal Meteorological Society |volume=27 |pages=1–62 |bibcode=1901QJRMS..27....1E |doi=10.1002/qj.49702711702 |number=117}}</ref> | |||
| During the late 20th century, a ] evolved that increasing concentrations of greenhouse gases in the atmosphere cause a substantial rise in global temperatures and changes to other parts of the climate system,<ref>{{Cite journal |last1=Cook |first1=J. |last2=Nuccitelli |first2=D. |last3=Green |first3=S.A. |last4=Richardson |first4=M. |last5=Winkler |first5=B.R. |last6=Painting |first6=R. |last7=Way |first7=R. |last8=Jacobs |first8=P. |last9=Skuce |first9=A. |year=2013 |title=Quantifying the consensus on anthropogenic global warming in the scientific literature |journal=Environmental Research Letters |volume=8 |issue=2 |page=024024 |bibcode=2013ERL.....8b4024C |doi=10.1088/1748-9326/8/2/024024 |doi-access=free}}</ref> with ] for the ] and for ]. | |||
| ==Other planets== | |||
| {{Further|Greenhouse effect#Bodies other than Earth}} | |||
| Greenhouse gases exist in many ], creating greenhouse effects on ], ], and particularly in the thick ].<ref>{{cite web |author=Eddie Schwieterman |title=Comparing the Greenhouse Effect on Earth, Mars, Venus, and Titan: Present Day and through Time |url=http://www.astro.washington.edu/users/eschwiet/essays/greenhouse_ASTR555.pdf |url-status=dead |archive-url=https://web.archive.org/web/20150130202450/http://www.astro.washington.edu/users/eschwiet/essays/greenhouse_ASTR555.pdf |archive-date=30 January 2015}}</ref> While Venus has been described as the ultimate end state of ], such a process would have virtually no chance of occurring from any increases in greenhouse gas concentrations caused by humans,<ref name="IPCC2009">{{cite report |url=https://www.ipcc.ch/site/assets/uploads/2018/03/inf3-6.pdf |title=Scoping of the IPCC 5th Assessment Report Cross Cutting Issues |work=Thirty-first Session of the IPCC Bali, 26–29 October 2009 |url-status=live |archive-url=https://web.archive.org/web/20091109215503/http://www.ipcc.ch/meetings/session31/inf3.pdf |archive-date=9 November 2009 |access-date=24 March 2019}}</ref> as the ]'s brightness is too low and it would likely need to increase by some tens of percents, which will take a few billion years.<ref name="Hansen et al 2013">{{cite journal |last1=Hansen |first1=James |first2=Makiko |last2=Sato |first3=Gary |last3=Russell |first4=Pushker |last4=Kharecha |date=2013 |title=Climate sensitivity, sea level and atmospheric carbon dioxide |journal= Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences |volume=371 |issue=2001 |at=20120294 |bibcode=2013RSPTA.37120294H |doi=10.1098/rsta.2012.0294 |pmid=24043864 |pmc=3785813|arxiv=1211.4846 }}</ref> | |||
| ==See also== | ==See also== | ||
| {{Portal|Climate change|Environment |
{{Portal|Climate change|Environment}} | ||
| {{Columns-list|colwidth=22em| | |||
| *{{ Annotated link | Carbon accounting }} | |||
| * ] | |||
| *{{ Annotated link | Carbon budget }} | |||
| * ] | |||
| *{{ Annotated link | Carbon sequestration }} | |||
| * ] | |||
| *{{ Annotated link | Climate change feedback }} | |||
| * ] | |||
| *{{ Annotated link | Greenhouse gas monitoring }} | |||
| * ] | |||
| *{{ Annotated link | Greenhouse gas inventory }} | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| }} | |||
| ==References== | ==References== | ||
| {{Reflist}} | {{Reflist}} | ||
| == Further reading == | |||
| <!-- NOTE TO EDITORS: | |||
| * please add new entries in alphabetical order of author's last name. | |||
| * These are the 'general references' to the source; do not incorporate quotes, etc. here. | |||
| * Some of these references are used as part of the ] template. Removing these references will break some of the citations in the article. | |||
| * Unreferenced sources belong in Further reading section. | |||
| --> | |||
| * {{citation | |||
| | title=Current Greenhouse Gas Concentrations | |||
| | last=Blasing | |||
| | first=T.J. | |||
| | doi=10.3334/CDIAC/atg.032 | |||
| | date=February 2013 | |||
| | url=http://cdiac.ornl.gov/pns/current_ghg.html | |||
| | access-date=30 October 2012 | |||
| | archive-date=16 July 2011 | |||
| | archive-url=https://web.archive.org/web/20110716073547/http://cdiac.ornl.gov/pns/current_ghg.html | |||
| | url-status=dead | |||
| | doi-access=free | |||
| }} | |||
| *{{Cite book |ref= {{harvid|IPCC AR6 WG1 Ch1|2021}} | |||
| |chapter= Chapter 1: Framing, context, and methods | |||
| |last1 = Chen |first1=D. | |||
| |last2 = Rojas |first2=M. | |||
| |last3 = Samset|first3=B.H. | |||
| |last4 = Cobb |first4=K. | |||
| |last5 = Diongue-Niang|first5=A. | |||
| |last6 = Edwards|first6=P. | |||
| |last7 = Emori|first7=S. | |||
| |last8 = Faria|first8=S.H. | |||
| |last9 = Hawkins|first9=E. | |||
| |last10 = Hope|first10=P. | |||
| |last11 = Huybrechts|first11=P. | |||
| |last12 = Meinshausen|first12=M. | |||
| |last13 = Mustafa|first13=S.K.E.A.R. | |||
| |last14 = Plattner|first14=G.-K. | |||
| |last15 = Treguier|first15=A.M. | |||
| |display-authors=4 | |||
| |chapter-url= https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_Chapter_01.pdf | |||
| |year= 2021 | |||
| |title= {{Harvnb|IPCC AR6 WG1|2021}} | |||
| |pages= 1-215 | |||
| }} | |||
| * {{Citation | |||
| |year = 2007 | |||
| |author = IPCC AR4 WG1 | |||
| |author-link = IPCC | |||
| |title = Climate Change 2007: The Physical Science Basis – Contribution of Working Group I (WG1) to the Fourth Assessment Report (AR4) of the Intergovernmental Panel on Climate Change (IPCC) | |||
| |editor = Solomon, S. |editor2=Qin, D. |editor3=Manning, M. |editor4=Chen, Z. |editor5=Marquis, M. |editor6=Averyt, K.B. |editor7=Tignor, M. |editor8=Miller, H.L. | |||
| |publisher = Cambridge University Press | |||
| |url = http://www.ipcc.ch/publications_and_data/ar4/wg1/en/contents.html | |||
| |isbn = 978-0521880091 | |||
| }} (pb: {{ISBN|978-0521705967}}) | |||
| * {{citation |year=2007 |last1=Rogner |first1=H.-H. |first2=D. |last2=Zhou |first3=R. |last3=Bradley |first4=P. |last4=Crabbé |first5=O. |last5=Edenhofer |first6=B. |last6=Hare |first7=L. |last7=Kuijpers |first8=M. |last8=Yamaguchi |title=Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change |editor1=B. Metz |editor2=O.R. Davidson |editor3=P.R. Bosch |editor4=R. Dave |editor5=L.A. Meyer |url=http://www.ipcc.ch/publications_and_data/ar4/wg3/en/ch1s1-3.html#1-3-1 |publisher=Cambridge University Press |isbn=978-0521880114 |access-date=14 January 2012 |archive-url=https://web.archive.org/web/20120121013117/http://www.ipcc.ch/publications_and_data/ar4/wg3/en/ch1s1-3.html#1-3-1 |archive-date=21 January 2012 |url-status=dead }} | |||
| ==External links== | ==External links== | ||
| * |
{{Wiktionary}} | ||
| *{{Commons category-inline}} | |||
| * |
*{{citation |title=Carbon Dioxide Information Analysis Center (CDIAC) |publisher=U.S. Department of Energy |url=https://cdiac.ess-dive.lbl.gov/ |access-date=2020-07-26}} | ||
| * from NOAA | |||
| * from the ] | |||
| *. {{Webarchive|url=https://web.archive.org/web/20130325100504/http://spectralcalc.com/ |date=25 March 2013 }}. | |||
| * {{curlie|Science/Environment/Global_Change/}} | |||
| * from ] | |||
| * | |||
| {{Global warming}} | {{Global warming}} | ||
Latest revision as of 02:30, 9 December 2024
Gas in an atmosphere with certain absorption characteristicsThis article is about the physical properties of greenhouse gases. For how human activities are adding to greenhouse gases, see Greenhouse gas emissions.
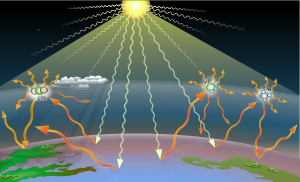

Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. What distinguishes them from other gases is that they absorb the wavelengths of radiation that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about −18 °C (0 °F), rather than the present average of 15 °C (59 °F).
The five most abundant greenhouse gases in Earth's atmosphere, listed in decreasing order of average global mole fraction, are: water vapor, carbon dioxide, methane, nitrous oxide, ozone. Other greenhouse gases of concern include chlorofluorocarbons (CFCs and HCFCs), hydrofluorocarbons (HFCs), perfluorocarbons, SF
6, and NF
3. Water vapor causes about half of the greenhouse effect, acting in response to other gases as a climate change feedback.
Human activities since the beginning of the Industrial Revolution (around 1750) have increased carbon dioxide by over 50%, and methane levels by 150%. Carbon dioxide emissions are causing about three-quarters of global warming, while methane emissions cause most of the rest. The vast majority of carbon dioxide emissions by humans come from the burning of fossil fuels, with remaining contributions from agriculture and industry. Methane emissions originate from agriculture, fossil fuel production, waste, and other sources. The carbon cycle takes thousands of years to fully absorb CO2 from the atmosphere, while methane lasts in the atmosphere for an average of only 12 years.
Natural flows of carbon happen between the atmosphere, terrestrial ecosystems, the ocean, and sediments. These flows have been fairly balanced over the past 1 million years, although greenhouse gas levels have varied widely in the more distant past. Carbon dioxide levels are now higher than they have been for 3 million years. If current emission rates continue then global warming will surpass 2.0 °C (3.6 °F) sometime between 2040 and 2070. This is a level which the Intergovernmental Panel on Climate Change (IPCC) says is "dangerous".
Properties and mechanisms

Greenhouse gases are infrared active, meaning that they absorb and emit infrared radiation in the same long wavelength range as what is emitted by the Earth's surface, clouds and atmosphere.
99% of the Earth's dry atmosphere (excluding water vapor) is made up of nitrogen (N
2) (78%) and oxygen (O
2) (21%). Because their molecules contain two atoms of the same element, they have no asymmetry in the distribution of their electrical charges, and so are almost totally unaffected by infrared thermal radiation, with only an extremely minor effect from collision-induced absorption. A further 0.9% of the atmosphere is made up by argon (Ar), which is monatomic, and so completely transparent to thermal radiation. On the other hand, carbon dioxide (0.04%), methane, nitrous oxide and even less abundant trace gases account for less than 0.1% of Earth's atmosphere, but because their molecules contain atoms of different elements, there is an asymmetry in electric charge distribution which allows molecular vibrations to interact with electromagnetic radiation. This makes them infrared active, and so their presence causes greenhouse effect.
Radiative forcing
Main article: Radiative forcing
Earth absorbs some of the radiant energy received from the sun, reflects some of it as light and reflects or radiates the rest back to space as heat. A planet's surface temperature depends on this balance between incoming and outgoing energy. When Earth's energy balance is shifted, its surface becomes warmer or cooler, leading to a variety of changes in global climate. Radiative forcing is a metric calculated in watts per square meter, which characterizes the impact of an external change in a factor that influences climate. It is calculated as the difference in top-of-atmosphere (TOA) energy balance immediately caused by such an external change. A positive forcing, such as from increased concentrations of greenhouse gases, means more energy arriving than leaving at the top-of-atmosphere, which causes additional warming, while negative forcing, like from sulfates forming in the atmosphere from sulfur dioxide, leads to cooling.
Within the lower atmosphere, greenhouse gases exchange thermal radiation with the surface and limit radiative heat flow away from it, which reduces the overall rate of upward radiative heat transfer. The increased concentration of greenhouse gases is also cooling the upper atmosphere, as it is much thinner than the lower layers, and any heat re-emitted from greenhouse gases is more likely to travel further to space than to interact with the fewer gas molecules in the upper layers. The upper atmosphere is also shrinking as the result.
Contributions of specific gases to the greenhouse effect
Main article: Greenhouse effectAnthropogenic changes to the natural greenhouse effect are sometimes referred to as the enhanced greenhouse effect.
This table shows the most important contributions to the overall greenhouse effect, without which the average temperature of Earth's surface would be about −18 °C (0 °F), instead of around 15 °C (59 °F). This table also specifies tropospheric ozone, because this gas has a cooling effect in the stratosphere, but a warming influence comparable to nitrous oxide and CFCs in the troposphere.
| K&T (1997) | Schmidt (2010) | |||
|---|---|---|---|---|
| Contributor | Clear Sky | With Clouds | Clear Sky | With Clouds |
| Water vapor | 60 | 41 | 67 | 50 |
| Clouds | 31 | 25 | ||
| CO2 | 26 | 18 | 24 | 19 |
| Tropospheric ozone (O3) | 8 | |||
| N2O + CH4 | 6 | |||
| Other | 9 | 9 | 7 | |
|
K&T (1997) used 353 ppm CO2 and calculated 125 W/m total clear-sky greenhouse effect; relied on single atmospheric profile and cloud model. "With Clouds" percentages are from Schmidt (2010) interpretation of K&T (1997). | ||||
Special role of water vapor

Water vapor is the most important greenhouse gas overall, being responsible for 41–67% of the greenhouse effect, but its global concentrations are not directly affected by human activity. While local water vapor concentrations can be affected by developments such as irrigation, it has little impact on the global scale due to its short residence time of about nine days. Indirectly, an increase in global temperatures cause will also increase water vapor concentrations and thus their warming effect, in a process known as water vapor feedback. It occurs because Clausius–Clapeyron relation establishes that more water vapor will be present per unit volume at elevated temperatures. Thus, local atmospheric concentration of water vapor varies from less than 0.01% in extremely cold regions and up to 3% by mass in saturated air at about 32 °C.
Global warming potential (GWP) and CO2 equivalents
This section is an excerpt from Global warming potential.
Global warming potential (GWP) is an index to measure how much infrared thermal radiation a greenhouse gas would absorb over a given time frame after it has been added to the atmosphere (or emitted to the atmosphere). The GWP makes different greenhouse gases comparable with regard to their "effectiveness in causing radiative forcing". It is expressed as a multiple of the radiation that would be absorbed by the same mass of added carbon dioxide (CO2), which is taken as a reference gas. Therefore, the GWP has a value of 1 for CO2. For other gases it depends on how strongly the gas absorbs infrared thermal radiation, how quickly the gas leaves the atmosphere, and the time frame being considered.
For example, methane has a GWP over 20 years (GWP-20) of 81.2 meaning that, for example, a leak of a tonne of methane is equivalent to emitting 81.2 tonnes of carbon dioxide measured over 20 years. As methane has a much shorter atmospheric lifetime than carbon dioxide, its GWP is much less over longer time periods, with a GWP-100 of 27.9 and a GWP-500 of 7.95.
The carbon dioxide equivalent (CO2e or CO2eq or CO2-e or CO2-eq) can be calculated from the GWP. For any gas, it is the mass of CO2 that would warm the earth as much as the mass of that gas. Thus it provides a common scale for measuring the climate effects of different gases. It is calculated as GWP times mass of the other gas.List of all greenhouse gases

The contribution of each gas to the enhanced greenhouse effect is determined by the characteristics of that gas, its abundance, and any indirect effects it may cause. For example, the direct radiative effect of a mass of methane is about 84 times stronger than the same mass of carbon dioxide over a 20-year time frame. Since the 1980s, greenhouse gas forcing contributions (relative to year 1750) are also estimated with high accuracy using IPCC-recommended expressions derived from radiative transfer models.
The concentration of a greenhouse gas is typically measured in parts per million (ppm) or parts per billion (ppb) by volume. A CO2 concentration of 420 ppm means that 420 out of every million air molecules is a CO2 molecule. The first 30 ppm increase in CO2 concentrations took place in about 200 years, from the start of the Industrial Revolution to 1958; however the next 90 ppm increase took place within 56 years, from 1958 to 2014. Similarly, the average annual increase in the 1960s was only 37% of what it was in 2000 through 2007.
Many observations are available online in a variety of Atmospheric Chemistry Observational Databases. The table below shows the most influential long-lived, well-mixed greenhouse gases, along with their tropospheric concentrations and direct radiative forcings, as identified by the Intergovernmental Panel on Climate Change (IPCC). Abundances of these trace gases are regularly measured by atmospheric scientists from samples collected throughout the world. It excludes water vapor because changes in its concentrations are calculated as a climate change feedback indirectly caused by changes in other greenhouse gases, as well as ozone, whose concentrations are only modified indirectly by various refrigerants that cause ozone depletion. Some short-lived gases (e.g. carbon monoxide, NOx) and aerosols (e.g. mineral dust or black carbon) are also excluded because of limited role and strong variation, along with minor refrigerants and other halogenated gases, which have been mass-produced in smaller quantities than those in the table. and Annex III of the 2021 IPCC WG1 Report
| Species | Lifetime
(years) |
100-yr | Mole Fraction + Radiative forcing | Concentrations
over time up to year 2022 | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline
Year 1750 |
TAR
Year 1998 |
AR4
Year 2005 |
AR5
Year 2011 |
AR6
Year 2019 | ||||
| CO2 | 1 | 278 | 365 (1.46) | 379 (1.66) | 391 (1.82) | 410 (2.16) | 
| |
| CH4 | 12.4 | 28 | 700 | 1,745 (0.48) | 1,774 (0.48) | 1,801 (0.48) | 1866 (0.54) | 
|
| N2O | 121 | 265 | 270 | 314 (0.15) | 319 (0.16) | 324 (0.17) | 332 (0.21) | 
|
| CFC-11 | 45 | 4,660 | 0 | 268 (0.07) | 251 (0.063) | 238 (0.062) | 226 (0.066) | 
|
| CFC-12 | 100 | 10,200 | 0 | 533 (0.17) | 538 (0.17) | 528 (0.17) | 503 (0.18) | 
|
| CFC-13 | 640 | 13,900 | 0 | 4 (0.001) | – | 2.7 (0.0007) | 3.28 (0.0009) | cfc13 |
| CFC-113 | 85 | 6,490 | 0 | 84 (0.03) | 79 (0.024) | 74 (0.022) | 70 (0.021) | 
|
| CFC-114 | 190 | 7,710 | 0 | 15 (0.005) | – | – | 16 (0.005) | cfc114 |
| CFC-115 | 1,020 | 5,860 | 0 | 7 (0.001) | – | 8.37 (0.0017) | 8.67 (0.0021) | cfc115 |
| HCFC-22 | 11.9 | 5,280 | 0 | 132 (0.03) | 169 (0.033) | 213 (0.0447) | 247 (0.0528) | 
|
| HCFC-141b | 9.2 | 2,550 | 0 | 10 (0.001) | 18 (0.0025) | 21.4 (0.0034) | 24.4 (0.0039) | 
|
| HCFC-142b | 17.2 | 5,020 | 0 | 11 (0.002) | 15 (0.0031) | 21.2 (0.0040) | 22.3 (0.0043) | 
|
| CH3CCl3 | 5 | 160 | 0 | 69 (0.004) | 19 (0.0011) | 6.32 (0.0004) | 1.6 (0.0001) | 
|
| CCl4 | 26 | 1,730 | 0 | 102 (0.01) | 93 (0.012) | 85.8 (0.0146) | 78 (0.0129) | 
|
| HFC-23 | 222 | 12,400 | 0 | 14 (0.002) | 18 (0.0033) | 24 (0.0043) | 32.4 (0.0062) | 
|
| HFC-32 | 5.2 | 677 | 0 | – | – | 4.92 (0.0005) | 20 (0.0022) | 
|
| HFC-125 | 28.2 | 3,170 | 0 | – | 3.7 (0.0009) | 9.58 (0.0022) | 29.4 (0.0069) | 
|
| HFC-134a | 13.4 | 1,300 | 0 | 7.5 (0.001) | 35 (0.0055) | 62.7 (0.0100) | 107.6 (0.018) | 
|
| HFC-143a | 47.1 | 4,800 | 0 | – | – | 12.0 (0.0019) | 24 (0.0040) | 
|
| HFC-152a | 1.5 | 138 | 0 | 0.5 (0.0000) | 3.9 (0.0004) | 6.4 (0.0006) | 7.1 (0.0007) | 
|
| CF4 (PFC-14) | 50,000 | 6,630 | 40 | 80 (0.003) | 74 (0.0034) | 79 (0.0040) | 85.5 (0.0051) | 
|
| C2F6 (PFC-116) | 10,000 | 11,100 | 0 | 3 (0.001) | 2.9 (0.0008) | 4.16 (0.0010) | 4.85 (0.0013) | 
|
| SF6 | 3,200 | 23,500 | 0 | 4.2 (0.002) | 5.6 (0.0029) | 7.28 (0.0041) | 9.95 (0.0056) | 
|
| SO2F2 | 36 | 4,090 | 0 | – | – | 1.71 (0.0003) | 2.5 (0.0005) | 
|
| NF3 | 500 | 16,100 | 0 | – | – | 0.9 (0.0002) | 2.05 (0.0004) | 
|
Mole fractions: μmol/mol = ppm = parts per million (10); nmol/mol = ppb = parts per billion (10); pmol/mol = ppt = parts per trillion (10).
The IPCC states that "no single atmospheric lifetime can be given" for CO2. This is mostly due to the rapid growth and cumulative magnitude of the disturbances to Earth's carbon cycle by the geologic extraction and burning of fossil carbon. As of year 2014, fossil CO2 emitted as a theoretical 10 to 100 GtC pulse on top of the existing atmospheric concentration was expected to be 50% removed by land vegetation and ocean sinks in less than about a century, as based on the projections of coupled models referenced in the AR5 assessment. A substantial fraction (20–35%) was also projected to remain in the atmosphere for centuries to millennia, where fractional persistence increases with pulse size.
Values are relative to year 1750. AR6 reports the effective radiative forcing which includes effects of rapid adjustments in the atmosphere and at the surface.
Factors affecting concentrations
Atmospheric concentrations are determined by the balance between sources (emissions of the gas from human activities and natural systems) and sinks (the removal of the gas from the atmosphere by conversion to a different chemical compound or absorption by bodies of water).
Airborne fraction

The proportion of an emission remaining in the atmosphere after a specified time is the "airborne fraction" (AF). The annual airborne fraction is the ratio of the atmospheric increase in a given year to that year's total emissions. The annual airborne fraction for CO2 had been stable at 0.45 for the past six decades even as the emissions have been increasing. This means that the other 0.55 of emitted CO2 is absorbed by the land and atmosphere carbon sinks within the first year of an emission. In the high-emission scenarios, the effectiveness of carbon sinks will be lower, increasing the atmospheric fraction of CO2 even though the raw amount of emissions absorbed will be higher than in the present.
Atmospheric lifetime

Major greenhouse gases are well mixed and take many years to leave the atmosphere.
The atmospheric lifetime of a greenhouse gas refers to the time required to restore equilibrium following a sudden increase or decrease in its concentration in the atmosphere. Individual atoms or molecules may be lost or deposited to sinks such as the soil, the oceans and other waters, or vegetation and other biological systems, reducing the excess to background concentrations. The average time taken to achieve this is the mean lifetime. This can be represented through the following formula, where the lifetime of an atmospheric species X in a one-box model is the average time that a molecule of X remains in the box.
can also be defined as the ratio of the mass (in kg) of X in the box to its removal rate, which is the sum of the flow of X out of the box (), chemical loss of X (), and deposition of X () (all in kg/s):
- .
If input of this gas into the box ceased, then after time , its concentration would decrease by about 63%.
Changes to any of these variables can alter the atmospheric lifetime of a greenhouse gas. For instance, methane's atmospheric lifetime is estimated to have been lower in the 19th century than now, but to have been higher in the second half of the 20th century than after 2000. Carbon dioxide has an even more variable lifetime, which cannot be specified down to a single number. Scientists instead say that while the first 10% of carbon dioxide's airborne fraction (not counting the ~50% absorbed by land and ocean sinks within the emission's first year) is removed "quickly", the vast majority of the airborne fraction – 80% – lasts for "centuries to millennia". The remaining 10% stays for tens of thousands of years. In some models, this longest-lasting fraction is as large as 30%.

During geologic time scales
This section is an excerpt from Carbon dioxide in Earth's atmosphere § Concentrations in the geologic past.

Estimates in 2023 found that the current carbon dioxide concentration in the atmosphere may be the highest it has been in the last 14 million years. However the IPCC Sixth Assessment Report estimated similar levels 3 to 3.3 million years ago in the mid-Pliocene warm period. This period can be a proxy for likely climate outcomes with current levels of CO2.
Carbon dioxide is believed to have played an important effect in regulating Earth's temperature throughout its 4.54 billion year history. Early in the Earth's life, scientists have found evidence of liquid water indicating a warm world even though the Sun's output is believed to have only been 70% of what it is today. Higher carbon dioxide concentrations in the early Earth's atmosphere might help explain this faint young sun paradox. When Earth first formed, Earth's atmosphere may have contained more greenhouse gases and CO2 concentrations may have been higher, with estimated partial pressure as large as 1,000 kPa (10 bar), because there was no bacterial photosynthesis to reduce the gas to carbon compounds and oxygen. Methane, a very active greenhouse gas, may have been more prevalent as well.Monitoring
Further information: Greenhouse gas monitoring, Greenhouse gas inventory, and Greenhouse gas emissionsGreenhouse gas monitoring involves the direct measurement of atmospheric concentrations and direct and indirect measurement of greenhouse gas emissions. Indirect methods calculate emissions of greenhouse gases based on related metrics such as fossil fuel extraction.
There are several different methods of measuring carbon dioxide concentrations in the atmosphere, including infrared analyzing and manometry. Methane and nitrous oxide are measured by other instruments, such as the range-resolved infrared differential absorption lidar (DIAL). Greenhouse gases are measured from space such as by the Orbiting Carbon Observatory and through networks of ground stations such as the Integrated Carbon Observation System.
The Annual Greenhouse Gas Index (AGGI) is defined by atmospheric scientists at NOAA as the ratio of total direct radiative forcing due to long-lived and well-mixed greenhouse gases for any year for which adequate global measurements exist, to that present in year 1990. These radiative forcing levels are relative to those present in year 1750 (i.e. prior to the start of the industrial era). 1990 is chosen because it is the baseline year for the Kyoto Protocol, and is the publication year of the first IPCC Scientific Assessment of Climate Change. As such, NOAA states that the AGGI "measures the commitment that (global) society has already made to living in a changing climate. It is based on the highest quality atmospheric observations from sites around the world. Its uncertainty is very low."
Data networks
This section is an excerpt from Carbon dioxide in Earth's atmosphere § Data networks. There are several surface measurement (including flasks and continuous in situ) networks including NOAA/ERSL, WDCGG, and RAMCES. The NOAA/ESRL Baseline Observatory Network, and the Scripps Institution of Oceanography Network data are hosted at the CDIAC at ORNL. The World Data Centre for Greenhouse Gases (WDCGG), part of GAW, data are hosted by the JMA. The Reseau Atmospherique de Mesure des Composes an Effet de Serre database (RAMCES) is part of IPSL.Types of sources
Natural sources
Further information: Carbon cycleThe natural flows of carbon between the atmosphere, ocean, terrestrial ecosystems, and sediments are fairly balanced; so carbon levels would be roughly stable without human influence. Carbon dioxide is removed from the atmosphere primarily through photosynthesis and enters the terrestrial and oceanic biospheres. Carbon dioxide also dissolves directly from the atmosphere into bodies of water (ocean, lakes, etc.), as well as dissolving in precipitation as raindrops fall through the atmosphere. When dissolved in water, carbon dioxide reacts with water molecules and forms carbonic acid, which contributes to ocean acidity. It can then be absorbed by rocks through weathering. It also can acidify other surfaces it touches or be washed into the ocean.
This section is an excerpt from Atmospheric carbon cycle.
Human-made sources
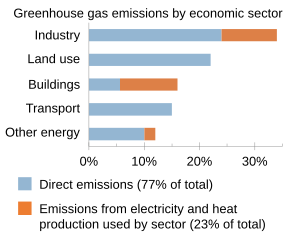
The vast majority of carbon dioxide emissions by humans come from the burning of fossil fuels. Additional contributions come from cement manufacturing, fertilizer production, and changes in land use like deforestation. Methane emissions originate from agriculture, fossil fuel production, waste, and other sources.
If current emission rates continue then temperature rises will surpass 2.0 °C (3.6 °F) sometime between 2040 and 2070, which is the level the United Nations' Intergovernmental Panel on Climate Change (IPCC) says is "dangerous".
Most greenhouse gases have both natural and human-caused sources. An exception are purely human-produced synthetic halocarbons which have no natural sources. During the pre-industrial Holocene, concentrations of existing gases were roughly constant, because the large natural sources and sinks roughly balanced. In the industrial era, human activities have added greenhouse gases to the atmosphere, mainly through the burning of fossil fuels and clearing of forests.
This section is an excerpt from Greenhouse gas emissions § Overview of main sources.The major anthropogenic (human origin) sources of greenhouse gases are carbon dioxide (CO2), nitrous oxide (N
2O), methane and three groups of fluorinated gases (sulfur hexafluoride (SF
6), hydrofluorocarbons (HFCs) and perfluorocarbons (PFCs, sulphur hexafluoride (SF6), and nitrogen trifluoride (NF3)). Though the greenhouse effect is heavily driven by water vapor, human emissions of water vapor are not a significant contributor to warming.
Reducing human-caused greenhouse gases
Main article: Climate change mitigationNeeded emissions cuts
This section is an excerpt from Climate change mitigation § Needed emissions cuts.
The annual "Emissions Gap Report" by UNEP stated in 2022 that it was necessary to almost halve emissions. "To get on track for limiting global warming to 1.5°C, global annual GHG emissions must be reduced by 45 per cent compared with emissions projections under policies currently in place in just eight years, and they must continue to decline rapidly after 2030, to avoid exhausting the limited remaining atmospheric carbon budget." The report commented that the world should focus on broad-based economy-wide transformations and not incremental change.
In 2022, the Intergovernmental Panel on Climate Change (IPCC) released its Sixth Assessment Report on climate change. It warned that greenhouse gas emissions must peak before 2025 at the latest and decline 43% by 2030 to have a good chance of limiting global warming to 1.5 °C (2.7 °F). Or in the words of Secretary-General of the United Nations António Guterres: "Main emitters must drastically cut emissions starting this year".Removal from the atmosphere through negative emissions
Main articles: Carbon dioxide removal, Net zero emissions, and Carbon sinkSeveral technologies remove greenhouse gas emissions from the atmosphere. Most widely analyzed are those that remove carbon dioxide from the atmosphere, either to geologic formations such as bio-energy with carbon capture and storage and carbon dioxide air capture, or to the soil as in the case with biochar. Many long-term climate scenario models require large-scale human-made negative emissions to avoid serious climate change.
Negative emissions approaches are also being studied for atmospheric methane, called atmospheric methane removal.
History of discovery
Further information: History of climate change science and Greenhouse effect § History
In the late 19th century, scientists experimentally discovered that N
2 and O
2 do not absorb infrared radiation (called, at that time, "dark radiation"), while water (both as true vapor and condensed in the form of microscopic droplets suspended in clouds) and CO2 and other poly-atomic gaseous molecules do absorb infrared radiation. In the early 20th century, researchers realized that greenhouse gases in the atmosphere made Earth's overall temperature higher than it would be without them. The term greenhouse was first applied to this phenomenon by Nils Gustaf Ekholm in 1901.
During the late 20th century, a scientific consensus evolved that increasing concentrations of greenhouse gases in the atmosphere cause a substantial rise in global temperatures and changes to other parts of the climate system, with consequences for the environment and for human health.
Other planets
Further information: Greenhouse effect § Bodies other than EarthGreenhouse gases exist in many atmospheres, creating greenhouse effects on Mars, Titan, and particularly in the thick atmosphere of Venus. While Venus has been described as the ultimate end state of runaway greenhouse effect, such a process would have virtually no chance of occurring from any increases in greenhouse gas concentrations caused by humans, as the Sun's brightness is too low and it would likely need to increase by some tens of percents, which will take a few billion years.
See also
- Carbon accounting – Processes used to measure emissions of carbon dioxide equivalents
- Carbon budget – Limit on carbon dioxide emission for a given climate impact
- Carbon sequestration – Storing carbon in a carbon pool
- Climate change feedback – Feedback related to climate changePages displaying short descriptions of redirect targets
- Greenhouse gas monitoring – Measurement of greenhouse gas emissions and levels
- Greenhouse gas inventory – Inventory for emissions of greenhouse gases
References
- Matthews, J.B.R.; Möller, V.; van Diemenn, R.; Fuglesvedt, J.R.; et al. (9 August 2021). "Annex VII: Glossary". In Masson-Delmotte, Valérie; Zhai, Panmao; Pirani, Anna; Connors, Sarah L.; Péan, Clotilde; et al. (eds.). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (PDF). IPCC / Cambridge University Press. pp. 2215–2256. doi:10.1017/9781009157896.022. ISBN 9781009157896.
- ^ Qiancheng Ma (March 1998). "Science Briefs: Greenhouse Gases: Refining the Role of Carbon Dioxide". NASA GISS. Archived from the original on 12 January 2005. Retrieved 26 April 2016.
- ^ Karl TR, Trenberth KE (2003). "Modern global climate change". Science. 302 (5651): 1719–23. Bibcode:2003Sci...302.1719K. doi:10.1126/science.1090228. PMID 14657489. S2CID 45484084. Archived from the original on 22 April 2021. Retrieved 26 July 2019 – via Zenodo.
- ^ Le Treut, H., R. Somerville, U. Cubasch, Y. Ding, C. Mauritzen, A. Mokssit, T. Peterson and M. Prather, 2007: "Chapter 1: Historical Overview of Climate Change". In: "Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change". . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- "Atmospheric Concentration of Greenhouse Gases" (PDF). U.S. Environmental Protection Agency. 1 August 2016. Archived (PDF) from the original on 19 October 2021. Retrieved 6 September 2021.
- "Inside the Earth's invisible blanket". sequestration.org. Archived from the original on 28 July 2020. Retrieved 5 March 2021.
- Gavin Schmidt (1 October 2010). "Taking the Measure of the Greenhouse Effect". NASA Goddard Institute for Space Studies – Science Briefs.
- ^ "Carbon dioxide now more than 50% higher than pre-industrial levels". National Oceanic and Atmospheric Administration. 3 June 2022. Retrieved 30 August 2022.
- "Understanding methane emissions". International Energy Agency.
The concentration of methane in the atmosphere is currently over two-and-a-half times greater than its pre-industrial levels
- "Global Greenhouse Gas Emissions Data". United States Environmental Protection Agency. 12 January 2016.
- ^ "Global Greenhouse Gas Emissions Data". U.S. Environmental Protection Agency. 12 January 2016. Archived from the original on 5 December 2019. Retrieved 30 December 2019.
The burning of coal, natural gas, and oil for electricity and heat is the largest single source of global greenhouse gas emissions.
- ^ Canadell, J.G., P.M.S. Monteiro, M.H. Costa, L. Cotrim da Cunha, P.M. Cox, A.V. Eliseev, S. Henson, M. Ishii, S. Jaccard, C. Koven, A. Lohila, P.K. Patra, S. Piao, J. Rogelj, S. Syampungani, S. Zaehle, and K. Zickfeld, 2021: Chapter 5: Global Carbon and other Biogeochemical Cycles and Feedbacks. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 673–816, doi:10.1017/9781009157896.007.
- ^ "Global Methane Tracker 2023". International Energy Agency. 21 February 2023.
- "Climate Change Indicators: Greenhouse Gases". United States Environmental Protection Agency. 16 December 2015.
Carbon dioxide's lifetime cannot be represented with a single value because the gas is not destroyed over time, but instead moves among different parts of the ocean–atmosphere–land system. Some of the excess carbon dioxide is absorbed quickly (for example, by the ocean surface), but some will remain in the atmosphere for thousands of years, due in part to the very slow process by which carbon is transferred to ocean sediments.
- "Understanding methane emissions". International Energy Agency.
- "Climate Change Indicators: Atmospheric Concentrations of Greenhouse Gases". EPA.gov. U.S. Environmental Protection Agency. 27 June 2016. Retrieved 20 June 2024.
- Lindsey, Rebecca. "Climate Change: Atmospheric Carbon Dioxide". climate.gov. Archived from the original on 24 June 2013. Retrieved 2 March 2020.
- ^ "Analysis: When might the world exceed 1.5C and 2C of global warming?". Carbon Brief. 4 December 2020. Archived from the original on 6 June 2021. Retrieved 17 June 2021.
- ^ IPCC, 2021: Annex VII: Glossary . In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 2215–2256, doi:10.1017/9781009157896.022.
- ^ Archer, David (2011). Global Warming: Understanding the Forecast, Chapter 4: Greenhouse Gases (PDF) (2 ed.). Wiley. ISBN 978-0470943410. Retrieved 14 June 2023.
- Wei, Peng-Sheng; Hsieh, Yin-Chih; Chiu, Hsuan-Han; Yen, Da-Lun; Lee, Chieh; Tsai, Yi-Cheng; Ting, Te-Chuan (6 October 2018). "Absorption coefficient of carbon dioxide across atmospheric troposphere layer". Heliyon. 4 (10): e00785. Bibcode:2018Heliy...400785W. doi:10.1016/j.heliyon.2018.e00785. ISSN 2405-8440. PMC 6174548. PMID 30302408.
- Höpfner, M.; Milz, M.; Buehler, S.; Orphall, J.; Stiller, G. (24 May 2012). "The natural greenhouse effect of atmospheric oxygen (O2) and nitrogen (N2)". Geophysical Research Letters. 39 (L10706). Bibcode:2012GeoRL..3910706H. doi:10.1029/2012GL051409. ISSN 1944-8007. S2CID 128823108.
- "Which Gases Are Greenhouse Gases?". American Chemical Society. Retrieved 31 May 2021.
- Höpfner, M.; Milz, M.; Buehler, S.; Orphall, J.; Stiller, G. (24 May 2012). "The natural greenhouse effect of atmospheric oxygen (O2) and nitrogen (N2)". Geophysical Research Letters. 39 (L10706). Bibcode:2012GeoRL..3910706H. doi:10.1029/2012GL051409. ISSN 1944-8007. S2CID 128823108.
- "Climate Change Indicators in the United States – Greenhouse Gases". U.S. Environmental Protection Agency (EPA). 2016. Archived from the original on 27 August 2016. Retrieved 5 September 2020..
- "Climate Change Indicators in the United States – Climate Forcing". U.S. Environmental Protection Agency (EPA). 2016. Archived from the original on 27 August 2016. Retrieved 5 September 2020. Archived 21 September 2020 at the Wayback Machine
- Wallace, J. M.; Hobbs, P. V. (2006). Atmospheric Science (2 ed.). Academic Press. ISBN 978-0-12-732951-2.
- Manabe, S.; Strickler, R. F. (1964). "Thermal Equilibrium of the Atmosphere with a Convective Adjustment". J. Atmos. Sci. 21 (4): 361–385. Bibcode:1964JAtS...21..361M. doi:10.1175/1520-0469(1964)021<0361:TEOTAW>2.0.CO;2.
- Hatfield, Miles (30 June 2021). "NASA Satellites See Upper Atmosphere Cooling and Contracting Due to Climate Change". NASA.
- "Atmospheric Concentration of Greenhouse Gases" (PDF). U.S. Environmental Protection Agency. 1 August 2016.
- ^ Kiehl, J.T.; Kevin E. Trenberth (1997). "Earth's annual global mean energy budget" (PDF). Bulletin of the American Meteorological Society. 78 (2): 197–208. Bibcode:1997BAMS...78..197K. doi:10.1175/1520-0477(1997)078<0197:EAGMEB>2.0.CO;2.
- ^ Schmidt, G.A.; R. Ruedy; R.L. Miller; A.A. Lacis (2010), "The attribution of the present-day total greenhouse effect" (PDF), J. Geophys. Res., vol. 115, no. D20, pp. D20106, Bibcode:2010JGRD..11520106S, doi:10.1029/2010JD014287, archived from the original (PDF) on 22 October 2011, D20106. Web page Archived 4 June 2012 at the Wayback Machine
- "NASA: Climate Forcings and Global Warming". 14 January 2009. Archived from the original on 18 April 2021. Retrieved 20 April 2014.
- "AGU Water Vapor in the Climate System". Eso.org. 27 April 1995. Archived from the original on 20 October 2012. Retrieved 11 September 2011.
- Held, Isaac M.; Soden, Brian J. (November 2000). "Water vapor feedback and global warming". Annual Review of Energy and the Environment. 25 (1): 441–475. CiteSeerX 10.1.1.22.9397. doi:10.1146/annurev.energy.25.1.441. ISSN 1056-3466.
- Evans, Kimberly Masters (2005). "The greenhouse effect and climate change". The environment: a revolution in attitudes. Detroit: Thomson Gale. ISBN 978-0787690823.
- IPCC, 2021: Annex VII: Glossary . In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 2215–2256, doi:10.1017/9781009157896.022.
- ^ 7.SM.6 Tables of greenhouse gas lifetimes, radiative efficiencies and metrics (PDF), IPCC, 2021, p. 7SM-24.
- "The NOAA Annual Greenhouse Gas Index (AGGI)". NOAA.gov. National Oceanic and Atmospheric Administration (NOAA). 2024. Archived from the original on 5 October 2024.
- "Annual Greenhouse Gas Index". U.S. Global Change Research Program. Archived from the original on 21 April 2021. Retrieved 5 September 2020.
- ^ Butler J. and Montzka S. (2020). "The NOAA Annual Greenhouse Gas Index (AGGI)". NOAA Global Monitoring Laboratory/Earth System Research Laboratories. Archived from the original on 22 September 2013. Retrieved 5 September 2020.
- ^ "Appendix 8.A" (PDF). Intergovernmental Panel on Climate Change Fifth Assessment Report. p. 731. Archived (PDF) from the original on 13 October 2017. Retrieved 6 November 2017.
- Butler J. and Montzka S. (2020). "The NOAA Annual Greenhouse Gas Index (AGGI)". NOAA Global Monitoring Laboratory/Earth System Research Laboratories.
- Charles J. Kibert (2016). "Background". Sustainable Construction: Green Building Design and Delivery. Wiley. ISBN 978-1119055327.
- "Full Mauna Loa CO2 record". Earth System Research Laboratories. 2005. Archived from the original on 28 April 2017. Retrieved 6 May 2017.
- Tans, Pieter (3 May 2008). "Annual CO2 mole fraction increase (ppm) for 1959–2007". National Oceanic and Atmospheric Administration Earth System Research Laboratories, Global Monitoring Division. "additional details". Archived from the original on 25 December 2018. Retrieved 15 May 2008.; see also Masarie, K.A.; Tans, P.P. (1995). "Extension and integration of atmospheric carbon dioxide data into a globally consistent measurement record". J. Geophys. Res. 100 (D6): 11593–610. Bibcode:1995JGR...10011593M. doi:10.1029/95JD00859. Archived from the original on 8 March 2021. Retrieved 26 July 2019.
- ^ "Chapter 8". AR5 Climate Change 2013: The Physical Science Basis.
- "Global Monitoring Laboratory". NOAA Earth System Research Laboratories. Retrieved 11 December 2020.
- "World Data Centre for Greenhouse Gases". World Meteorological Organization Global Atmosphere Watch Programme and Japan Meteorological Agency. Retrieved 11 December 2020.
- "Advanced Global Atmospheric Gas Experiment". Massachusetts Institute of Technology. Retrieved 11 December 2020.
- ^ Dentener F. J.; B. Hall; C. Smith, eds. (9 August 2021), "Annex III: Tables of historical and projected well-mixed greenhouse gas mixing ratios and effective radiative forcing of all climate forcers" (PDF), Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press
- "Long-term global trends of atmospheric trace gases". NOAA Earth System Research Laboratories. Retrieved 11 February 2021.
- "AGAGE Data and Figures". Massachusetts Institute of Technology. Retrieved 11 February 2021.
- "Chapter 6". TAR Climate Change 2001: The Scientific Basis. p. 358.
- "Chapter 2". AR4 Climate Change 2007: The Physical Science Basis. p. 141.
- ^ Friedlingstein, Pierre; O'Sullivan, Michael; Jones, Matthew W.; Andrew, Robbie M.; Hauck, Judith; Olsen, Are; Peters, Glen P.; Peters, Wouter; Pongratz, Julia; Sitch, Stephen; Le Quéré, Corinne; Canadell, Josep G.; Ciais, Philippe; Jackson, Robert B.; Alin, Simone (2020). "Global Carbon Budget 2020". Earth System Science Data. 12 (4): 3269–3340. Bibcode:2020ESSD...12.3269F. doi:10.5194/essd-12-3269-2020. hdl:20.500.11850/458765. ISSN 1866-3516.
- "Figure 8.SM.4" (PDF). Intergovernmental Panel on Climate Change Fifth Assessment Report – Supplemental Material. p. 8SM-16.
- Archer, David (2009). "Atmospheric lifetime of fossil fuel carbon dioxide". Annual Review of Earth and Planetary Sciences. 37 (1): 117–34. Bibcode:2009AREPS..37..117A. doi:10.1146/annurev.earth.031208.100206. hdl:2268/12933.
- Joos, F.; Roth, R.; Fuglestvedt, J.D.; et al. (2013). "Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis". Atmospheric Chemistry and Physics. 13 (5): 2793–2825. doi:10.5194/acpd-12-19799-2012. hdl:20.500.11850/58316.
- Hansen, J.; Sato, M.; Ruedy, R.; et al. (2005). "Efficacy of Climate Forcings". Journal of Geophysical Research: Atmospheres. 119 (D18104). Bibcode:2005JGRD..11018104H. doi:10.1029/2005JD005776.
- Denman, K.L., G. Brasseur, A. Chidthaisong, P. Ciais, P.M. Cox, R.E. Dickinson, D. Hauglustaine, C. Heinze, E. Holland, D. Jacob, U. Lohmann, S Ramachandran, P.L. da Silva Dias, S.C. Wofsy and X. Zhang, 2007: Chapter 7: Couplings Between Changes in the Climate System and Biogeochemistry. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- Canadell, J. G.; Monteiro, P. M. S.; Costa, M. H.; Cotrim da Cunha, L.; Ishii, M.; Jaccard, S.; Cox, P. M.; Eliseev, A. V.; Henson, S.; Koven, C.; Lohila, A.; Patra, P. K.; Piao, S.; Rogelj, J.; Syampungani, S.; Zaehle, S.; Zickfeld, K. (2021). "Global Carbon and Other Biogeochemical Cycles and Feedbacks" (PDF). IPCC Sixth Assessment Report: Working Group 1.
- ^ Arora, Vivek K.; Melton, Joe R.; Plummer, David (1 August 2018). "An assessment of natural methane fluxes simulated by the CLASS-CTEM model" (PDF). Biogeosciences. 15 (15): 4683–4709. Bibcode:2018BGeo...15.4683A. doi:10.5194/bg-15-4683-2018.
- Betts (2001). "6.3 Well-mixed Greenhouse Gases". Chapter 6 Radiative Forcing of Climate Change. Working Group I: The Scientific Basis IPCC Third Assessment Report – Climate Change 2001. UNEP/GRID-Arendal – Publications. Archived from the original on 29 June 2011. Retrieved 16 October 2010.
- ^ Jacob, Daniel (1999). Introduction to atmospheric chemistry. Princeton University Press. pp. 25–26. ISBN 978-0691001852. Archived from the original on 2 September 2011.
- "How long will global warming last?". RealClimate. 15 March 2005. Archived from the original on 4 March 2021. Retrieved 12 June 2012.
- "How long will global warming last?". MIT Climate Portal. 17 January 2023.
- Atkinson, Kate (19 July 2023). "How long will global warming last?". Australian Associated Press.
- Ahmed, Issam. "Current carbon dioxide levels last seen 14 million years ago". phys.org. Retrieved 8 February 2024.
- Gulev, S.K., P.W. Thorne, J. Ahn, F.J. Dentener, C.M. Domingues, S. Gerland, D. Gong, D.S. Kaufman, H.C. Nnamchi, J. Quaas, J.A. Rivera, S. Sathyendranath, S.L. Smith, B. Trewin, K. von Schuckmann, and R.S. Vose, 2021: Chapter 2: Changing State of the Climate System. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 287–422, doi:10.1017/9781009157896.004.
- Walker, James C.G. (June 1985). "Carbon dioxide on the early earth" (PDF). Origins of Life and Evolution of the Biosphere. 16 (2): 117–27. Bibcode:1985OrLi...16..117W. doi:10.1007/BF01809466. hdl:2027.42/43349. PMID 11542014. S2CID 206804461. Archived (PDF) from the original on 14 September 2012. Retrieved 30 January 2010.
- Pavlov, Alexander A.; Kasting, James F.; Brown, Lisa L.; Rages, Kathy A.; Freedman, Richard (May 2000). "Greenhouse warming by CH4 in the atmosphere of early Earth". Journal of Geophysical Research. 105 (E5): 11981–90. Bibcode:2000JGR...10511981P. doi:10.1029/1999JE001134. PMID 11543544.
- Harris, Daniel C. (2010). "Charles David Keeling and the Story of Atmospheric CO2 Measurements". Analytical Chemistry. 82 (19): 7865–7870. doi:10.1021/ac1001492. ISSN 0003-2700. PMID 20536268.
- Innocenti, Fabrizio; Robinson, Rod; Gardiner, Tom; Finlayson, Andrew; Connor, Andy (2017). "Differential Absorption Lidar (DIAL) Measurements of Landfill Methane Emissions" (PDF). Remote Sensing. 9 (9): 953. Bibcode:2017RemS....9..953I. doi:10.3390/rs9090953.
- LuAnn Dahlman (14 August 2020). "Climate change: annual greenhouse gas index". NOAA Climate.gov science news & Information for a climate smart nation. Archived from the original on 16 August 2013. Retrieved 5 September 2020.
- "The NOAA Annual Greenhouse Gas Index (AGGI) – An Introduction". NOAA Global Monitoring Laboratory/Earth System Research Laboratories. Archived from the original on 27 November 2020. Retrieved 5 September 2020.
- "NOAA CCGG page Retrieved 2 March 2016". Archived from the original on 11 August 2011. Retrieved 14 March 2023.
- WDCGG webpage Archived 6 April 2016 at the Wayback Machine Retrieved 2 March 2016
- RAMCES webpage
- "CDIAC CO2 page Retrieved 9 February 2016". Archived from the original on 13 August 2011. Retrieved 14 March 2023.
- Prentice, I.C. (2001). "The carbon cycle and atmospheric carbon dioxide". In Houghton, J.T. (ed.). Climate change 2001: the scientific basis: contribution of Working Group I to the Third Assessment Report of the Intergouvernmental Panel on Climate Change. hdl:10067/381670151162165141.
- "An Introduction to the Global Carbon Cycle" (PDF). University of New Hampshire. 2009. Archived (PDF) from the original on 8 October 2016. Retrieved 6 February 2016.
- "Many Planets, One Earth // Section 4: Carbon Cycling and Earth's Climate". Many Planets, One Earth. 4. Archived from the original on 17 April 2012. Retrieved 24 June 2012.
- Friedlingstein, Pierre; O'Sullivan, Michael; Jones, Matthew W.; Andrew, Robbie M.; Hauck, Judith; Olsen, Are; Peters, Glen P.; Peters, Wouter; Pongratz, Julia; Sitch, Stephen; Le Quéré, Corinne; Canadell, Josep G.; Ciais, Philippe; Jackson, Robert B.; Alin, Simone (2020). "Global Carbon Budget 2020". Earth System Science Data. 12 (4): 3269–3340. Bibcode:2020ESSD...12.3269F. doi:10.5194/essd-12-3269-2020. hdl:20.500.11850/458765. ISSN 1866-3516.
- Falkowski, P.; Scholes, R. J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; MacKenzie, F. T.; Moore III, B.; Pedersen, T.; Rosenthal, Y.; Seitzinger, S.; Smetacek, V.; Steffen, W. (2000). "The Global Carbon Cycle: A Test of Our Knowledge of Earth as a System". Science. 290 (5490): 291–296. Bibcode:2000Sci...290..291F. doi:10.1126/science.290.5490.291. PMID 11030643.
- Riebeek, Holli (16 June 2011). "The Carbon Cycle". Earth Observatory. NASA. Archived from the original on 5 March 2016. Retrieved 5 April 2018.
- "AR4 SYR Synthesis Report Summary for Policymakers – 2 Causes of change". ipcc.ch. Archived from the original on 28 February 2018. Retrieved 9 October 2015.
- "Chapter 3, IPCC Special Report on Emissions Scenarios, 2000" (PDF). Intergovernmental Panel on Climate Change. 2000. Archived (PDF) from the original on 20 August 2018. Retrieved 16 October 2010.
- Dhakal, S., J.C. Minx, F.L. Toth, A. Abdel-Aziz, M.J. Figueroa Meza, K. Hubacek, I.G.C. Jonckheere, Yong-Gun Kim, G.F. Nemet, S. Pachauri, X.C. Tan, T. Wiedmann, 2022: Chapter 2: Emissions Trends and Drivers. In IPCC, 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change . Cambridge University Press, Cambridge, UK and New York, NY, USA. doi: 10.1017/9781009157926.004
- "Water Vapor". earthobservatory.nasa.gov. 30 June 2023. Retrieved 16 August 2023.
- Johnston, Chris; Milman, Oliver; Vidal, John (15 October 2016). "Climate change: global deal reached to limit use of hydrofluorocarbons". The Guardian. Retrieved 21 August 2018.
- "Climate change: 'Monumental' deal to cut HFCs, fastest growing greenhouse gases". BBC News. 15 October 2016. Retrieved 15 October 2016.
- "Nations, Fighting Powerful Refrigerant That Warms Planet, Reach Landmark Deal". The New York Times. 15 October 2016. Retrieved 15 October 2016.
- Vaara, Miska (2003), Use of ozone depleting substances in laboratories, TemaNord, p. 170, ISBN 978-9289308847, archived from the original on 6 August 2011
- Montreal Protocol
- ^ United Nations Environment Programme (2022). Emissions Gap Report 2022: The Closing Window — Climate crisis calls for rapid transformation of societies. Nairobi.
- "It's over for fossil fuels: IPCC spells out what's needed to avert climate disaster". The Guardian. 4 April 2022. Retrieved 4 April 2022.
- "The evidence is clear: the time for action is now. We can halve emissions by 2030". IPCC. 4 April 2022. Retrieved 4 April 2022.
- "Ambitious Action Key to Resolving Triple Planetary Crisis of Climate Disruption, Nature Loss, Pollution, Secretary-General Says in Message for International Mother Earth Day | Meetings Coverage and Press Releases". www.un.org. Retrieved 10 June 2022.
- ^ "Geoengineering the climate: science, governance and uncertainty". The Royal Society. 2009. Archived from the original on 7 September 2009. Retrieved 12 September 2009.
- Fisher, B.S., N. Nakicenovic, K. Alfsen, J. Corfee Morlot, F. de la Chesnaye, J.-Ch. Hourcade, K. Jiang, M. Kainuma, E. La Rovere, A. Matysek, A. Rana, K. Riahi, R. Richels, S. Rose, D. van Vuuren, R. Warren, 2007: Chapter 3: Issues related to mitigation in the long term context, In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Inter-governmental Panel on Climate Change , Cambridge University Press, Cambridge,
- Jackson, Robert B.; Abernethy, Sam; Canadell, Josep G.; Cargnello, Matteo; Davis, Steven J.; Féron, Sarah; Fuss, Sabine; Heyer, Alexander J.; Hong, Chaopeng; Jones, Chris D.; Damon Matthews, H.; O'Connor, Fiona M.; Pisciotta, Maxwell; Rhoda, Hannah M.; de Richter, Renaud (15 November 2021). "Atmospheric methane removal: a research agenda". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 379 (2210): 20200454. Bibcode:2021RSPTA.37900454J. doi:10.1098/rsta.2020.0454. ISSN 1364-503X. PMC 8473948. PMID 34565221.
- "Coal Consumption Affecting Climate". Rodney and Otamatea Times, Waitemata and Kaipara Gazette. Warkworth, New Zealand. 14 August 1912. p. 7. Text was earlier published in Popular Mechanics, March 1912, p. 341.
- Arrhenius, Svante (1896). "On the influence of carbonic acid in the air upon the temperature of the ground" (PDF). The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 41 (251): 237–276. doi:10.1080/14786449608620846. Archived (PDF) from the original on 18 November 2020. Retrieved 1 December 2020.
- Arrhenius, Svante (1897). "On the Influence of Carbonic Acid in the Air Upon the Temperature of the Ground". Publications of the Astronomical Society of the Pacific. 9 (54): 14. Bibcode:1897PASP....9...14A. doi:10.1086/121158.
- Easterbrook, Steve (18 August 2015). "Who first coined the term "Greenhouse Effect"?". Serendipity. Archived from the original on 13 November 2015. Retrieved 11 November 2015.
- Ekholm N (1901). "On The Variations Of The Climate Of The Geological And Historical Past And Their Causes". Quarterly Journal of the Royal Meteorological Society. 27 (117): 1–62. Bibcode:1901QJRMS..27....1E. doi:10.1002/qj.49702711702.
- Cook, J.; Nuccitelli, D.; Green, S.A.; Richardson, M.; Winkler, B.R.; Painting, R.; Way, R.; Jacobs, P.; Skuce, A. (2013). "Quantifying the consensus on anthropogenic global warming in the scientific literature". Environmental Research Letters. 8 (2): 024024. Bibcode:2013ERL.....8b4024C. doi:10.1088/1748-9326/8/2/024024.
- Eddie Schwieterman. "Comparing the Greenhouse Effect on Earth, Mars, Venus, and Titan: Present Day and through Time" (PDF). Archived from the original (PDF) on 30 January 2015.
- Scoping of the IPCC 5th Assessment Report Cross Cutting Issues (PDF). Thirty-first Session of the IPCC Bali, 26–29 October 2009 (Report). Archived (PDF) from the original on 9 November 2009. Retrieved 24 March 2019.
- Hansen, James; Sato, Makiko; Russell, Gary; Kharecha, Pushker (2013). "Climate sensitivity, sea level and atmospheric carbon dioxide". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 371 (2001). 20120294. arXiv:1211.4846. Bibcode:2013RSPTA.37120294H. doi:10.1098/rsta.2012.0294. PMC 3785813. PMID 24043864.
External links
 Media related to Greenhouse gases at Wikimedia Commons
Media related to Greenhouse gases at Wikimedia Commons- Carbon Dioxide Information Analysis Center (CDIAC), U.S. Department of Energy, retrieved 26 July 2020
- Annual Greenhouse Gas Index (AGGI) from NOAA
- Atmospheric spectra of GHGs and other trace gases. Archived 25 March 2013 at the Wayback Machine.
 of an atmospheric
of an atmospheric  (in kg) of X in the box to its removal rate, which is the sum of the flow of X out of the box
(
(in kg) of X in the box to its removal rate, which is the sum of the flow of X out of the box
( ),
chemical loss of X
(
),
chemical loss of X
( ),
and
),
and  )
(all in kg/s):
)
(all in kg/s):
 .
.