| Revision as of 13:17, 23 July 2022 editPrime Lemur (talk | contribs)Extended confirmed users773 edits →External links: Original URL was dead & redirected to enrolments page. Internal search returned zero hits, Google search, however, found new URL at same institution.Tags: Mobile edit Mobile web edit Advanced mobile edit← Previous edit | Latest revision as of 03:05, 24 February 2023 edit undoSimLibrarian (talk | contribs)Extended confirmed users124,334 editsm capitalization fixTag: Visual edit | ||
| Line 53: | Line 53: | ||
| * Many ]s and other aromatic ]s. | * Many ]s and other aromatic ]s. | ||
| *The folate precursor ''para''-aminobenzoate ] | *The folate precursor ''para''-aminobenzoate ] | ||
| * The biosynthesis of ] and ] in plants and microorganisms. | * The biosynthesis of ] and ] in plants and microorganisms. | ||
| The name chorismic acid derives from a classical Greek word {{lang|grc|χωρίζω}} meaning "to separate",<ref>{{cite book|isbn=0-19-864226-1|title=A Greek-English Lexicon|author=Henry George Liddell|author2=Robert Scott|author3=Henry Stuart Jones|author4=Roderick McKenzie|name-list-style=amp|title-link=A Greek-English Lexicon}}</ref> because the compound plays a role as a branch-point in aromatic amino acid biosynthesis.<ref>{{Cite journal | last1 = Gibson | first1 = F. | title = The elusive branch-point compound of aromatic amino acid biosynthesis | doi = 10.1016/S0968-0004(98)01330-9 | journal = Trends in Biochemical Sciences | volume = 24 | issue = 1 | pages = 36–38 | year = 1999 | pmid = 10087921}}</ref> | The name chorismic acid derives from a classical Greek word {{lang|grc|χωρίζω}} meaning "to separate",<ref>{{cite book|isbn=0-19-864226-1|title=A Greek-English Lexicon|author=Henry George Liddell|author2=Robert Scott|author3=Henry Stuart Jones|author4=Roderick McKenzie|name-list-style=amp|title-link=A Greek-English Lexicon}}</ref> because the compound plays a role as a branch-point in aromatic amino acid biosynthesis.<ref>{{Cite journal | last1 = Gibson | first1 = F. | title = The elusive branch-point compound of aromatic amino acid biosynthesis | doi = 10.1016/S0968-0004(98)01330-9 | journal = Trends in Biochemical Sciences | volume = 24 | issue = 1 | pages = 36–38 | year = 1999 | pmid = 10087921}}</ref> | ||
Latest revision as of 03:05, 24 February 2023

| |

| |
| Names | |
|---|---|
| IUPAC name (3R,4R)-3--4-hydroxycyclohexa-1,5-diene-1-carboxylic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.164.204 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H10O6 |
| Molar mass | 226.184 g·mol |
| Melting point | 140 °C (284 °F; 413 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H302, H312, H315, H319, H332, H335, H350, H361 |
| Precautionary statements | P201, P202, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Chorismic acid, more commonly known as its anionic form chorismate, is an important biochemical intermediate in plants and microorganisms. It is a precursor for:
- The aromatic amino acids phenylalanine, tryptophan, and tyrosine
- Indole, indole derivatives and tryptophan
- 2,3-Dihydroxybenzoic acid (DHB) used for enterobactin biosynthesis
- The plant hormone salicylic acid
- Many alkaloids and other aromatic metabolites.
- The folate precursor para-aminobenzoate (pABA)
- The biosynthesis of vitamin K and folate in plants and microorganisms.
The name chorismic acid derives from a classical Greek word χωρίζω meaning "to separate", because the compound plays a role as a branch-point in aromatic amino acid biosynthesis.
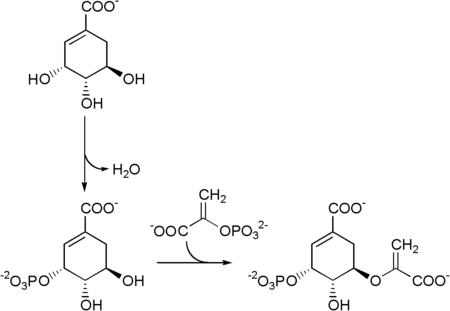
Biosynthesis
Shikimate → shikimate-3-phosphate → 5-enolpyruvylshikimate-3-phosphate (5-O-(1-carboxyvinyl)-3-phosphoshikimate)
Chorismate synthase is an enzyme that catalyzes the final chemical reaction:
- 5-O-(1-carboxyvinyl)-3-phosphoshikimate → chorismate + phosphate.
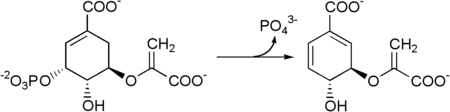
Metabolism
Chorismate is transformed into para-aminobenzoic acid by the enzymes 4-amino-4-deoxychorismate synthase and 4-amino-4-deoxychorismate lyase.
Chorismate lyase is an enzyme that transforms chorismate into 4-hydroxybenzoate and pyruvate. This enzyme catalyses the first step in ubiquinone biosynthesis in Escherichia coli and other Gram-negative bacteria.
See also
References
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001). "Isochorismate synthase is required to synthesize salicylic acid for plant defence". Nature. 414 (6863): 562–5. Bibcode:2001Natur.414..562W. doi:10.1038/35107108. PMID 11734859.
- Henry George Liddell; Robert Scott; Henry Stuart Jones & Roderick McKenzie. A Greek-English Lexicon. ISBN 0-19-864226-1.
- Gibson, F. (1999). "The elusive branch-point compound of aromatic amino acid biosynthesis". Trends in Biochemical Sciences. 24 (1): 36–38. doi:10.1016/S0968-0004(98)01330-9. PMID 10087921.