| Revision as of 13:32, 28 November 2023 editBon courage (talk | contribs)Extended confirmed users66,166 edits →Timing of spillover: rmv. use of primary to undercut secondary as prohitied by MEDRS← Previous edit | Latest revision as of 16:56, 21 December 2024 edit undoRMCD bot (talk | contribs)Bots, Template editors996,715 edits Removing notice of move discussion | ||
| (58 intermediate revisions by 22 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|none}} | |||
| {{medref|date=November 2023}} | {{medref|date=November 2023}} | ||
| {{Merge to|Origin of SARS-CoV-2|date=December 2024}} | |||
| {{short description|Theories on how COVID-19 originated in animals}} | |||
| {{Merge to|Origin of COVID-19|discuss=Talk:Origin of COVID-19#Proposed merge of COVID-19 zoonosis theories into Origin of COVID-19|date=November 2023}} | |||
| {{COVID-19 pandemic sidebar}} | {{COVID-19 pandemic sidebar}} | ||
| {{Use mdy dates|date=February 2024}} | |||
| ], the causative agent of ], was first introduced to humans through ] (transmission of a pathogen to a human from an animal), and a zoonotic spillover event is the ] that is considered most plausible by the scientific community.{{efn| :{{sfn|Balloux|Tan|Swadling|Richard|2022|p=4}} ... "the proximal phylogenetic origin of SARS-CoV-2 and its mode of introduction into human circulation remain unclear. There is no evidence for SARS-CoV-2 having been 'engineered' in a lab. Conversely, the escape of a strain from a lab or an accidental contamination during field work cannot formally be ruled out at this stage. However, a zoonotic spillover event in nature is considered as the most plausible scenario in the scientific community"}} Human ] including ] are zoonotic diseases that are often acquired through ] from animals.{{sfn|Kane|Wong|Gao|2023|p=15}} | ], the causative agent of ], was first introduced to humans through ] (transmission of a pathogen to a human from an animal), and a zoonotic spillover event is the ] that is considered most plausible by the scientific community.{{efn| :{{sfn|Balloux|Tan|Swadling|Richard|2022|p=4}} ... "the proximal phylogenetic origin of SARS-CoV-2 and its mode of introduction into human circulation remain unclear. There is no evidence for SARS-CoV-2 having been 'engineered' in a lab. Conversely, the escape of a strain from a lab or an accidental contamination during field work cannot formally be ruled out at this stage. However, a zoonotic spillover event in nature is considered as the most plausible scenario in the scientific community"}} Human ] including ] are zoonotic diseases that are often acquired through ] from animals.{{sfn|Kane|Wong|Gao|2023|p=15}} | ||
| ==Background== | ==Background== | ||
| Previous emergence of SARS-CoV-1 and ] showed that ] represent a risk for emergence of diseases threatening to humans.{{sfn|Kane|Wong|Gao|2023|p=23}}{{sfn|Kane|Wong|Gao|2023|pp=20,21,23}} Increased awareness due to the ] motivated research into the potential for other coronavirus outbreaks and the ] which could lead to them.{{sfn|Graham|Baric|2010|p=3135}} Bats, in particular, are known to harbor persistent populations of coronaviruses, and under conditions of persistent infection, coronaviruses tend to accumulate mutations that allow their receptor binding domains to interact with cross-species ] of the ].{{sfn|Graham|Baric|2010|pp=3139-3140}} Based on serological and molecular studies, ] were identified as the most likely reservoir for SARS-CoV-1.{{sfn|Kane|Wong|Gao|2023|p=12}} Bats were also a likely reservoir for the related betacoronavirus ], though the evidence for this is less conclusive than the role of ] as a reservoir for MERS.{{sfn|Kane|Wong|Gao|2023|p=13}} By 2010, ''in vitro'' experiments had confirmed that modifications to the ] binding domain could enable human infection by several SARS-related coronaviruses.{{sfn|Graham|Baric|2010}} Virologists Rachel Graham and ] at that time wrote, "that the question of emergence of another pathogenic human coronavirus from bat reservoirs might be more appropriately expressed as 'when' than as 'if'."{{sfn|Graham|Baric|2010|pp=3142}}{{Better source needed|reason=The current source is out-of-date for its intended impact. MERS appeared after publication. We need one that was post-2015 which, I am sure, we can find.|date=November 2023}} |
Previous emergence of SARS-CoV-1 and ] showed that ] represent a risk for emergence of diseases threatening to humans.{{sfn|Kane|Wong|Gao|2023|p=23}}{{sfn|Kane|Wong|Gao|2023|pp=20,21,23}} Increased awareness due to the ] motivated research into the potential for other coronavirus outbreaks and the ] which could lead to them.{{sfn|Graham|Baric|2010|p=3135}} Bats, in particular, are known to harbor persistent populations of coronaviruses, and under conditions of persistent infection, coronaviruses tend to accumulate mutations that allow their receptor binding domains to interact with cross-species ] of the ].{{sfn|Graham|Baric|2010|pp=3139-3140}} Based on serological and molecular studies, ] were identified as the most likely reservoir for SARS-CoV-1.{{sfn|Kane|Wong|Gao|2023|p=12}} Bats were also a likely reservoir for the related betacoronavirus ], though the evidence for this is less conclusive than the role of ] as a reservoir for MERS.{{sfn|Kane|Wong|Gao|2023|p=13}} By 2010, ''in vitro'' experiments had confirmed that modifications to the ] binding domain could enable human infection by several SARS-related coronaviruses.{{sfn|Graham|Baric|2010}} Virologists Rachel Graham and ] at that time wrote, "that the question of emergence of another pathogenic human coronavirus from bat reservoirs might be more appropriately expressed as 'when' than as 'if'."{{sfn|Graham|Baric|2010|pp=3142}}{{Better source needed|reason=The current source is out-of-date for its intended impact. MERS appeared after publication. We need one that was post-2015 which, I am sure, we can find.|date=November 2023}} | ||
| In 2013, researchers identified for the first time a virus (designated ]) infecting bats that was capable of using human ] for cell entry without further adaptation in its receptor binding domain.{{sfn|Ge|Li|Yang|Chmura|2013}} To study its infectivity, Menachery ''et al.'' created a ] virus incorporating the WIV1 spike protein into a backbone of mouse-adapted SARS-CoV-1.{{sfn|Menachery|Yount|Sims|Baric|2016}} They confirmed its ability to infect human airway ] in culture. It also infected ] in vivo but was rapidly cleared by the immune system. Menachery ''et al.'' concluded that the WIV1 clade represented a threat for future human pathogen emergence, but it would require additional adaptations to be capable of epidemic spread.{{sfn|Menachery|Yount|Sims|Baric|2016|p=3052}} | |||
| ==Origin in bats== | ==Origin in bats== | ||
| The likely origin of ] from bats aligns with the origins of other coronaviruses in its genus ], subgenus ].{{sfn|Kane|Wong|Gao|2023|pp=2-3}}{{sfn|Cagliani|Forni|Clerici|Sironi|2020|p=1}}{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020|p=1}}{{sfn|Kane|Wong|Gao|2023|pp=12,23}} Several bat species have special cellular mechanisms to resist proinflammatory ] associated with Betacoronavirus ],{{sfn|Kane|Wong|Gao|2023|p=17}} for example, ] in ]es coevolve with bat ] receptors in an evolutionary arms race.{{sfn|Guo|Hu|Yang|Zeng|2020 |
The likely origin of ] from bats aligns with the origins of other coronaviruses in its genus ], subgenus ].{{sfn|Kane|Wong|Gao|2023|pp=2-3}}{{sfn|Cagliani|Forni|Clerici|Sironi|2020|p=1}}{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020|p=1}}{{sfn|Kane|Wong|Gao|2023|pp=12,23}} Several bat species have special cellular mechanisms to resist proinflammatory ] associated with Betacoronavirus ],{{sfn|Kane|Wong|Gao|2023|p=17}} for example, ] in ]es coevolve with bat ] receptors in an evolutionary arms race.{{sfn|Guo|Hu|Yang|Zeng|2020}} | ||
| ] of SARS-CoV-2 and closely related betacoronaviruses (left) and their geographic context (right)]] | ] of SARS-CoV-2 and closely related betacoronaviruses (left) and their geographic context (right)]] | ||
| Bats, along with their viruses, have large overlapping geographic ranges in Southeast Asia,{{sfn|Lytras|Hughes|Martin|Swanepoel|2022}} and there is a particularly great concentration and diversity of bat-related coronaviruses in Southern and Southwest China.{{sfn|Kane|Wong|Gao|2023|p=17}} The most similar known viruses to SARS-CoV-2 include bat coronaviruses |
Bats, along with their viruses, have large overlapping geographic ranges in Southeast Asia,{{sfn|Lytras|Hughes|Martin|Swanepoel|2022}} and there is a particularly great concentration and diversity of bat-related coronaviruses in Southern and Southwest China.{{sfn|Kane|Wong|Gao|2023|p=17}} The most similar known viruses to SARS-CoV-2 include bat coronaviruses ] with 94.5% identity,{{sfn|Kane|Wong|Gao|2023|p=17}} and ] with 93% identity{{sfn|Kane|Wong|Gao|2023|p=3}} RaTG13 was not the direct progenitor of SARS-CoV-2.{{sfn|Holmes|Goldstein|Rasmussen|Robertson|2021|p=4850}} Temmam ''et al.'' found no serological evidence for exposure to BANAL-52 among bat handlers and guano collectors in the area of ] where it was sampled.{{sfn|Temmam|Montagutelli|Herate|Donati|2023|p=8,10}} Lytras ''et al.'' wrote that "SARS-CoV-2 can be unambiguously traced to horseshoe bats".{{sfn|Lytras|Hughes|Martin|Swanepoel|2022|p=1}} They estimated SARS-CoV-2's ]s with RmYN02 and RaTG13 to have diverged 40 and 50 years ago, respectively.{{sfn|Lytras|Hughes|Martin|Swanepoel|2022|p=4}} | ||
| ==Novel features of SARS-CoV-2== | ==Novel features of SARS-CoV-2== | ||
| The ] binding domain of the SARS-CoV-2 ] has an insertion of ] between its S1 and S2 subunits.{{sfn|Kane|Wong|Gao|2023|pp=3,14 |
The ] binding domain of the SARS-CoV-2 ] has an insertion of ] between its S1 and S2 subunits.{{sfn|Kane|Wong|Gao|2023|pp=3,14}} Among Sarbecoviruses, only SARS-CoV-2 and RmYN02 have such an insertion, suggesting differences in reservoir species, intermediate hosts, or evolutionary pathway.{{sfn|Kane|Wong|Gao|2023|p=3}} The receptor binding motif is the portion of SARS-CoV-2 that is most diverged from RaTG13.{{sfn|Cagliani|Forni|Clerici|Sironi|2020|p=5}} The SARS-CoV-2 receptor binding domain is more similar to those of ] coronaviruses.{{sfn|Kane|Wong|Gao|2023|p=3}} Viruses including BANAL-52 isolated from bats in ] showed high similarity to the SARS-CoV-2 receptor binding domain in amino acid residues but less than 76.4% nucleotide identity across the spike protein.{{sfn|Kane|Wong|Gao|2023|p=15}} The observed binding of ] by the NTD<ref>{{cite journal | url=https://doi.org/10.1126/science.abm3125 | doi=10.1126/science.abm3125 | title=Pathogen-sugar interactions revealed by universal saturation transfer analysis | date=2022 | last1=Buchanan | first1=Charles J. | last2=Gaunt | first2=Ben | last3=Harrison | first3=Peter J. | last4=Yang | first4=Yun | last5=Liu | first5=Jiwei | last6=Khan | first6=Aziz | last7=Giltrap | first7=Andrew M. | last8=Le Bas | first8=Audrey | last9=Ward | first9=Philip N. | last10=Gupta | first10=Kapil | last11=Dumoux | first11=Maud | last12=Tan | first12=Tiong Kit | last13=Schimaski | first13=Lisa | last14=Daga | first14=Sergio | last15=Picchiotti | first15=Nicola | last16=Baldassarri | first16=Margherita | last17=Benetti | first17=Elisa | last18=Fallerini | first18=Chiara | last19=Fava | first19=Francesca | last20=Giliberti | first20=Annarita | last21=Koukos | first21=Panagiotis I. | last22=Davy | first22=Matthew J. | last23=Lakshminarayanan | first23=Abirami | last24=Xue | first24=Xiaochao | last25=Papadakis | first25=Georgios | last26=Deimel | first26=Lachlan P. | last27=Casablancas-Antràs | first27=Virgínia | last28=Claridge | first28=Timothy D. W. | last29=Bonvin | first29=Alexandre M. J. J. | last30=Sattentau | first30=Quentin J. | journal=Science | volume=377 | issue=6604 | pages=eabm3125 | pmid=35737812 | hdl=1983/355cbd8f-c424-4cc0-adb2-881c04ab3bf0 | display-authors=1 | hdl-access=free }}</ref> of the ] and loss of that binding through mutation of the corresponding sugar binding pocket in emergent variants of concern has suggested a potential role for transient sugar-binding in the zoonosis of SARS-CoV-2, consistent with prior evolutionary proposals.<ref>{{cite journal | doi=10.1016/S0021-9258(18)63732-9 | doi-access=free | title=The Canyon Hypothesis | date=1989 | last1=Rossmann | first1=M. G. | journal=Journal of Biological Chemistry | volume=264 | issue=25 | pages=14587–14590 }}</ref> | ||
| ⚫ | |||
| ⚫ | ] | ||
| ⚫ | Within genus ''Betacoronavirus'', furin cleavage sites are common in subgenera '']'' and '']''.{{sfn|Wu|Zhao|2021}} Furin cleavage sites have independently evolved six times in different clades of Betacoronavirus.{{sfn|Wu|Zhao|2021}} Furin cleavage sites also evolved in Alphacoronaviruses and Gammacoronaviruses independently of Betacoronavirus.{{sfn|Wu|Zhao|2021}} | ||
| ⚫ | Furin cleavage contributes strongly to the transmissibility and pathogenicity of SARS-CoV-2 in humans. |
||
| ⚫ | ] | ||
| ⚫ | Furin cleavage contributes strongly to the transmissibility and pathogenicity of SARS-CoV-2 in humans.{{sfn|Hossain|Tang|Akter|Zheng|2021|p=1816}} SARS-CoV-2 variants lacking the furin cleavage site are transmissible between humans, but much less effectively.{{sfn|Hossain|Tang|Akter|Zheng|2021|p=1817}} The furin cleavage site is sometimes described as "polybasic" on account of its particular motif of ] amino acids.{{sfn|Hossain|Tang|Akter|Zheng|2021|p=1816}} SARS-CoV-2 shares amino acid identity with a furin cleavage site of human ] α subunit.{{sfn|Anand|Puranik|Aravamudan|Venkatakrishnan|2020}}{{sfn|Harrison|Sachs|2022}}{{efn|Harrison and Sachs' analysis of the furin cleavage site have been disputed in a letter to the editor {{cite journal|last=Garry|first=Robert F.|title=SARS-CoV-2 furin cleavage site was not engineered|journal=PNAS|volume=119|number=40|date=September 29, 2022|pages=e2211107119 |doi=10.1073/pnas.2211107119|doi-access=free |pmid=36173950 |pmc=9546612 |bibcode=2022PNAS..11911107G }}, with a reply from the authors {{cite journal|last1=Harrison|first1=Neil L.|last2=Sachs|first2=Jeffery D.|title=Reply to Garry: The origin of SARS-CoV-2 remains unresolved|journal=PNAS|volume=119|number=45|date=November 2, 2022a|pages=e2215826119 |doi=10.1073/pnas.2215826119|pmid=36322733 |pmc=9659384 |bibcode=2022PNAS..11915826H }} }} Human ENaC is identical only to that of a few ] and ].{{sfn|Harrison|Sachs|2022|p=4}} | ||
| The furin cleavage site could hypothetically have arisen in several ways: ] between an ancestor of SARS-CoV-2 and a virus already possessing a furin cleavage site, ] in an intermediate host, or adaptation by an asymptomatic early human infection.{{sfn|Temmam|Vongphayloth|Baquero|Munier|2022|p=335}} | |||
| SARS-CoV-2 is also distinct among human coronaviruses for having a single intact ORF8 gene rather than "a" and "b" subunits.{{sfn|Balloux|Tan|Swadling|Richard|2022|p=4}} | SARS-CoV-2 is also distinct among human coronaviruses for having a single intact ORF8 gene rather than "a" and "b" subunits.{{sfn|Balloux|Tan|Swadling|Richard|2022|p=4}} | ||
| ==Processes of host adaptation== | ==Processes of host adaptation== | ||
| SARS-CoV-2 has an expanded host range compared to SARS-CoV-1 and MERS-CoV.{{sfn|Kane|Wong|Gao|2023|p=14}}{{sfn|Li|Du|Liu|Li|2023}} SARS-CoV-2 (along with SARS-CoV-1 and MERS-CoV) are generalist viruses, not specifically adapted to humans, meaning they have potential to spill over to many species and establish new natural reservoirs after adaptive evolutionary changes.{{sfn|Li|Du|Liu|Li|2023|p=557}} | |||
| Within a single host, a variety of ] arise through random mutations and ] giving rise to viral ].{{sfn|Biancolella|Colona|Luzzatto|Watt|2023|p=3}} SARS-CoV-2 mutates at a slower rate than is typical of RNA viruses.{{sfn|Biancolella|Colona|Luzzatto|Watt|2023|p=3}} The main host-derived driver of mutation is editing by ] proteins.{{sfn|Biancolella|Colona|Luzzatto|Watt|2023|p=3}} Negative selection by host immune processes causes convergent evolution towards immune escape.{{sfn|Biancolella|Colona|Luzzatto|Watt|2023|p=3}} Persistence of infection is correlated with quasispecies diversity, but the direction of causality for this is unknown.{{sfn|Biancolella|Colona|Luzzatto|Watt|2023|p=3}} | Within a single host, a variety of ] arise through random mutations and ] giving rise to viral ].{{sfn|Biancolella|Colona|Luzzatto|Watt|2023|p=3}} SARS-CoV-2 mutates at a slower rate than is typical of RNA viruses.{{sfn|Biancolella|Colona|Luzzatto|Watt|2023|p=3}} The main host-derived driver of mutation is editing by ] proteins.{{sfn|Biancolella|Colona|Luzzatto|Watt|2023|p=3}} Negative selection by host immune processes causes convergent evolution towards immune escape.{{sfn|Biancolella|Colona|Luzzatto|Watt|2023|p=3}} Persistence of infection is correlated with quasispecies diversity, but the direction of causality for this is unknown.{{sfn|Biancolella|Colona|Luzzatto|Watt|2023|p=3}} | ||
| Actual host susceptibility can differ significantly from '']'' predictions.{{sfn|Li|Du|Liu|Li|2023|p=557}}{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020|p=3}} The process of host adaptation has been studied in ] as well as by generating mouse-adapted viral strains through ].{{sfn|Kane|Wong|Gao|2023|p=8}} ] mice are only weakly |
Actual host susceptibility can differ significantly from '']'' predictions.{{sfn|Li|Du|Liu|Li|2023|p=557}}{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020|p=3}} The process of host adaptation has been studied in ] as well as by generating mouse-adapted viral strains through ].{{sfn|Kane|Wong|Gao|2023|p=8}} ] mice are only weakly susceptible to the original SARS-CoV-2 strain.{{sfn|Kane|Wong|Gao|2023|p=8}} | ||
| ===Recombination=== | ===Recombination=== | ||
| Compared to other ], coronaviruses have increased tendency to undergo ], which allows them to exchange genetic material with close relatives co-infecting the same organism.{{sfn|Kane|Wong|Gao|2023|p=19}} The origin of SARS-CoV-1 is believed to involve multiple recombination events.{{sfn|Graham|Baric|2010|p=3139}} Recombination between strains of SARS-CoV-1 is common.{{sfn|Kane|Wong|Gao|2023|p=20}} Recombination between various SARS-CoV-2 ] has been reported.{{sfn|Kane|Wong|Gao|2023|p=4}} | Compared to other ], coronaviruses have increased tendency to undergo ], which allows them to exchange genetic material with close relatives co-infecting the same organism.{{sfn|Kane|Wong|Gao|2023|p=19}} The origin of SARS-CoV-1 is believed to involve multiple recombination events.{{sfn|Graham|Baric|2010|p=3139}} Recombination between strains of SARS-CoV-1 is common.{{sfn|Kane|Wong|Gao|2023|p=20}} Inferred phylogenies of SARS2-related coronaviruses might be explained by recombination.{{sfn|Qian|Li|Tang|Lu|2022|p=8}} Recombination between various SARS-CoV-2 ] has been reported.{{sfn|Kane|Wong|Gao|2023|p=4}} | ||
| ] | ] | ||
| Lytras ''et al.'' identified the SARS-CoV-2 spike open reading frame as a recombination hotspot.{{sfn|Lytras|Hughes|Martin|Swanepoel|2022|p=3}} They speculate that the SARS-CoV-2 genome may involve repeated recombination events overprinting regions that were already themselves products of recombination.{{sfn|Lytras|Hughes|Martin|Swanepoel|2022|p=6}} Temmam ''et al.'' wrote that due to the limited diffusion of bat viruses in mammals, co-infection required for recombination in mammals was unlikely.{{sfn|Temmam|Montagutelli|Herate|Donati|2023|p=10}} Therefore, they considered it more likely that the furin cleavage site arose in a bat reservoir prior to spillover. | |||
| ===Selection=== | ===Selection=== | ||
| Line 45: | Line 41: | ||
| ] with one ] highlighted. The ACE2 binding domain is magenta.]] | ] with one ] highlighted. The ACE2 binding domain is magenta.]] | ||
| Strong evidence of positive selection was found however in the spike protein S1 subunit, which contains the receptor binding domain.{{sfn|Cagliani|Forni|Clerici|Sironi|2020|p=5}}{{sfn|Qian|Li|Tang|Lu|2022 |
Strong evidence of positive selection was found however in the spike protein S1 subunit, which contains the receptor binding domain.{{sfn|Cagliani|Forni|Clerici|Sironi|2020|p=5}}{{sfn|Qian|Li|Tang|Lu|2022}} | ||
| ] in the spike protein is typical of coronaviruses in general, favoring the production of numerous spike protein variants.{{sfn|Kane|Wong|Gao|2023|p=4}} The receptor binding domain is a significant factor in ], or the variety of species a virus can infect.{{sfn|Kane|Wong|Gao|2023|p=16}}{{sfn|Li|Du|Liu|Li|2023|p=554}} Coronavirus adaptation to a new host often requires mutations in the receptor binding domain.{{sfn|Kane|Wong|Gao|2023|p=21 |
] in the spike protein is typical of coronaviruses in general, favoring the production of numerous spike protein variants.{{sfn|Kane|Wong|Gao|2023|p=4}} The receptor binding domain is a significant factor in ], or the variety of species a virus can infect.{{sfn|Kane|Wong|Gao|2023|p=16}}{{sfn|Li|Du|Liu|Li|2023|p=554}} Coronavirus adaptation to a new host often requires mutations in the receptor binding domain.{{sfn|Kane|Wong|Gao|2023|p=21}} Kang ''et al.'' identified a ] relative to RaTG13 in the spike protein, consistent among all of more than 180,000 SARS-CoV-2 samples, affecting ] of the receptor binding domain.{{sfn|Kang|He|Sharp|Wang|2021|p=4396}} Using a reverse genetics system to generate an ancestral-like mutant, they confirmed that the putative ancestral form of this SNP was much less transmissible in human cells.{{sfn|Kang|He|Sharp|Wang|2021|p=4397}} | ||
| Early in the pandemic, SARS-CoV-2 diverged into two lineages differing from one another in two positions with strong ].{{sfn|Qian|Li|Tang|Lu|2022|p=11,15}} This leads to either a leucine or serine in a particular position, causing the lineages to be named "L" and "S". They have also been called "A" and "B", with "A" corresponding with "S" and "B" with "L". Qiang ''et al.'' write that the persistence of the linkage between the two sites suggests ], though the mechanism is unknown.{{sfn|Qian|Li|Tang|Lu|2022|p=15}} The adaptations required for a functional furin cleavage site also come at a cost to the protein's structural stability.{{sfn|Lubinski|Whittaker|2023}} Pathogenic mutations are therefore accompanied by simultaneous epistatic mutations compensating for this instability. Consequently, the furin cleavage site is quickly lost to random mutations in cell culture. Different solutions to this stability problem underlie the divergence of lineages A and B. It is also integral to the emergence of ]. | |||
| ===Host editing=== | |||
| From December 2019 through February 2020, SARS-CoV-2 mutations showed a bias towards converting ] to ].{{sfn|Tai|Sun|Tsung|Li|2022|p=5}} Tai ''et al.'' wrote that this may have been due to a CpG targeting mechanism involving zinc finger ].{{sfn|Tai|Sun|Tsung|Li|2022|p=7}} In contrast, the divergence of SARS-CoV-2 and RaTG13 from ancestral sarbecoviruses involved the opposite bias.{{sfn|Tai|Sun|Tsung|Li|2022|p=7}} Qiang ''et al.'' wrote that editing by APOBECs could account for the codon bias.{{sfn|Qian|Li|Tang|Lu|2022|p=10}} MacLean ''et al.'' wrote that the codon bias is evidence that a common progenitor of SARS-CoV-2 and RmYN02 underwent positive selection in bats without circulation in other species.{{sfn|MacLean|Lytras|Weaver|Singer|2021|pp=1,7-9}} The S/A lineage is more similar to presumed ancestral sequences in its two nucleotide variations.{{sfn|Qian|Li|Tang|Lu|2022|p=11}} Pekar ''et al.'' wrote that lineage L/B was the earlier strain in humans, and that lineage S/A reverted to ancestral nucleotides due to mutational codon bias.{{sfn|Pekar|Magee|Parker|Moshiri|2022|p=2}} | |||
| ===Host proteases=== | ===Host proteases=== | ||
| The majority of sarbecoviruses are not dependent on ACE2 for cell entry.{{sfn|Guo|Li|Dong|Su|2022}} Guo ''et al.'' divide the sarbecoviruses into four clades, the first being ACE2-using respiratory viruses including SARS-CoV-1, SARS-CoV-2, and WIV1. Relative to clade 1, clades 3 and 4 have a one residue deletion in the receptor binding domain and diminished ability to use ACE2. Clade 2 has two deletions and does not interact with ACE2. Clades 2 through 4 are more difficult to isolate or propagate in cell culture, and consequently have been less studied. ACE2-independent infection by sarbecoviruses depends on high levels of ], a digestive protein, in the host environment. Trypsin may compensate for other missing or compatible host proteases. The ubiquity of furin compared to tripsin would allow the gain of a furin cleavage site to expand viral tissue tropism.{{sfn|Jaimes|Millet|Whittaker|2020|p=2}} Guo ''et al.'' identified clade 1 and 2 sarbecoviruses in ''Rhinolophus'' fecal samples, suggesting that both types naturally replicate in the bat digestive tract.{{sfn|Guo|Li|Dong|Su|2022}} Bats with SARS-like infections in the wild showed no viral replication in respiratory droplets.{{sfn|Graham|Baric|2010|p=3141}} A fecal-oral transmission is an alternative route for some respiratory viruses.{{sfn|Guo|Li|Dong|Su|2022}} No higher incidence of Sarbecoviruses has been reported in workers who come into direct contact with guano.{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020|p=1}} Temmam ''et al.'' found that BANAL-236, a SARS-CoV-2-related virus isolated from bats in Laos, acts as an enteric virus in ].{{sfn|Temmam|Montagutelli|Herate|Donati|2023|p=5 |
The majority of sarbecoviruses are not dependent on ACE2 for cell entry.{{sfn|Guo|Li|Dong|Su|2022}} Guo ''et al.'' divide the sarbecoviruses into four clades, the first being ACE2-using respiratory viruses including SARS-CoV-1, SARS-CoV-2, and WIV1. Relative to clade 1, clades 3 and 4 have a one residue deletion in the receptor binding domain and diminished ability to use ACE2. Clade 2 has two deletions and does not interact with ACE2. Clades 2 through 4 are more difficult to isolate or propagate in cell culture, and consequently have been less studied. ACE2-independent infection by sarbecoviruses depends on high levels of ], a digestive protein, in the host environment. Trypsin may compensate for other missing or compatible host proteases. The ubiquity of furin compared to tripsin would allow the gain of a furin cleavage site to expand viral tissue tropism.{{sfn|Jaimes|Millet|Whittaker|2020|p=2}} Guo ''et al.'' identified clade 1 and 2 sarbecoviruses in ''Rhinolophus'' fecal samples, suggesting that both types naturally replicate in the bat digestive tract.{{sfn|Guo|Li|Dong|Su|2022}} Bats with SARS-like infections in the wild showed no viral replication in respiratory droplets.{{sfn|Graham|Baric|2010|p=3141}} A fecal-oral transmission is an alternative route for some respiratory viruses.{{sfn|Guo|Li|Dong|Su|2022}} No higher incidence of Sarbecoviruses has been reported in workers who come into direct contact with guano.{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020|p=1}} Temmam ''et al.'' found that BANAL-236, a SARS-CoV-2-related virus isolated from bats in Laos, acts as an enteric virus in ].{{sfn|Temmam|Montagutelli|Herate|Donati|2023|p=5}} | ||
| ==Timing of spillover== | ==Timing of spillover== | ||
| {{See also|COVID-19 pandemic in Hubei}} | {{See also|COVID-19 pandemic in Hubei}} | ||
| Based on epidemiological simulations, the most recent common ancestor of circulating human strains was in a non-human animal prior to spillover.{{sfn|Pekar|Magee|Parker|Moshiri|2022}} It is estimated that lineage B/L spilled over to humans between October 23 and December 8, and lineage A/S separately spilled over between October 29 and December 14. | |||
| In contrast to SARS-CoV-1, the initial outbreak of SARS-CoV-2 was reported in only one city.{{sfn|Berche|2023|p=1,5}} Chinese epidemiological surveillance did not report any other pneumonia outbreaks in autumn 2019.{{sfn|Berche|2023|p=5}} Graham and Baric wrote that in the case of SARS-CoV-1, virus populations circulated and adapted to civets and humans over the course of two years before the recognized outbreak.{{sfn|Graham|Baric|2010|p=3141}} | In contrast to SARS-CoV-1, the initial outbreak of SARS-CoV-2 was reported in only one city.{{sfn|Berche|2023|p=1,5}} Chinese epidemiological surveillance did not report any other pneumonia outbreaks in autumn 2019.{{sfn|Berche|2023|p=5}} Graham and Baric wrote that in the case of SARS-CoV-1, virus populations circulated and adapted to civets and humans over the course of two years before the recognized outbreak.{{sfn|Graham|Baric|2010|p=3141}} | ||
| Line 66: | Line 55: | ||
| ==Reverse zoonosis== | ==Reverse zoonosis== | ||
| Transmission of SARS-CoV-2 from humans to animals, known as ] or anthroponosis, is possible.{{sfn|Qian|Li|Tang|Lu|2022|p=16}} Reverse zoonotic or experimental infection with SARS-CoV-2 has been reported in 31 animal species.{{sfn|Reggiani|Rugna|Bonilauri|2022|p=4}} It is not believed that wildlife plays a significant role in the ongoing circulation of SARS-CoV-2 among humans.{{sfn|Reggiani|Rugna|Bonilauri|2022|p=2}} | Transmission of SARS-CoV-2 from humans to animals, known as ] or anthroponosis, is possible.{{sfn|Qian|Li|Tang|Lu|2022|p=16}} Reverse zoonotic or experimental infection with SARS-CoV-2 has been reported in 31 animal species.{{sfn|Reggiani|Rugna|Bonilauri|2022|p=4}} It is not believed that wildlife plays a significant role in the ongoing circulation of SARS-CoV-2 among humans.{{sfn|Reggiani|Rugna|Bonilauri|2022|p=2}} | ||
| ] | ] | ||
| ] were found co-circulating among humans and farmed mink.{{sfn|Kane|Wong|Gao|2023|p=15}} Mink are the only animal in Europe or North America to experience widespread SARS-CoV-2 outbreaks. Transmission from human to mink has occurred multiple times, in most cases not resulting in a sustained mink outbreak.{{sfn|Tai|Sun|Tsung|Li|2022|p=2}} Strong evidence was seen of positive selection in mink after spillover, concentrated in the receptor binding motif.{{sfn|Tai|Sun|Tsung|Li|2022|p=2-3}} | ] were found co-circulating among humans and farmed mink.{{sfn|Kane|Wong|Gao|2023|p=15}} Mink are the only animal in Europe or North America to experience widespread SARS-CoV-2 outbreaks. Transmission from human to mink has occurred multiple times, in most cases not resulting in a sustained mink outbreak.{{sfn|Tai|Sun|Tsung|Li|2022|p=2}} Strong evidence was seen of positive selection in mink after spillover, concentrated in the receptor binding motif.{{sfn|Tai|Sun|Tsung|Li|2022|p=2-3}} | ||
| Line 76: | Line 65: | ||
| {{See also|COVID-19 in animals}} | {{See also|COVID-19 in animals}} | ||
| In the outbreak of SARS-CoV-1, palm civets, raccoon dogs, ferret badgers, red foxes, domestic cats, and rice field rats were possible vectors.{{sfn|Kane|Wong|Gao|2023|p=12}} Graham and Baric wrote that human and civet infections likely stemmed from an unknown common progenitor.{{sfn|Graham|Baric|2010|pp=3141,3142}} Patrick Berche wrote that the emergences of SARS-CoV-1 and MERS-CoV appeared to be sequential processes involving intermediate hosts, co-infections, and recombination.{{sfn|Berche|2023|p=1}} In contrast with the rapid identification of animal hosts for SARS-CoV-1 and MERS-CoV, no direct animal source for SARS-CoV-2 has been found.{{sfn|Deng|Xing|He|2022}} Holmes ''et al.'' wrote that the lack of intermediate host is likely because the right animal has not been tested so far.{{sfn|Holmes|Goldstein|Rasmussen|Robertson|2021|p=4850}} Frutos ''et al.'' proposed that rather than a discrete spillover event, SARS-CoV-2 arose in accordance with a circulation model, involving repeated horizontal transfer among humans, bats, and other mammals without establishing significant reservoirs in any of them until the pandemic.{{sfn|Frutos|Pliez|Gavotte|Devaux|2021}} | In the outbreak of SARS-CoV-1, palm civets, raccoon dogs, ferret badgers, red foxes, domestic cats, and rice field rats were possible vectors.{{sfn|Kane|Wong|Gao|2023|p=12}} Graham and Baric wrote that human and civet infections likely stemmed from an unknown common progenitor.{{sfn|Graham|Baric|2010|pp=3141,3142}} Patrick Berche wrote that the emergences of SARS-CoV-1 and MERS-CoV appeared to be sequential processes involving intermediate hosts, co-infections, and recombination.{{sfn|Berche|2023|p=1}} In contrast with the rapid identification of animal hosts for SARS-CoV-1 and MERS-CoV, no direct animal source for SARS-CoV-2 has been found.{{sfn|Deng|Xing|He|2022}} Holmes ''et al.'' wrote that the lack of intermediate host is likely because the right animal has not been tested so far.{{sfn|Holmes|Goldstein|Rasmussen|Robertson|2021|p=4850}} Frutos ''et al.'' proposed that rather than a discrete spillover event, SARS-CoV-2 arose in accordance with a circulation model, involving repeated horizontal transfer among humans, bats, and other mammals without establishing significant reservoirs in any of them until the pandemic.{{sfn|Frutos|Pliez|Gavotte|Devaux|2021}} | ||
| ] | ] | ||
| ] have been considered a possible reservoir of ].{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020}} Pangolins are sometimes sold in wet markets in China, where they are considered a culinary delicacy and a component of traditional medicine.{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020|p=1}} The highest sequence similarity to the SARS-CoV-2 spike receptor binding domain was found in a coronavirus infecting ] in ] province.{{sfn|Kane|Wong|Gao|2023|p=14}} Pangolins are frequently smuggled to China.{{sfn|Lytras|Hughes|Martin|Swanepoel |
] have been considered a possible reservoir of ].{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020}} Pangolins are sometimes sold in wet markets in China, where they are considered a culinary delicacy and a component of traditional medicine.{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020|p=1}} The highest sequence similarity to the SARS-CoV-2 spike receptor binding domain was found in a coronavirus infecting ] in ] province.{{sfn|Kane|Wong|Gao|2023|p=14}} Pangolins are frequently smuggled to China.{{sfn|Lytras|Hughes|Martin|Swanepoel|2022|p=6}} Lytras ''et al.'' wrote that, consistent with a lack of reported infections of pangolins in Malaysia, they were likely infected after being trafficked into China.{{sfn|Lytras|Hughes|Martin|Swanepoel|2022|p=6}} | ||
| The SARS-CoV-2 receptor binding domain shares more ] with a pangolin-CoV than RaTG13.{{sfn|Cagliani|Forni|Clerici|Sironi|2020|p=6}} The binding potential of SARS-CoV-2 to pangolin ACE2 however is very low.{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020|p=3}} |
The SARS-CoV-2 receptor binding domain shares more ] with a pangolin-CoV than RaTG13.{{sfn|Cagliani|Forni|Clerici|Sironi|2020|p=6}} The binding potential of SARS-CoV-2 to pangolin ACE2 however is very low.{{sfn|Frutos|Serra-Cobo|Chen|Devaux|2020|p=3}} The receptor binding domain of SARS-CoV-2 has been hypothesized to originate from recombination of the relevant portion of pangolin-CoV with an RaTG13-like virus.{{sfn|Kane|Wong|Gao|2023|pp=19-20}} | ||
| ] are highly |
] are highly susceptible to SARS-CoV-2, making them potential reservoir or intermediate hosts.{{sfn|Kane|Wong|Gao|2023|p=10}} | ||
| ] could be capable of transmitting SARS-CoV-2 to other animals under farm-like conditions.{{sfn|Kane|Wong|Gao|2023|p=11}} Transmission between raccoon dogs was shown under laboratory conditions.{{sfn|Reggiani|Rugna|Bonilauri|2022|p=4}} | ] could be capable of transmitting SARS-CoV-2 to other animals under farm-like conditions.{{sfn|Kane|Wong|Gao|2023|p=11}} Transmission between raccoon dogs was shown under laboratory conditions.{{sfn|Reggiani|Rugna|Bonilauri|2022|p=4}} | ||
| ] are a potential vector for SARS-CoV-2.{{sfn|Kane|Wong|Gao|2023|p=11}} | ] are a potential vector for SARS-CoV-2.{{sfn|Kane|Wong|Gao|2023|p=11}} | ||
| ==Wet market hypothesis== | ==Wet market hypothesis== | ||
| Spillover has been proposed to have occurred at one or more ] in China.{{sfn|Lytras|Hughes|Martin|Swanepoel|2022|p=2}} Wild and semi-wild ] are commonly traded and consumed in China, and this practice has expanded in recent decades. The close contact among animals in unsanitary conditions creates the potential for diseases to thrive. In the 2002 outbreak in Guangdong, most living animals in the markets showed serological evidence of exposure to SARS-CoV-1.{{sfn|Berche|2023|p=1 |
Spillover has been proposed to have occurred at one or more ] in China.{{sfn|Lytras|Hughes|Martin|Swanepoel|2022|p=2}} Wild and semi-wild ] are commonly traded and consumed in China, and this practice has expanded in recent decades. The close contact among animals in unsanitary conditions creates the potential for diseases to thrive. In the 2002 outbreak in Guangdong, most living animals in the markets showed serological evidence of exposure to SARS-CoV-1.{{sfn|Berche|2023|p=1}} | ||
| ] | ] | ||
| Many early cases were associated with the ] in Wuhan.{{sfn|Lytras|Hughes|Martin|Swanepoel|2022|p=1}} The first documented COVID-19 infection was in a worker at a seafood stall in the Huanan market.{{sfn|Pekar|Magee|Parker|Moshiri|2022|p=4}} Sequences from that patient have not been published; however, SARS-CoV-2 belonging to lineage B/L were detected in environmental samples from the patient's stall.{{sfn|Pekar|Magee|Parker|Moshiri|2022|p=4}} No bats or pangolins were reported to be at the market between May 2017 and November 2019. Raccoon dogs, which are hypothesized to be |
Many early cases were associated with the ] in Wuhan.{{sfn|Lytras|Hughes|Martin|Swanepoel|2022|p=1}} The first documented COVID-19 infection was in a worker at a seafood stall in the Huanan market.{{sfn|Pekar|Magee|Parker|Moshiri|2022|p=4}} Sequences from that patient have not been published; however, SARS-CoV-2 belonging to lineage B/L were detected in environmental samples from the patient's stall.{{sfn|Pekar|Magee|Parker|Moshiri|2022|p=4}} No bats or pangolins were reported to be at the market between May 2017 and November 2019. Raccoon dogs, which are hypothesized to be susceptible to SARS-CoV-2, were at the market.{{sfn|Liu|Liu|Lei|Jia|2023|p=3}} A survey by He ''et al.'' in 2021 identified 102 mammal-affecting viruses in Chinese game animals, 65 of which were identified then for the first time.{{sfn|He|Hou|Zhao|Sun|2022}} They did not find any SARS-like viruses, but did find evidence for the transmission of a MERS-like virus from bats to hedgehogs.{{sfn|He|Hou|Zhao|Sun|2022}} | ||
| Between January 1 and March 30, epidemiologists from China CDC collected 457 samples from animal matter including carcasses and feces and collected 923 environmental samples. All animal samples tested negative for SARS-CoV-2.{{sfn|Liu|Liu|Lei|Jia|2023|p=7}} SARS-CoV-2 was found in 73 environmental samples. Live virus was isolated from three samples, two of which came from stalls belonging to known patients.{{sfn|Liu|Liu|Lei|Jia|2023|p=7}} No significant association was found between environmental virus titer and the type of product sold at particular stalls.{{sfn|Liu|Liu|Lei|Jia|2023|p=6}} SARS-CoV-2 was identified in all four sewer wells in the market. Overground drainage from the surrounding area concentrated under the market. | Between January 1 and March 30, epidemiologists from China CDC collected 457 samples from animal matter including carcasses and feces and collected 923 environmental samples. All animal samples tested negative for SARS-CoV-2.{{sfn|Liu|Liu|Lei|Jia|2023|p=7}} SARS-CoV-2 was found in 73 environmental samples. Live virus was isolated from three samples, two of which came from stalls belonging to known patients.{{sfn|Liu|Liu|Lei|Jia|2023|p=7}} No significant association was found between environmental virus titer and the type of product sold at particular stalls.{{sfn|Liu|Liu|Lei|Jia|2023|p=6}} SARS-CoV-2 was identified in all four sewer wells in the market. Overground drainage from the surrounding area concentrated under the market. | ||
| Line 100: | Line 89: | ||
| The evolution of the ] appeared to be more rapid than other variants.{{sfn|Kane|Wong|Gao|2023|p=21}} Theories for the origin of the omicron variant include long-term circulation among humans outside areas where genetic surveillance was performed, mutation in an ] individual, or adaptation in an animal species after reverse zoonosis.{{sfn|Qian|Li|Tang|Lu|2022|p=14}}{{sfn|Reggiani|Rugna|Bonilauri|2022|p=3}} The Omicron variant is proposed to have originated in mice.{{sfn|Kane|Wong|Gao|2023|p=20}}{{sfn|Reggiani|Rugna|Bonilauri|2022|p=3}} | The evolution of the ] appeared to be more rapid than other variants.{{sfn|Kane|Wong|Gao|2023|p=21}} Theories for the origin of the omicron variant include long-term circulation among humans outside areas where genetic surveillance was performed, mutation in an ] individual, or adaptation in an animal species after reverse zoonosis.{{sfn|Qian|Li|Tang|Lu|2022|p=14}}{{sfn|Reggiani|Rugna|Bonilauri|2022|p=3}} The Omicron variant is proposed to have originated in mice.{{sfn|Kane|Wong|Gao|2023|p=20}}{{sfn|Reggiani|Rugna|Bonilauri|2022|p=3}} | ||
| == |
==Notes== | ||
| ⚫ | {{notelist}} | ||
| ] | |||
| Origins of COVID-19 either in the wildlife trade or a laboratory in China would be considered embarrassing to the Chinese government.{{sfn|Cohen|2022}} A number of Chinese publications have argued that COVID-19 originated (in the words of Jon Cohen) "anywhere but here,"{{sfn|Cohen|2022}} by routes including imported frozen food products or competitors in the ]. Most scientists outside China consider these scenarios to be implausible.{{sfn|Cohen|2022}} Some contributors to the ] have said that the resulting report reflected political pressure from the Chinese government.{{sfn|Cohen|2022}} After the WHO-convened report was widely criticized internationally, China withdrew most support for further studies of COVID-19's origins.{{sfn|Cohen|2022}} Scientists including ] have expressed skepticism that studies which found no SARS-CoV-2-related viruses in Chinese wildlife were free from political interference.{{sfn|Cohen|2022}} Former China CDC director ] expressed willingness to consider any origin theory but stressed a lack of evidence. Gao said, "Once the scientists can do free science, we might get the answer. But now, everything is politicized."{{sfn|Eban|2023}} Speculation about wet markets and associated culinary practices in the origin of COVID-19 has contributed to ].{{sfn|Fernando|Mumphrey|2020}}{{sfn|Palmer|2020}} | |||
| ⚫ | ==References== | ||
| A 2020 paper by Andersen ''et al.''{{sfn|Anderson|Rambaut|Lipkin|Holmes|2020}} was widely cited in news media as conclusively supporting a natural origin.{{sfn|Tobias|2023}} | |||
| ⚫ | ===Citations=== | ||
| ⚫ | {{reflist}} | ||
| ⚫ | ==Citations== | ||
| ===Bibliography=== | |||
| {{refbegin|30em|indent=yes}} | |||
| * {{cite journal|last1=Anand|first1=Praveen|last2=Puranik|first2=Arjun|last3=Aravamudan|first3=Murali|last4=Venkatakrishnan|first4=AJ|title=SARS-CoV-2 strategically mimics proteolytic activation of human ENaC|journal=eLife|date=May 26, 2020|volume=9 |doi=10.7554/eLife.58603 |pmid=32452762 |pmc=7343387 |doi-access=free }} | * {{cite journal|last1=Anand|first1=Praveen|last2=Puranik|first2=Arjun|last3=Aravamudan|first3=Murali|last4=Venkatakrishnan|first4=AJ|title=SARS-CoV-2 strategically mimics proteolytic activation of human ENaC|journal=eLife|date=May 26, 2020|volume=9 |doi=10.7554/eLife.58603 |pmid=32452762 |pmc=7343387 |doi-access=free }} | ||
| * {{cite journal|last1=Anderson|first1=Kristian G.|last2=Rambaut|first2=Andrew|last3=Lipkin|first3=W. Ian|last4=Holmes|first4=Edward C.|title=The proximal origin of SARS-CoV-2|journal=Nature|date=March 17, 2020|volume=26 |issue=4 |pages=450–452 |doi=10.1038/s41591-020-0820-9|pmid=32284615 |pmc=7095063 }} | * {{cite journal|last1=Anderson|first1=Kristian G.|last2=Rambaut|first2=Andrew|last3=Lipkin|first3=W. Ian|last4=Holmes|first4=Edward C.|title=The proximal origin of SARS-CoV-2|journal=Nature|date=March 17, 2020|volume=26 |issue=4 |pages=450–452 |doi=10.1038/s41591-020-0820-9|pmid=32284615 |pmc=7095063 }} | ||
| * {{cite journal|last1=Balloux|first1=François|last2=Tan|first2=Cedric|last3=Swadling|first3=Leo|last4=Richard|first4=Damien|title=The past, current and future epidemiological dynamic of SARS-CoV-2|journal=Oxford Open Immunology|volume=3|issue=1|date=June 20, 2022|pages=iqac003 |doi=10.1093/oxfimm/iqac003|pmid=35872966 |pmc=9278178 }} | * {{cite journal|last1=Balloux|first1=François|last2=Tan|first2=Cedric|last3=Swadling|first3=Leo|last4=Richard|first4=Damien|title=The past, current and future epidemiological dynamic of SARS-CoV-2|journal=Oxford Open Immunology|volume=3|issue=1|date=June 20, 2022|pages=iqac003 |doi=10.1093/oxfimm/iqac003|pmid=35872966 |pmc=9278178 }} | ||
| * {{cite journal|last1=Berche|first1=Patrick|title=Gain-of-function and origin of Covid19|journal=La Presse Médicale|volume=52|issue=1|date=March 2023|doi=10.1016/j.lpm.2023.104167|pmid=37269978|pmc=10234839 }} | * {{cite journal|last1=Berche|first1=Patrick|title=Gain-of-function and origin of Covid19|journal=La Presse Médicale|volume=52|issue=1|date=March 2023|doi=10.1016/j.lpm.2023.104167|pmid=37269978|pmc=10234839 }} | ||
| * {{cite journal|last1=Biancolella|first1=Michela|last2=Colona|first2=Vito Luigi|last3= |
* {{cite journal|last1=Biancolella|first1=Michela|last2=Colona|first2=Vito Luigi|last3=Luzzatto|first3=Lucio|last4=Watt|first4=Jessica Lee|title=COVID-19 annual update: a narrative review|journal=Human Genomics|date=July 24, 2023|volume=17 |issue=1 |page=68 |doi=10.1186/s40246-023-00515-2 |pmid=37488607 |pmc=10367267 |doi-access=free }} | ||
| * {{cite journal|last1=Cagliani|first1=Rachele|last2=Forni|first2=Diego|last3=Clerici|first3=Mario|last4=Sironi|first4=Manuela|title=Computational Inference of Selection Underlying the Evolution of the Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2|journal=Journal of Virology|date=April 1, 2020|volume=94 |issue=12 |doi=10.1128/JVI.00411-20|pmid=32238584 |pmc=7307108 }} | * {{cite journal|last1=Cagliani|first1=Rachele|last2=Forni|first2=Diego|last3=Clerici|first3=Mario|last4=Sironi|first4=Manuela|title=Computational Inference of Selection Underlying the Evolution of the Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2|journal=Journal of Virology|date=April 1, 2020|volume=94 |issue=12 |doi=10.1128/JVI.00411-20|pmid=32238584 |pmc=7307108 }} | ||
| * {{cite journal|last1=Canuti|first1=Marta|last2=Bianchi|first2=Silvia|last3=Kolbi|first3=Otto|last4=Pond|first4=Sergei L.K.|title=Waiting for the truth: is reluctance in accepting an early origin hypothesis for SARS-CoV-2 delaying our understanding of viral emergence?|journal=BMJ Global Health|date=March 16, 2022|volume=7 |issue=3 |pages=e008386 |doi=10.1136/bmjgh-2021-008386|pmid=35296465 |pmc=8927931 }} | * {{cite journal|last1=Canuti|first1=Marta|last2=Bianchi|first2=Silvia|last3=Kolbi|first3=Otto|last4=Pond|first4=Sergei L.K.|title=Waiting for the truth: is reluctance in accepting an early origin hypothesis for SARS-CoV-2 delaying our understanding of viral emergence?|journal=BMJ Global Health|date=March 16, 2022|volume=7 |issue=3 |pages=e008386 |doi=10.1136/bmjgh-2021-008386|pmid=35296465 |pmc=8927931 }} | ||
| Line 118: | Line 108: | ||
| * {{cite journal|last1=Deng|first1=Shanjun|last2=Xing|first2=Ke|last3=He|first3=Xionglei|title=Mutation signatures inform the natural host of SARS-CoV-2|journal=National Science Review|volume=9|issue=2|date=February 2022|pages=nwab220 |doi=10.1093/nsr/nwab220|pmid=35211321 |pmc=8690307 }} | * {{cite journal|last1=Deng|first1=Shanjun|last2=Xing|first2=Ke|last3=He|first3=Xionglei|title=Mutation signatures inform the natural host of SARS-CoV-2|journal=National Science Review|volume=9|issue=2|date=February 2022|pages=nwab220 |doi=10.1093/nsr/nwab220|pmid=35211321 |pmc=8690307 }} | ||
| * {{cite magazine|last1=Eban|first1=Katherine|title=Inside the COVID Origins Raccoon Dog Cage Match|magazine=Vanity Fair|date=June 1, 2023|url=https://www.vanityfair.com/news/2023/06/raccoon-dog-george-gao-covid-origins|archive-url=https://web.archive.org/web/20230701093013/https://www.vanityfair.com/news/2023/06/raccoon-dog-george-gao-covid-origins|archive-date=July 1, 2023}} | * {{cite magazine|last1=Eban|first1=Katherine|title=Inside the COVID Origins Raccoon Dog Cage Match|magazine=Vanity Fair|date=June 1, 2023|url=https://www.vanityfair.com/news/2023/06/raccoon-dog-george-gao-covid-origins|archive-url=https://web.archive.org/web/20230701093013/https://www.vanityfair.com/news/2023/06/raccoon-dog-george-gao-covid-origins|archive-date=July 1, 2023}} | ||
| * {{cite journal|last1=Frutos|first1=Roger|last2=Pliez|first2=Olivier|last3=Gavotte|first3=Laurent|last4=Devaux|first4=Christian A.|title=There is no "origin" to SARS-CoV-2|journal=Environmental Research|volume=207|date=October 6, 2021|doi=10.1016/j.envres.2021.112173|pmid=34626592 |s2cid=238412878 }} | * {{cite journal|last1=Frutos|first1=Roger|last2=Pliez|first2=Olivier|last3=Gavotte|first3=Laurent|last4=Devaux|first4=Christian A.|title=There is no "origin" to SARS-CoV-2|journal=Environmental Research|volume=207|date=October 6, 2021|doi=10.1016/j.envres.2021.112173|pmid=34626592 |pmc=8493644 |s2cid=238412878 }} | ||
| * {{cite journal|last1=Frutos|first1=Roger|last2=Serra-Cobo|first2=Jordi|last3=Chen|first3=Tianmu|last4=Devaux|first4=Christian A.|title=COVID-19: Time to exonerate the pangolin from the transmission of SARS-CoV-2 to humans|journal=Infection, Genetics and Evolution|date=August 5, 2020|volume=84 |doi=10.1016/j.meegid.2020.104493|pmid=32768565 |s2cid=220971074 }} | * {{cite journal|last1=Frutos|first1=Roger|last2=Serra-Cobo|first2=Jordi|last3=Chen|first3=Tianmu|last4=Devaux|first4=Christian A.|title=COVID-19: Time to exonerate the pangolin from the transmission of SARS-CoV-2 to humans|journal=Infection, Genetics and Evolution|date=August 5, 2020|volume=84 |doi=10.1016/j.meegid.2020.104493|pmid=32768565 |pmc=7405773 |bibcode=2020InfGE..8404493F |s2cid=220971074 }} | ||
| * {{cite journal|last1=Ge|first1=Xing-Yi|last2=Li|first2=Jia-Lu|last3=Yang|first3=Xing-Lou|last4=Chmura|first4=Aleksei A.|title=Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor|journal=Nature|date=October 30, 2013|volume=503 |issue=7477 |pages=535–538 |doi=10.1038/nature12711|pmid=24172901 |bibcode=2013Natur.503..535G |s2cid=4464714 }} | * {{cite journal|last1=Ge|first1=Xing-Yi|last2=Li|first2=Jia-Lu|last3=Yang|first3=Xing-Lou|last4=Chmura|first4=Aleksei A.|title=Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor|journal=Nature|date=October 30, 2013|volume=503 |issue=7477 |pages=535–538 |doi=10.1038/nature12711|pmid=24172901 |pmc=5389864 |bibcode=2013Natur.503..535G |s2cid=4464714 }} | ||
| * {{cite web|last=Goodman|first=Brenda|title=Scientists at House hearing refute claims they were bribed and influenced to deny Covid-19 'lab leak' theory|work=CNN|date=July 11, 2023|url=https://www.cnn.com/2023/07/11/health/covid-origins-researchers-house-subcommittee-hearing/index.html}} | * {{cite web|last=Goodman|first=Brenda|title=Scientists at House hearing refute claims they were bribed and influenced to deny Covid-19 'lab leak' theory|work=CNN|date=July 11, 2023|url=https://www.cnn.com/2023/07/11/health/covid-origins-researchers-house-subcommittee-hearing/index.html}} | ||
| * {{cite journal|last1=Graham|first1=Rachel L.|last2=Baric|first2=Ralph S.|title=Recombination, Reservoirs, and the Modular Spike: Mechanisms of Coronavirus Cross-Species Transmission|journal=Journal of Virology|volume=84|number=7|date=April 2010|pages=3134–3146 |doi=10.1128/JVI.01394-09|pmid=19906932 |pmc=2838128 }} | * {{cite journal|last1=Graham|first1=Rachel L.|last2=Baric|first2=Ralph S.|title=Recombination, Reservoirs, and the Modular Spike: Mechanisms of Coronavirus Cross-Species Transmission|journal=Journal of Virology|volume=84|number=7|date=April 2010|pages=3134–3146 |doi=10.1128/JVI.01394-09|pmid=19906932 |pmc=2838128 }} | ||
| * {{cite journal|last1=Guo|first1=Hua|last2=Hu|first2=Bing-Jie|last3=Yang|first3=Xing-Lou|last4=Zeng|first4=Lei-Ping|title=Evolutionary Arms Race between Virus and Host Drives Genetic Diversity in Bat Severe Acute Respiratory Syndrome-Related Coronavirus Spike Genes|journal=Journal of Virology|date=September 29, 2020|volume=94 |issue=20 |doi=10.1128/JVI.00902-20|pmid=32699095 |s2cid=220716415 }} | * {{cite journal|last1=Guo|first1=Hua|last2=Hu|first2=Bing-Jie|last3=Yang|first3=Xing-Lou|last4=Zeng|first4=Lei-Ping|title=Evolutionary Arms Race between Virus and Host Drives Genetic Diversity in Bat Severe Acute Respiratory Syndrome-Related Coronavirus Spike Genes|journal=Journal of Virology|date=September 29, 2020|volume=94 |issue=20 |doi=10.1128/JVI.00902-20|pmid=32699095 |pmc=7527062 |s2cid=220716415 }} | ||
| * {{cite journal|last1=Guo|first1=Hua|last2=Li|first2=Ang|last3=Dong|first3=Tian-Yi|last4=Su|first4=Jia|title=ACE2-Independent Bat Sarbecovirus Entry and Replication in Human and Bat Cells|journal=Virology|date=November 21, 2022|volume=13 |issue=6 |pages=e0256622 |doi=10.1128/mbio.02566-22|pmid=36409074 |pmc=9765407 }} | * {{cite journal|last1=Guo|first1=Hua|last2=Li|first2=Ang|last3=Dong|first3=Tian-Yi|last4=Su|first4=Jia|title=ACE2-Independent Bat Sarbecovirus Entry and Replication in Human and Bat Cells|journal=Virology|date=November 21, 2022|volume=13 |issue=6 |pages=e0256622 |doi=10.1128/mbio.02566-22|pmid=36409074 |pmc=9765407 }} | ||
| * {{cite journal|last1=Harrison|first1=Neil L.|last2=Sachs|first2=Jeffery D.|title=A call for an independent inquiry into the origin of the SARS-CoV-2 virus|journal=PNAS|volume=119|number=21|date=May 19, 2022|pages=e2202769119 |doi=10.1073/pnas.2202769119|pmid=35588448 |pmc=9173817 |bibcode=2022PNAS..11902769H }} | * {{cite journal|last1=Harrison|first1=Neil L.|last2=Sachs|first2=Jeffery D.|title=A call for an independent inquiry into the origin of the SARS-CoV-2 virus|journal=PNAS|volume=119|number=21|date=May 19, 2022|pages=e2202769119 |doi=10.1073/pnas.2202769119|doi-access=free |pmid=35588448 |pmc=9173817 |bibcode=2022PNAS..11902769H }} | ||
| * {{cite journal|last1=He|first1=Wan-Ting|last2=Hou|first2=Xin|last3=Zhao|first3=Jin|last4=Sun|first4=Jiumeng|title=Virome characterization of game animals in China reveals a spectrum of emerging pathogens|journal=Cell|date=February 16, 2022|volume=185 |issue=7 |pages=1117–1129.e8 |doi=10.1016/j.cell.2022.02.014|pmid=35298912 |pmc=9942426 }} | * {{cite journal|last1=He|first1=Wan-Ting|last2=Hou|first2=Xin|last3=Zhao|first3=Jin|last4=Sun|first4=Jiumeng|title=Virome characterization of game animals in China reveals a spectrum of emerging pathogens|journal=Cell|date=February 16, 2022|volume=185 |issue=7 |pages=1117–1129.e8 |doi=10.1016/j.cell.2022.02.014|pmid=35298912 |pmc=9942426 }} | ||
| * {{cite journal|last1=Holmes|first1=Edward C.|last2=Goldstein|first2=Stephen A.|last3=Rasmussen|first3=Angela L.|last4=Robertson|first4=David L.|title=The origins of SARS-CoV-2: A critical review|journal=Cell|volume=184|issue=19|date=September 16, 2021|pages=4848–4856 |doi=10.1016/j.cell.2021.08.017|pmid=34480864 |pmc=8373617 }} | * {{cite journal|last1=Holmes|first1=Edward C.|last2=Goldstein|first2=Stephen A.|last3=Rasmussen|first3=Angela L.|last4=Robertson|first4=David L.|title=The origins of SARS-CoV-2: A critical review|journal=Cell|volume=184|issue=19|date=September 16, 2021|pages=4848–4856 |doi=10.1016/j.cell.2021.08.017|pmid=34480864 |pmc=8373617 }} | ||
| * {{cite journal|last1=Hossain|first1=Golzar|last2=Tang|first2=Yan-dong|last3=Akter|first3=Sharmin|last4=Zheng|first4=Chunfu|title=Roles of the polybasic furin cleavage site of spike protein in SARS-CoV-2 replication, pathogenesis, and host immune responses and vaccination|journal=Journal of Medical Virology|date=December 22, 2021|volume=94 |issue=5 |pages=1815–1820 |doi=10.1002/jmv.27539|pmid=34936124 |s2cid=245430230 }} | * {{cite journal|last1=Hossain|first1=Golzar|last2=Tang|first2=Yan-dong|last3=Akter|first3=Sharmin|last4=Zheng|first4=Chunfu|title=Roles of the polybasic furin cleavage site of spike protein in SARS-CoV-2 replication, pathogenesis, and host immune responses and vaccination|journal=Journal of Medical Virology|date=December 22, 2021|volume=94 |issue=5 |pages=1815–1820 |doi=10.1002/jmv.27539|pmid=34936124 |s2cid=245430230 }} | ||
| * {{cite journal|last1=Jaimes|first1=Javier A.|last2=Millet|first2=Jean K.|last3=Whittaker|first3=Gary R.|title=Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the Novel S1/S2 Site|journal=iScience|date=June 26, 2020|volume=23 |issue=6 |doi=10.1016/j.isci.2020.101212|pmid=32512386 |pmc=7255728 |bibcode=2020iSci...23j1212J }} | * {{cite journal|last1=Jaimes|first1=Javier A.|last2=Millet|first2=Jean K.|last3=Whittaker|first3=Gary R.|title=Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the Novel S1/S2 Site|journal=iScience|date=June 26, 2020|volume=23 |issue=6 |doi=10.1016/j.isci.2020.101212|pmid=32512386 |pmc=7255728 |bibcode=2020iSci...23j1212J }} | ||
| * {{cite journal|last1=Kane|first1=Yakhouba|last2=Wong|first2=Gary|last3=Gao|first3=George F.|title=Animal Models, Zoonotic Reservoirs, and Cross-Species Transmission of Emerging Human-Infecting Coronaviruses|journal=Annual Review of Animal Biosciences|date=February 2023|volume=11 |pages=1–31 |doi=10.1146/annurev-animal-020420-025011|pmid=36790890 |s2cid=256869353 }} | * {{cite journal|last1=Kane|first1=Yakhouba|last2=Wong|first2=Gary|last3=Gao|first3=George F.|title=Animal Models, Zoonotic Reservoirs, and Cross-Species Transmission of Emerging Human-Infecting Coronaviruses|journal=Annual Review of Animal Biosciences|date=February 2023|volume=11 |pages=1–31 |doi=10.1146/annurev-animal-020420-025011|pmid=36790890 |s2cid=256869353 |doi-access=free}} | ||
| * {{cite journal|last1=Kang|first1=Lin|last2=He|first2=Guijuan|last3=Sharp|first3=Amanda K.|last4=Wang|first4=Xiaofeng|title=A selective sweep in the Spike gene has driven SARS-CoV-2 human adaptation|journal=Cell|date=July 6, 2021|volume=184 |issue=17 |pages=4392–4400.e4 |doi=10.1016/j.cell.2021.07.007|pmid=34289344 |pmc=8260498 }} | * {{cite journal|last1=Kang|first1=Lin|last2=He|first2=Guijuan|last3=Sharp|first3=Amanda K.|last4=Wang|first4=Xiaofeng|title=A selective sweep in the Spike gene has driven SARS-CoV-2 human adaptation|journal=Cell|date=July 6, 2021|volume=184 |issue=17 |pages=4392–4400.e4 |doi=10.1016/j.cell.2021.07.007|pmid=34289344 |pmc=8260498 }} | ||
| * {{cite journal|last1=Kumar|first1=Sudhir|last2=Tao|first2=Qiqing|last3=Weaver|first3=Steven|last4=Sanderford|first4=Maxwell|title=An Evolutionary Portrait of the Progenitor SARS-CoV-2 and Its Dominant Offshoots in COVID-19 Pandemic|journal=Molecular Biology and Evolution|volume=38|issue=8|date=August 8, 2021|pages=3046–3059 |doi=10.1093/molbev/msab118|pmid=33942847 }} | * {{cite journal|last1=Kumar|first1=Sudhir|last2=Tao|first2=Qiqing|last3=Weaver|first3=Steven|last4=Sanderford|first4=Maxwell|title=An Evolutionary Portrait of the Progenitor SARS-CoV-2 and Its Dominant Offshoots in COVID-19 Pandemic|journal=Molecular Biology and Evolution|volume=38|issue=8|date=August 8, 2021|pages=3046–3059 |doi=10.1093/molbev/msab118|pmid=33942847 |pmc=8135569 }} | ||
| * {{cite journal|last1=Li|first1=Hongying|last2=Mendelsohn|first2=Emma|last3=Zong|first3=Chen|last4=Zhang|first4=Wei|title=Human-animal interactions and bat coronavirus spillover potential among rural residents in Southern China|journal=Biosafety and Health|volume=1|issue=2|date=September 2019|pages=84–90 |doi=10.1016/j.bsheal.2019.10.004|pmid=32501444 |pmc=7148670 }} | * {{cite journal|last1=Li|first1=Hongying|last2=Mendelsohn|first2=Emma|last3=Zong|first3=Chen|last4=Zhang|first4=Wei|title=Human-animal interactions and bat coronavirus spillover potential among rural residents in Southern China|journal=Biosafety and Health|volume=1|issue=2|date=September 2019|pages=84–90 |doi=10.1016/j.bsheal.2019.10.004|pmid=32501444 |pmc=7148670 }} | ||
| * {{cite journal|last1=Li|first1=Meng|last2=Du|first2=Juan|last3=Liu|first3=Weiqiang|last4=Li|first4=Zihao|title=Comparative susceptibility of SARS-CoV-2, SARS-CoV, and MERS-CoV across mammals|journal=ISME|date=January 23, 2023|volume=17 |issue=4 |pages=549–560 |doi=10.1038/s41396-023-01368-2|pmid=36690780 |pmc=9869846 }} | * {{cite journal|last1=Li|first1=Meng|last2=Du|first2=Juan|last3=Liu|first3=Weiqiang|last4=Li|first4=Zihao|title=Comparative susceptibility of SARS-CoV-2, SARS-CoV, and MERS-CoV across mammals|journal=ISME|date=January 23, 2023|volume=17 |issue=4 |pages=549–560 |doi=10.1038/s41396-023-01368-2|pmid=36690780 |pmc=9869846 |bibcode=2023ISMEJ..17..549L }} | ||
| * {{cite journal|last1=Liu|first1=William J.|last2=Liu|first2=Peipei|last3=Lei|first3=Wenwen|last4=Jia|first4=Zhiyuan|title=Surveillance of SARS-CoV-2 at the Huanan Seafood Market|journal=Nature|date=April 5, 2023|doi=10.1038/s41586-023-06043-2|pmid=37019149 |s2cid=257983307 }} | * {{cite journal|last1=Liu|first1=William J.|last2=Liu|first2=Peipei|last3=Lei|first3=Wenwen|last4=Jia|first4=Zhiyuan|title=Surveillance of SARS-CoV-2 at the Huanan Seafood Market|journal=Nature|date=April 5, 2023|volume=631 |issue=8020 |pages=402–408 |doi=10.1038/s41586-023-06043-2|pmid=37019149 |s2cid=257983307 |doi-access=free}} | ||
| * {{cite journal|last1=Lytras|first1=Spyros|last2=Hughes|first2=Joseph|last3=Martin|first3=Darren|last4=Swanepoel|first4=Phillip|title=Exploring the Natural Origins of SARS-CoV-2 in the Light of Recombination|journal=Genome Biology and Evolution|date=February 8, 2022|volume=14 |issue=2 |doi=10.1093/gbe/evac018|pmid=35137080 |pmc=8882382 }} | * {{cite journal|last1=Lytras|first1=Spyros|last2=Hughes|first2=Joseph|last3=Martin|first3=Darren|last4=Swanepoel|first4=Phillip|title=Exploring the Natural Origins of SARS-CoV-2 in the Light of Recombination|journal=Genome Biology and Evolution|date=February 8, 2022|volume=14 |issue=2 |doi=10.1093/gbe/evac018|pmid=35137080 |pmc=8882382 }} | ||
| * {{cite journal|last1=Lubinski|first1=Bailey|last2=Whittaker|first2=Gary R|title=The SARS-CoV-2 furin cleavage site: natural selection or smoking gun?|journal=The Lancet Microbe|volume=4|issue=8|date=August 2023|pages=e570 |doi=10.1016/S2666-5247(23)00144-1|pmid=37236215 |pmc=10205058 }} | * {{cite journal|last1=Lubinski|first1=Bailey|last2=Whittaker|first2=Gary R|title=The SARS-CoV-2 furin cleavage site: natural selection or smoking gun?|journal=The Lancet Microbe|volume=4|issue=8|date=August 2023|pages=e570 |doi=10.1016/S2666-5247(23)00144-1|pmid=37236215 |pmc=10205058 }} | ||
| * {{cite journal|last1=MacLean|first1=Oscar A.|last2=Lytras|first2=Spyros|last3=Weaver|first3=Steven|last4=Singer|first4=Joshua B.|title=Natural selection in the evolution of SARS-CoV-2 in bats created a generalist virus and highly capable human pathogen|journal=PLOS Biology|date=March 12, 2021|volume=19 |issue=3 |pages=e3001115 |doi=10.1371/journal.pbio.3001115 |pmid=33711012 |pmc=7990310 |doi-access=free }} | * {{cite journal|last1=MacLean|first1=Oscar A.|last2=Lytras|first2=Spyros|last3=Weaver|first3=Steven|last4=Singer|first4=Joshua B.|title=Natural selection in the evolution of SARS-CoV-2 in bats created a generalist virus and highly capable human pathogen|journal=PLOS Biology|date=March 12, 2021|volume=19 |issue=3 |pages=e3001115 |doi=10.1371/journal.pbio.3001115 |pmid=33711012 |pmc=7990310 |doi-access=free }} | ||
| * {{cite web|last1=McCarthy|first1=Simone|last2=Baptista|first2=Eduardo|title=Science turns nasty in Covid-19 origins argument on Twitter|work=South China Morning Post|date=October 15, 2021|url=https://www.scmp.com/news/china/science/article/3151906/science-turns-nasty-covid-19-origins-argument-twitter|archive-url=https://web.archive.org/web/ |
* {{cite web|last1=McCarthy|first1=Simone|last2=Baptista|first2=Eduardo|title=Science turns nasty in Covid-19 origins argument on Twitter|work=South China Morning Post|date=October 15, 2021|url=https://www.scmp.com/news/china/science/article/3151906/science-turns-nasty-covid-19-origins-argument-twitter|url-access=subscription|archive-url=https://web.archive.org/web/20240310032509/https://condosingapore.com/showthread.php/33079-Science-turns-nasty-in-Covid-19-origins-argument-on-Twitter|archive-date=March 10, 2024}}{{void|Fabrickator|comment|story as posted at South China Morning Post requires subscription, archive copies from South China Morning Post website are not usable, archive version is from 3rd-party reprint of story at condosingapore.com}} | ||
| * {{cite journal|last1=Menachery|first1=Vineet D.|last2=Yount|first2=Boyd L. |
* {{cite journal|last1=Menachery|first1=Vineet D.|last2=Yount|first2=Boyd L. Jr.|last3=Sims|first3=Amy C.|last4=Baric|first4=Ralph S.|title=SARS-like WIV1-CoV poised for human emergence|journal=PNAS|volume=113|number=11|date=March 14, 2016|pages=3048–3053 |doi=10.1073/pnas.1517719113 |pmid=26976607 |pmc=4801244 |bibcode=2016PNAS..113.3048M |doi-access=free }} | ||
| * {{cite web|last=Palmer|first=James|title=Don't Blame Bat Soup for the Coronavirus|work=Foreign Policy|date=January 27, 2020|url=https://foreignpolicy.com/2020/01/27/coronavirus-covid19-dont-blame-bat-soup-for-the-virus/}} | * {{cite web|last=Palmer|first=James|title=Don't Blame Bat Soup for the Coronavirus|work=Foreign Policy|date=January 27, 2020|url=https://foreignpolicy.com/2020/01/27/coronavirus-covid19-dont-blame-bat-soup-for-the-virus/}} | ||
| * {{cite journal|last1=Pekar|first1=Jonathan E.|last2=Magee|first2=Andrew|last3=Parker|first3=Edyth|last4=Moshiri|first4=Niema|title=The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2|journal=Science|volume=377|issue=6609|date=July 26, 2022|pages=960–966 |doi=10.1126/science.abp8337|pmid=35881005 |pmc=9348752 |bibcode=2022Sci...377..960P }} | * {{cite journal|last1=Pekar|first1=Jonathan E.|last2=Magee|first2=Andrew|last3=Parker|first3=Edyth|last4=Moshiri|first4=Niema|title=The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2|journal=Science|volume=377|issue=6609|date=July 26, 2022|pages=960–966 |doi=10.1126/science.abp8337|pmid=35881005 |pmc=9348752 |bibcode=2022Sci...377..960P }} | ||
| Line 147: | Line 137: | ||
| * {{cite web|last=Smith|first=David|title="It's just gotten crazy": how the origins of Covid became a toxic US political debate|work=The Guardian|date=February 28, 2023|url=https://www.theguardian.com/world/2023/feb/28/lab-leak-natural-spillover-how-origins-covid-us-political-debate}} | * {{cite web|last=Smith|first=David|title="It's just gotten crazy": how the origins of Covid became a toxic US political debate|work=The Guardian|date=February 28, 2023|url=https://www.theguardian.com/world/2023/feb/28/lab-leak-natural-spillover-how-origins-covid-us-political-debate}} | ||
| * {{cite journal|last1=Tai|first1=Jui-Hung|last2=Sun|first2=Hsaio-Yu|last3=Tsung|first3=Yi-Cheng|last4=Li|first4=Guanghao|title=Contrasting Patterns in the Early Stage of SARS-CoV-2 Evolution between Humans and Minks|journal=Journal of Virology|date=August 8, 2022|volume=94 |issue=12 |doi=10.1128/JVI.00411-20|pmid=32238584 |pmc=7307108 }} | * {{cite journal|last1=Tai|first1=Jui-Hung|last2=Sun|first2=Hsaio-Yu|last3=Tsung|first3=Yi-Cheng|last4=Li|first4=Guanghao|title=Contrasting Patterns in the Early Stage of SARS-CoV-2 Evolution between Humans and Minks|journal=Journal of Virology|date=August 8, 2022|volume=94 |issue=12 |doi=10.1128/JVI.00411-20|pmid=32238584 |pmc=7307108 }} | ||
| * {{cite journal|last1=Temmam|first1=Sarah|last2=Montagutelli|first2=Xavier|last3=Herate|first3=Cécile|last4=Donati|first4=Flora|title=SARS-CoV-2-related bat virus behavior in the human-relevant models sheds light on the origin of COVID-19|journal=EMBO Reports|date=April 5, 2023|volume=24 |issue=4 |pages=e56055 |doi=10.15252/embr.202256055|pmid=36876574 |pmc=10074129 |
* {{cite journal|last1=Temmam|first1=Sarah|last2=Montagutelli|first2=Xavier|last3=Herate|first3=Cécile|last4=Donati|first4=Flora|title=SARS-CoV-2-related bat virus behavior in the human-relevant models sheds light on the origin of COVID-19|journal=EMBO Reports|date=April 5, 2023|volume=24 |issue=4 |pages=e56055 |doi=10.15252/embr.202256055|pmid=36876574 |pmc=10074129 }} | ||
| * {{cite journal|last1=Temmam|first1=Sarah|last2=Vongphayloth|first2=Khamsing|last3=Baquero|first3=Eduard|last4=Munier|first4=Sandie|title=Bat coronaviruses related to SARS-CoV-2 and infectious for human cells|journal=Nature|date=February 16, 2022|volume=604 |issue=7905 |pages=330–336 |doi=10.1038/s41586-022-04532-4|pmid=35172323 |bibcode=2022Natur.604..330T |s2cid=246902858 }} | * {{cite journal|last1=Temmam|first1=Sarah|last2=Vongphayloth|first2=Khamsing|last3=Baquero|first3=Eduard|last4=Munier|first4=Sandie|title=Bat coronaviruses related to SARS-CoV-2 and infectious for human cells|journal=Nature|date=February 16, 2022|volume=604 |issue=7905 |pages=330–336 |doi=10.1038/s41586-022-04532-4|pmid=35172323 |bibcode=2022Natur.604..330T |s2cid=246902858 |doi-access=free}} | ||
| * {{cite web|last1=Tobias|first1=Jimmy|title=Amid Partisan Politicking, Revelations on a Covid Origins Article|work=The Nation|date=July 12, 2023|url=https://www.thenation.com/article/politics/covid-origins-congress-hearing/}} | * {{cite web|last1=Tobias|first1=Jimmy|title=Amid Partisan Politicking, Revelations on a Covid Origins Article|work=The Nation|date=July 12, 2023|url=https://www.thenation.com/article/politics/covid-origins-congress-hearing/}} | ||
| * {{cite journal|last=Willman|first=David|title=The US quietly terminates a controversial $125m wildlife virus hunting programme amid safety fears|journal=BMJ|date=September 7, 2023|volume=382 |pages=2002 |doi=10.1136/bmj.p2002|pmid=37678902 |s2cid=261557718 }} | * {{cite journal|last=Willman|first=David|title=The US quietly terminates a controversial $125m wildlife virus hunting programme amid safety fears|journal=BMJ|date=September 7, 2023|volume=382 |pages=2002 |doi=10.1136/bmj.p2002|pmid=37678902 |s2cid=261557718 |doi-access=free}} | ||
| * {{cite news|last1=Willman|first1=David|last2=Warrick|first2=Joby|title=Research with exotic viruses risks a deadly outbreak, scientists warn|newspaper=Washington Post|date=April 10, 2023|url=https://www.washingtonpost.com/investigations/interactive/2023/virus-research-risk-outbreak/}} | * {{cite news|last1=Willman|first1=David|last2=Warrick|first2=Joby|title=Research with exotic viruses risks a deadly outbreak, scientists warn|newspaper=Washington Post|date=April 10, 2023|url=https://www.washingtonpost.com/investigations/interactive/2023/virus-research-risk-outbreak/}} | ||
| * {{cite journal|last1=Wu|first1=Yiran|last2=Zhao|first2=Suwen|title=Furin cleavage sites naturally occur in coronaviruses|journal=Stem Cell Research|date=January 2021|volume=50 |doi=10.1016/j.scr.2020.102115|pmid=33340798 |pmc=7836551 }} | * {{cite journal|last1=Wu|first1=Yiran|last2=Zhao|first2=Suwen|title=Furin cleavage sites naturally occur in coronaviruses|journal=Stem Cell Research|date=January 2021|volume=50 |doi=10.1016/j.scr.2020.102115|pmid=33340798 |pmc=7836551 }} | ||
| * {{cite news|title=An acrimonious debate about covid's origins will rumble on|newspaper=The Economist|date=June 26, 2023|url=https://www.economist.com/science-and-technology/2023/06/26/an-acrimonious-debate-about-covids-origins-will-rumble-on|archive-url=https://web.archive.org/web/20231107234206/https://www.economist.com/science-and-technology/2023/06/26/an-acrimonious-debate-about-covids-origins-will-rumble-on|archive-date=November 7, 2023|ref={{harvid|Economist|2023}} }} | * {{cite news|title=An acrimonious debate about covid's origins will rumble on|newspaper=The Economist|date=June 26, 2023|url=https://www.economist.com/science-and-technology/2023/06/26/an-acrimonious-debate-about-covids-origins-will-rumble-on|archive-url=https://web.archive.org/web/20231107234206/https://www.economist.com/science-and-technology/2023/06/26/an-acrimonious-debate-about-covids-origins-will-rumble-on|archive-date=November 7, 2023|ref={{harvid|Economist|2023}} }} | ||
| {{refend}} | |||
| ==Notes== | |||
| ⚫ | {{notelist}} | ||
| ⚫ | ==References== | ||
| ⚫ | {{reflist}} | ||
| {{COVID-19 pandemic}} | {{COVID-19 pandemic}} | ||
| {{Portal bar|COVID-19|Medicine|Viruses}} | {{Portal bar|COVID-19|Medicine|Viruses}} | ||
| {{authority control}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Latest revision as of 16:56, 21 December 2024
| This article needs more reliable medical references for verification or relies too heavily on primary sources. Please review the contents of the article and add the appropriate references if you can. Unsourced or poorly sourced material may be challenged and removed. Find sources: "Zoonotic origins of COVID-19" – news · newspapers · books · scholar · JSTOR (November 2023) |  |
| It has been suggested that this article be merged into Origin of SARS-CoV-2. (Discuss) Proposed since December 2024. |
SARS-CoV-2, the causative agent of COVID-19, was first introduced to humans through zoonosis (transmission of a pathogen to a human from an animal), and a zoonotic spillover event is the origin of SARS-CoV-2 that is considered most plausible by the scientific community. Human coronaviruses including SARS-CoV-2 are zoonotic diseases that are often acquired through spillover infection from animals.
Background
Previous emergence of SARS-CoV-1 and MERS-CoV showed that Betacoronaviruses represent a risk for emergence of diseases threatening to humans. Increased awareness due to the 2002–2004 SARS outbreak motivated research into the potential for other coronavirus outbreaks and the animal reservoirs which could lead to them. Bats, in particular, are known to harbor persistent populations of coronaviruses, and under conditions of persistent infection, coronaviruses tend to accumulate mutations that allow their receptor binding domains to interact with cross-species orthologs of the target receptors. Based on serological and molecular studies, Chinese horseshoe bats were identified as the most likely reservoir for SARS-CoV-1. Bats were also a likely reservoir for the related betacoronavirus MERS-CoV, though the evidence for this is less conclusive than the role of camels as a reservoir for MERS. By 2010, in vitro experiments had confirmed that modifications to the spike protein receptor binding domain could enable human infection by several SARS-related coronaviruses. Virologists Rachel Graham and Ralph S. Baric at that time wrote, "that the question of emergence of another pathogenic human coronavirus from bat reservoirs might be more appropriately expressed as 'when' than as 'if'."
Origin in bats
The likely origin of SARS-CoV-2 from bats aligns with the origins of other coronaviruses in its genus Betacoronavirus, subgenus Sarbecovirus. Several bat species have special cellular mechanisms to resist proinflammatory cytokines associated with Betacoronavirus virulence, for example, spike proteins in SARS-related coronaviruses coevolve with bat ACE2 receptors in an evolutionary arms race.

Bats, along with their viruses, have large overlapping geographic ranges in Southeast Asia, and there is a particularly great concentration and diversity of bat-related coronaviruses in Southern and Southwest China. The most similar known viruses to SARS-CoV-2 include bat coronaviruses RpYN06 with 94.5% identity, and RmYN02 with 93% identity RaTG13 was not the direct progenitor of SARS-CoV-2. Temmam et al. found no serological evidence for exposure to BANAL-52 among bat handlers and guano collectors in the area of Laos where it was sampled. Lytras et al. wrote that "SARS-CoV-2 can be unambiguously traced to horseshoe bats". They estimated SARS-CoV-2's most recent common ancestors with RmYN02 and RaTG13 to have diverged 40 and 50 years ago, respectively.
Novel features of SARS-CoV-2
The receptor binding domain of the SARS-CoV-2 spike protein has an insertion of amino acids between its S1 and S2 subunits. Among Sarbecoviruses, only SARS-CoV-2 and RmYN02 have such an insertion, suggesting differences in reservoir species, intermediate hosts, or evolutionary pathway. The receptor binding motif is the portion of SARS-CoV-2 that is most diverged from RaTG13. The SARS-CoV-2 receptor binding domain is more similar to those of pangolin coronaviruses. Viruses including BANAL-52 isolated from bats in Laos showed high similarity to the SARS-CoV-2 receptor binding domain in amino acid residues but less than 76.4% nucleotide identity across the spike protein. The observed binding of N-acetylneuraminic acid by the NTD of the spike protein and loss of that binding through mutation of the corresponding sugar binding pocket in emergent variants of concern has suggested a potential role for transient sugar-binding in the zoonosis of SARS-CoV-2, consistent with prior evolutionary proposals.
Within genus Betacoronavirus, furin cleavage sites are common in subgenera Merbecovirus and Embecovirus. Furin cleavage sites have independently evolved six times in different clades of Betacoronavirus. Furin cleavage sites also evolved in Alphacoronaviruses and Gammacoronaviruses independently of Betacoronavirus.
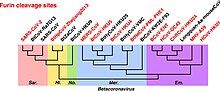
Furin cleavage contributes strongly to the transmissibility and pathogenicity of SARS-CoV-2 in humans. SARS-CoV-2 variants lacking the furin cleavage site are transmissible between humans, but much less effectively. The furin cleavage site is sometimes described as "polybasic" on account of its particular motif of basic amino acids. SARS-CoV-2 shares amino acid identity with a furin cleavage site of human ENaC α subunit. Human ENaC is identical only to that of a few great apes and Pipistrellus kuhli.
SARS-CoV-2 is also distinct among human coronaviruses for having a single intact ORF8 gene rather than "a" and "b" subunits.
Processes of host adaptation
SARS-CoV-2 has an expanded host range compared to SARS-CoV-1 and MERS-CoV. SARS-CoV-2 (along with SARS-CoV-1 and MERS-CoV) are generalist viruses, not specifically adapted to humans, meaning they have potential to spill over to many species and establish new natural reservoirs after adaptive evolutionary changes.
Within a single host, a variety of single-nucleotide variations arise through random mutations and genetic drift giving rise to viral quasispecies. SARS-CoV-2 mutates at a slower rate than is typical of RNA viruses. The main host-derived driver of mutation is editing by APOBEC proteins. Negative selection by host immune processes causes convergent evolution towards immune escape. Persistence of infection is correlated with quasispecies diversity, but the direction of causality for this is unknown.
Actual host susceptibility can differ significantly from in silico predictions. The process of host adaptation has been studied in humanized mice as well as by generating mouse-adapted viral strains through serial passage. Wild-type mice are only weakly susceptible to the original SARS-CoV-2 strain.
Recombination
Compared to other single-stranded RNA viruses, coronaviruses have increased tendency to undergo genetic recombination, which allows them to exchange genetic material with close relatives co-infecting the same organism. The origin of SARS-CoV-1 is believed to involve multiple recombination events. Recombination between strains of SARS-CoV-1 is common. Inferred phylogenies of SARS2-related coronaviruses might be explained by recombination. Recombination between various SARS-CoV-2 variants of concern has been reported.

Lytras et al. identified the SARS-CoV-2 spike open reading frame as a recombination hotspot. They speculate that the SARS-CoV-2 genome may involve repeated recombination events overprinting regions that were already themselves products of recombination. Temmam et al. wrote that due to the limited diffusion of bat viruses in mammals, co-infection required for recombination in mammals was unlikely. Therefore, they considered it more likely that the furin cleavage site arose in a bat reservoir prior to spillover.
Selection
After cross-species transmission of a virus, rapid evolution and positive selection are expected. Several studies found only weak signs of adaptive evolution early in the COVID-19 pandemic. Kang et al. wrote that SARS-CoV-2 had exhibited relatively little genetic variation by 2021. Tai et al. wrote that population expansion rather than positive selection explained the mutation frequency spectrum during the early pandemic. Cagliani et al. wrote that the SARS-CoV-2 genome overall shows evidence of "strong to moderate" purifying selection. Accessory open reading frames, especially ORF8, showed weak to neutral selection. The general lack of positive selection during the early outbreak of SARS-CoV-2 contrasted with the evolutionary course of SARS-CoV-1.
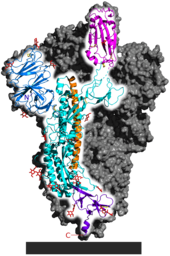
Strong evidence of positive selection was found however in the spike protein S1 subunit, which contains the receptor binding domain.
Genome instability in the spike protein is typical of coronaviruses in general, favoring the production of numerous spike protein variants. The receptor binding domain is a significant factor in host tropism, or the variety of species a virus can infect. Coronavirus adaptation to a new host often requires mutations in the receptor binding domain. Kang et al. identified a single nucleotide polymorphism relative to RaTG13 in the spike protein, consistent among all of more than 180,000 SARS-CoV-2 samples, affecting glycosylation of the receptor binding domain. Using a reverse genetics system to generate an ancestral-like mutant, they confirmed that the putative ancestral form of this SNP was much less transmissible in human cells.
Host proteases
The majority of sarbecoviruses are not dependent on ACE2 for cell entry. Guo et al. divide the sarbecoviruses into four clades, the first being ACE2-using respiratory viruses including SARS-CoV-1, SARS-CoV-2, and WIV1. Relative to clade 1, clades 3 and 4 have a one residue deletion in the receptor binding domain and diminished ability to use ACE2. Clade 2 has two deletions and does not interact with ACE2. Clades 2 through 4 are more difficult to isolate or propagate in cell culture, and consequently have been less studied. ACE2-independent infection by sarbecoviruses depends on high levels of trypsin, a digestive protein, in the host environment. Trypsin may compensate for other missing or compatible host proteases. The ubiquity of furin compared to tripsin would allow the gain of a furin cleavage site to expand viral tissue tropism. Guo et al. identified clade 1 and 2 sarbecoviruses in Rhinolophus fecal samples, suggesting that both types naturally replicate in the bat digestive tract. Bats with SARS-like infections in the wild showed no viral replication in respiratory droplets. A fecal-oral transmission is an alternative route for some respiratory viruses. No higher incidence of Sarbecoviruses has been reported in workers who come into direct contact with guano. Temmam et al. found that BANAL-236, a SARS-CoV-2-related virus isolated from bats in Laos, acts as an enteric virus in macaques.
Timing of spillover
See also: COVID-19 pandemic in HubeiIn contrast to SARS-CoV-1, the initial outbreak of SARS-CoV-2 was reported in only one city. Chinese epidemiological surveillance did not report any other pneumonia outbreaks in autumn 2019. Graham and Baric wrote that in the case of SARS-CoV-1, virus populations circulated and adapted to civets and humans over the course of two years before the recognized outbreak.
Reverse zoonosis
Transmission of SARS-CoV-2 from humans to animals, known as reverse zoonosis or anthroponosis, is possible. Reverse zoonotic or experimental infection with SARS-CoV-2 has been reported in 31 animal species. It is not believed that wildlife plays a significant role in the ongoing circulation of SARS-CoV-2 among humans.

SARS-CoV-2 variants adapted to mink were found co-circulating among humans and farmed mink. Mink are the only animal in Europe or North America to experience widespread SARS-CoV-2 outbreaks. Transmission from human to mink has occurred multiple times, in most cases not resulting in a sustained mink outbreak. Strong evidence was seen of positive selection in mink after spillover, concentrated in the receptor binding motif.
Transmission back to humans has been documented for mink, hamsters, and cats. Transmission to humans from deer is suspected. Transmission of SARS-CoV-2 among domestic cats has been confirmed.
Li et al. wrote that SARS-CoV-2 may be less able than SARS-CoV-1 to pass from humans to animals.
Hypothesized intermediate hosts
See also: COVID-19 in animalsIn the outbreak of SARS-CoV-1, palm civets, raccoon dogs, ferret badgers, red foxes, domestic cats, and rice field rats were possible vectors. Graham and Baric wrote that human and civet infections likely stemmed from an unknown common progenitor. Patrick Berche wrote that the emergences of SARS-CoV-1 and MERS-CoV appeared to be sequential processes involving intermediate hosts, co-infections, and recombination. In contrast with the rapid identification of animal hosts for SARS-CoV-1 and MERS-CoV, no direct animal source for SARS-CoV-2 has been found. Holmes et al. wrote that the lack of intermediate host is likely because the right animal has not been tested so far. Frutos et al. proposed that rather than a discrete spillover event, SARS-CoV-2 arose in accordance with a circulation model, involving repeated horizontal transfer among humans, bats, and other mammals without establishing significant reservoirs in any of them until the pandemic.

Pangolins have been considered a possible reservoir of SARS-CoV-2. Pangolins are sometimes sold in wet markets in China, where they are considered a culinary delicacy and a component of traditional medicine. The highest sequence similarity to the SARS-CoV-2 spike receptor binding domain was found in a coronavirus infecting Sunda pangolins in Guangdong province. Pangolins are frequently smuggled to China. Lytras et al. wrote that, consistent with a lack of reported infections of pangolins in Malaysia, they were likely infected after being trafficked into China.
The SARS-CoV-2 receptor binding domain shares more synonymous substitutions with a pangolin-CoV than RaTG13. The binding potential of SARS-CoV-2 to pangolin ACE2 however is very low. The receptor binding domain of SARS-CoV-2 has been hypothesized to originate from recombination of the relevant portion of pangolin-CoV with an RaTG13-like virus.
Deer mice are highly susceptible to SARS-CoV-2, making them potential reservoir or intermediate hosts. Raccoon dogs could be capable of transmitting SARS-CoV-2 to other animals under farm-like conditions. Transmission between raccoon dogs was shown under laboratory conditions. White-tailed deer are a potential vector for SARS-CoV-2.
Wet market hypothesis
Spillover has been proposed to have occurred at one or more wet markets in China. Wild and semi-wild game animals are commonly traded and consumed in China, and this practice has expanded in recent decades. The close contact among animals in unsanitary conditions creates the potential for diseases to thrive. In the 2002 outbreak in Guangdong, most living animals in the markets showed serological evidence of exposure to SARS-CoV-1.

Many early cases were associated with the Huanan seafood market in Wuhan. The first documented COVID-19 infection was in a worker at a seafood stall in the Huanan market. Sequences from that patient have not been published; however, SARS-CoV-2 belonging to lineage B/L were detected in environmental samples from the patient's stall. No bats or pangolins were reported to be at the market between May 2017 and November 2019. Raccoon dogs, which are hypothesized to be susceptible to SARS-CoV-2, were at the market. A survey by He et al. in 2021 identified 102 mammal-affecting viruses in Chinese game animals, 65 of which were identified then for the first time. They did not find any SARS-like viruses, but did find evidence for the transmission of a MERS-like virus from bats to hedgehogs.
Between January 1 and March 30, epidemiologists from China CDC collected 457 samples from animal matter including carcasses and feces and collected 923 environmental samples. All animal samples tested negative for SARS-CoV-2. SARS-CoV-2 was found in 73 environmental samples. Live virus was isolated from three samples, two of which came from stalls belonging to known patients. No significant association was found between environmental virus titer and the type of product sold at particular stalls. SARS-CoV-2 was identified in all four sewer wells in the market. Overground drainage from the surrounding area concentrated under the market.
One environmental sample contained lineage A/S SARS-CoV-2, while all others belonged to lineage B/L. The sample containing lineage A/S also contained evidence of humans and livestock, but no wild animals. Overall, wildlife including racoon dogs were detected at very low levels and mostly associated with negative samples. Liu et al. wrote that there was not enough information to determine the origin of the virus. In particular, they wrote that the evidence does not prove the presence of an infected raccoon dog or the occurrence of multiple zoonotic spillovers at the market as proposed by Pekar et al.
Origins of variants
The World Health Organization defines variants of concern as a variant with evidence of increase transmissibility, severity, or immune escape. All variants of concern so far independently evolved from the original strain, rather than from each other.
The evolution of the Omicron variant appeared to be more rapid than other variants. Theories for the origin of the omicron variant include long-term circulation among humans outside areas where genetic surveillance was performed, mutation in an immunocompromised individual, or adaptation in an animal species after reverse zoonosis. The Omicron variant is proposed to have originated in mice.
Notes
- : ... "the proximal phylogenetic origin of SARS-CoV-2 and its mode of introduction into human circulation remain unclear. There is no evidence for SARS-CoV-2 having been 'engineered' in a lab. Conversely, the escape of a strain from a lab or an accidental contamination during field work cannot formally be ruled out at this stage. However, a zoonotic spillover event in nature is considered as the most plausible scenario in the scientific community"
- Harrison and Sachs' analysis of the furin cleavage site have been disputed in a letter to the editor Garry, Robert F. (September 29, 2022). "SARS-CoV-2 furin cleavage site was not engineered". PNAS. 119 (40): e2211107119. Bibcode:2022PNAS..11911107G. doi:10.1073/pnas.2211107119. PMC 9546612. PMID 36173950., with a reply from the authors Harrison, Neil L.; Sachs, Jeffery D. (November 2, 2022a). "Reply to Garry: The origin of SARS-CoV-2 remains unresolved". PNAS. 119 (45): e2215826119. Bibcode:2022PNAS..11915826H. doi:10.1073/pnas.2215826119. PMC 9659384. PMID 36322733.
- : "Weak signs of adaptive evolution during the early phase of the epidemic of SARS-CoV-2 in humans have been observed in many studies (Chaw et al. 2020; Tang et al. 2020; MacLean et al. 2021; Martin et al. 2021)."
References
Citations
- ^ Balloux et al. 2022, p. 4.
- ^ Kane, Wong & Gao 2023, p. 15.
- Kane, Wong & Gao 2023, p. 23.
- Kane, Wong & Gao 2023, pp. 20, 21, 23.
- Graham & Baric 2010, p. 3135.
- Graham & Baric 2010, pp. 3139–3140.
- ^ Kane, Wong & Gao 2023, p. 12.
- Kane, Wong & Gao 2023, p. 13.
- Graham & Baric 2010.
- Graham & Baric 2010, pp. 3142.
- Kane, Wong & Gao 2023, pp. 2–3.
- Cagliani et al. 2020, p. 1.
- ^ Frutos et al. 2020, p. 1.
- Kane, Wong & Gao 2023, pp. 12, 23.
- ^ Kane, Wong & Gao 2023, p. 17.
- Guo et al. 2020.
- Lytras et al. 2022.
- ^ Kane, Wong & Gao 2023, p. 3.
- ^ Holmes et al. 2021, p. 4850.
- Temmam et al. 2023, p. 8,10.
- ^ Lytras et al. 2022, p. 1.
- Lytras et al. 2022, p. 4.
- Kane, Wong & Gao 2023, pp. 3, 14.
- ^ Cagliani et al. 2020, p. 5.
- Buchanan, Charles J.; et al. (2022). "Pathogen-sugar interactions revealed by universal saturation transfer analysis". Science. 377 (6604): eabm3125. doi:10.1126/science.abm3125. hdl:1983/355cbd8f-c424-4cc0-adb2-881c04ab3bf0. PMID 35737812.
- Rossmann, M. G. (1989). "The Canyon Hypothesis". Journal of Biological Chemistry. 264 (25): 14587–14590. doi:10.1016/S0021-9258(18)63732-9.
- ^ Wu & Zhao 2021.
- ^ Hossain et al. 2021, p. 1816.
- Hossain et al. 2021, p. 1817.
- Anand et al. 2020.
- Harrison & Sachs 2022.
- Harrison & Sachs 2022, p. 4.
- ^ Kane, Wong & Gao 2023, p. 14.
- Li et al. 2023.
- ^ Li et al. 2023, p. 557.
- ^ Biancolella et al. 2023, p. 3.
- ^ Frutos et al. 2020, p. 3.
- ^ Kane, Wong & Gao 2023, p. 8.
- Kane, Wong & Gao 2023, p. 19.
- Graham & Baric 2010, p. 3139.
- ^ Kane, Wong & Gao 2023, p. 20.
- Qian et al. 2022, p. 8.
- ^ Kane, Wong & Gao 2023, p. 4.
- Lytras et al. 2022, p. 3.
- ^ Lytras et al. 2022, p. 6.
- Temmam et al. 2023, p. 10.
- ^ Kang et al. 2021, p. 4393.
- Tai et al. 2022, p. 4.
- Tai et al. 2022, p. 8.
- Cagliani et al. 2020, p. 3.
- ^ Tai et al. 2022, p. 2.
- Qian et al. 2022.
- Kane, Wong & Gao 2023, p. 16.
- ^ Li et al. 2023, p. 554.
- ^ Kane, Wong & Gao 2023, p. 21.
- Kang et al. 2021, p. 4396.
- Kang et al. 2021, p. 4397.
- ^ Guo et al. 2022.
- Jaimes, Millet & Whittaker 2020, p. 2.
- ^ Graham & Baric 2010, p. 3141.
- Temmam et al. 2023, p. 5.
- Berche 2023, p. 1,5.
- Berche 2023, p. 5.
- Qian et al. 2022, p. 16.
- ^ Reggiani, Rugna & Bonilauri 2022, p. 4.
- ^ Reggiani, Rugna & Bonilauri 2022, p. 2.
- Tai et al. 2022, p. 2-3.
- ^ Kane, Wong & Gao 2023, p. 11.
- Graham & Baric 2010, pp. 3141, 3142.
- ^ Berche 2023, p. 1.
- Deng, Xing & He 2022.
- Frutos et al. 2021.
- Frutos et al. 2020.
- Cagliani et al. 2020, p. 6.
- Kane, Wong & Gao 2023, pp. 19–20.
- Kane, Wong & Gao 2023, p. 10.
- Lytras et al. 2022, p. 2.
- ^ Pekar et al. 2022, p. 4.
- Liu et al. 2023, p. 3.
- ^ He et al. 2022.
- ^ Liu et al. 2023, p. 7.
- Liu et al. 2023, p. 6.
- Liu et al. 2023, pp. 8–9.
- Liu et al. 2023, p. 10.
- ^ Liu et al. 2023, p. 12.
- Qian et al. 2022, p. 13.
- Qian et al. 2022, p. 14.
- ^ Reggiani, Rugna & Bonilauri 2022, p. 3.
Bibliography
- Anand, Praveen; Puranik, Arjun; Aravamudan, Murali; Venkatakrishnan, AJ (May 26, 2020). "SARS-CoV-2 strategically mimics proteolytic activation of human ENaC". eLife. 9. doi:10.7554/eLife.58603. PMC 7343387. PMID 32452762.
- Anderson, Kristian G.; Rambaut, Andrew; Lipkin, W. Ian; Holmes, Edward C. (March 17, 2020). "The proximal origin of SARS-CoV-2". Nature. 26 (4): 450–452. doi:10.1038/s41591-020-0820-9. PMC 7095063. PMID 32284615.
- Balloux, François; Tan, Cedric; Swadling, Leo; Richard, Damien (June 20, 2022). "The past, current and future epidemiological dynamic of SARS-CoV-2". Oxford Open Immunology. 3 (1): iqac003. doi:10.1093/oxfimm/iqac003. PMC 9278178. PMID 35872966.
- Berche, Patrick (March 2023). "Gain-of-function and origin of Covid19". La Presse Médicale. 52 (1). doi:10.1016/j.lpm.2023.104167. PMC 10234839. PMID 37269978.
- Biancolella, Michela; Colona, Vito Luigi; Luzzatto, Lucio; Watt, Jessica Lee (July 24, 2023). "COVID-19 annual update: a narrative review". Human Genomics. 17 (1): 68. doi:10.1186/s40246-023-00515-2. PMC 10367267. PMID 37488607.
- Cagliani, Rachele; Forni, Diego; Clerici, Mario; Sironi, Manuela (April 1, 2020). "Computational Inference of Selection Underlying the Evolution of the Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2". Journal of Virology. 94 (12). doi:10.1128/JVI.00411-20. PMC 7307108. PMID 32238584.
- Canuti, Marta; Bianchi, Silvia; Kolbi, Otto; Pond, Sergei L.K. (March 16, 2022). "Waiting for the truth: is reluctance in accepting an early origin hypothesis for SARS-CoV-2 delaying our understanding of viral emergence?". BMJ Global Health. 7 (3): e008386. doi:10.1136/bmjgh-2021-008386. PMC 8927931. PMID 35296465.
- Cohen, Jon (August 19, 2022). "Anywhere but Here". Science. 377 (6608): 805–809. Bibcode:2022Sci...377..805C. doi:10.1126/science.ade4235. PMID 35981032. S2CID 251670601.
- Fernando, Christine; Mumphrey, Cheyanne (December 20, 2020). "Racism targets Asian food, business during COVID-19 pandemic". PBS News Weekend.
- Deng, Shanjun; Xing, Ke; He, Xionglei (February 2022). "Mutation signatures inform the natural host of SARS-CoV-2". National Science Review. 9 (2): nwab220. doi:10.1093/nsr/nwab220. PMC 8690307. PMID 35211321.
- Eban, Katherine (June 1, 2023). "Inside the COVID Origins Raccoon Dog Cage Match". Vanity Fair. Archived from the original on July 1, 2023.
- Frutos, Roger; Pliez, Olivier; Gavotte, Laurent; Devaux, Christian A. (October 6, 2021). "There is no "origin" to SARS-CoV-2". Environmental Research. 207. doi:10.1016/j.envres.2021.112173. PMC 8493644. PMID 34626592. S2CID 238412878.
- Frutos, Roger; Serra-Cobo, Jordi; Chen, Tianmu; Devaux, Christian A. (August 5, 2020). "COVID-19: Time to exonerate the pangolin from the transmission of SARS-CoV-2 to humans". Infection, Genetics and Evolution. 84. Bibcode:2020InfGE..8404493F. doi:10.1016/j.meegid.2020.104493. PMC 7405773. PMID 32768565. S2CID 220971074.
- Ge, Xing-Yi; Li, Jia-Lu; Yang, Xing-Lou; Chmura, Aleksei A. (October 30, 2013). "Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor". Nature. 503 (7477): 535–538. Bibcode:2013Natur.503..535G. doi:10.1038/nature12711. PMC 5389864. PMID 24172901. S2CID 4464714.
- Goodman, Brenda (July 11, 2023). "Scientists at House hearing refute claims they were bribed and influenced to deny Covid-19 'lab leak' theory". CNN.
- Graham, Rachel L.; Baric, Ralph S. (April 2010). "Recombination, Reservoirs, and the Modular Spike: Mechanisms of Coronavirus Cross-Species Transmission". Journal of Virology. 84 (7): 3134–3146. doi:10.1128/JVI.01394-09. PMC 2838128. PMID 19906932.
- Guo, Hua; Hu, Bing-Jie; Yang, Xing-Lou; Zeng, Lei-Ping (September 29, 2020). "Evolutionary Arms Race between Virus and Host Drives Genetic Diversity in Bat Severe Acute Respiratory Syndrome-Related Coronavirus Spike Genes". Journal of Virology. 94 (20). doi:10.1128/JVI.00902-20. PMC 7527062. PMID 32699095. S2CID 220716415.
- Guo, Hua; Li, Ang; Dong, Tian-Yi; Su, Jia (November 21, 2022). "ACE2-Independent Bat Sarbecovirus Entry and Replication in Human and Bat Cells". Virology. 13 (6): e0256622. doi:10.1128/mbio.02566-22. PMC 9765407. PMID 36409074.
- Harrison, Neil L.; Sachs, Jeffery D. (May 19, 2022). "A call for an independent inquiry into the origin of the SARS-CoV-2 virus". PNAS. 119 (21): e2202769119. Bibcode:2022PNAS..11902769H. doi:10.1073/pnas.2202769119. PMC 9173817. PMID 35588448.
- He, Wan-Ting; Hou, Xin; Zhao, Jin; Sun, Jiumeng (February 16, 2022). "Virome characterization of game animals in China reveals a spectrum of emerging pathogens". Cell. 185 (7): 1117–1129.e8. doi:10.1016/j.cell.2022.02.014. PMC 9942426. PMID 35298912.
- Holmes, Edward C.; Goldstein, Stephen A.; Rasmussen, Angela L.; Robertson, David L. (September 16, 2021). "The origins of SARS-CoV-2: A critical review". Cell. 184 (19): 4848–4856. doi:10.1016/j.cell.2021.08.017. PMC 8373617. PMID 34480864.
- Hossain, Golzar; Tang, Yan-dong; Akter, Sharmin; Zheng, Chunfu (December 22, 2021). "Roles of the polybasic furin cleavage site of spike protein in SARS-CoV-2 replication, pathogenesis, and host immune responses and vaccination". Journal of Medical Virology. 94 (5): 1815–1820. doi:10.1002/jmv.27539. PMID 34936124. S2CID 245430230.
- Jaimes, Javier A.; Millet, Jean K.; Whittaker, Gary R. (June 26, 2020). "Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the Novel S1/S2 Site". iScience. 23 (6). Bibcode:2020iSci...23j1212J. doi:10.1016/j.isci.2020.101212. PMC 7255728. PMID 32512386.
- Kane, Yakhouba; Wong, Gary; Gao, George F. (February 2023). "Animal Models, Zoonotic Reservoirs, and Cross-Species Transmission of Emerging Human-Infecting Coronaviruses". Annual Review of Animal Biosciences. 11: 1–31. doi:10.1146/annurev-animal-020420-025011. PMID 36790890. S2CID 256869353.
- Kang, Lin; He, Guijuan; Sharp, Amanda K.; Wang, Xiaofeng (July 6, 2021). "A selective sweep in the Spike gene has driven SARS-CoV-2 human adaptation". Cell. 184 (17): 4392–4400.e4. doi:10.1016/j.cell.2021.07.007. PMC 8260498. PMID 34289344.
- Kumar, Sudhir; Tao, Qiqing; Weaver, Steven; Sanderford, Maxwell (August 8, 2021). "An Evolutionary Portrait of the Progenitor SARS-CoV-2 and Its Dominant Offshoots in COVID-19 Pandemic". Molecular Biology and Evolution. 38 (8): 3046–3059. doi:10.1093/molbev/msab118. PMC 8135569. PMID 33942847.
- Li, Hongying; Mendelsohn, Emma; Zong, Chen; Zhang, Wei (September 2019). "Human-animal interactions and bat coronavirus spillover potential among rural residents in Southern China". Biosafety and Health. 1 (2): 84–90. doi:10.1016/j.bsheal.2019.10.004. PMC 7148670. PMID 32501444.
- Li, Meng; Du, Juan; Liu, Weiqiang; Li, Zihao (January 23, 2023). "Comparative susceptibility of SARS-CoV-2, SARS-CoV, and MERS-CoV across mammals". ISME. 17 (4): 549–560. Bibcode:2023ISMEJ..17..549L. doi:10.1038/s41396-023-01368-2. PMC 9869846. PMID 36690780.
- Liu, William J.; Liu, Peipei; Lei, Wenwen; Jia, Zhiyuan (April 5, 2023). "Surveillance of SARS-CoV-2 at the Huanan Seafood Market". Nature. 631 (8020): 402–408. doi:10.1038/s41586-023-06043-2. PMID 37019149. S2CID 257983307.
- Lytras, Spyros; Hughes, Joseph; Martin, Darren; Swanepoel, Phillip (February 8, 2022). "Exploring the Natural Origins of SARS-CoV-2 in the Light of Recombination". Genome Biology and Evolution. 14 (2). doi:10.1093/gbe/evac018. PMC 8882382. PMID 35137080.
- Lubinski, Bailey; Whittaker, Gary R (August 2023). "The SARS-CoV-2 furin cleavage site: natural selection or smoking gun?". The Lancet Microbe. 4 (8): e570. doi:10.1016/S2666-5247(23)00144-1. PMC 10205058. PMID 37236215.
- MacLean, Oscar A.; Lytras, Spyros; Weaver, Steven; Singer, Joshua B. (March 12, 2021). "Natural selection in the evolution of SARS-CoV-2 in bats created a generalist virus and highly capable human pathogen". PLOS Biology. 19 (3): e3001115. doi:10.1371/journal.pbio.3001115. PMC 7990310. PMID 33711012.
- McCarthy, Simone; Baptista, Eduardo (October 15, 2021). "Science turns nasty in Covid-19 origins argument on Twitter". South China Morning Post. Archived from the original on March 10, 2024.
- Menachery, Vineet D.; Yount, Boyd L. Jr.; Sims, Amy C.; Baric, Ralph S. (March 14, 2016). "SARS-like WIV1-CoV poised for human emergence". PNAS. 113 (11): 3048–3053. Bibcode:2016PNAS..113.3048M. doi:10.1073/pnas.1517719113. PMC 4801244. PMID 26976607.
- Palmer, James (January 27, 2020). "Don't Blame Bat Soup for the Coronavirus". Foreign Policy.
- Pekar, Jonathan E.; Magee, Andrew; Parker, Edyth; Moshiri, Niema (July 26, 2022). "The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2". Science. 377 (6609): 960–966. Bibcode:2022Sci...377..960P. doi:10.1126/science.abp8337. PMC 9348752. PMID 35881005.
- Qian, Zhaohui; Li, Pei; Tang, Xiaolu; Lu, Jian (March 1, 2022). "Evolutionary dynamics of the severe acute respiratory syndrome coronavirus 2 genomes". Medical Review. 2 (1): 3–22. doi:10.1515/mr-2021-0035. PMC 9047652. PMID 35658106.
- Reggiani, Alessandro; Rugna, Gianluca; Bonilauri, Paolo (December 15, 2022). "SARS-CoV-2 and animals, a long story that doesn't have to end now: What we need to learn from the emergence of the Omicron variant". Frontiers in Veterinary Science. 9. doi:10.3389/fvets.2022.1085613. PMC 9798331. PMID 36590812.
- Smith, David (February 28, 2023). ""It's just gotten crazy": how the origins of Covid became a toxic US political debate". The Guardian.
- Tai, Jui-Hung; Sun, Hsaio-Yu; Tsung, Yi-Cheng; Li, Guanghao (August 8, 2022). "Contrasting Patterns in the Early Stage of SARS-CoV-2 Evolution between Humans and Minks". Journal of Virology. 94 (12). doi:10.1128/JVI.00411-20. PMC 7307108. PMID 32238584.
- Temmam, Sarah; Montagutelli, Xavier; Herate, Cécile; Donati, Flora (April 5, 2023). "SARS-CoV-2-related bat virus behavior in the human-relevant models sheds light on the origin of COVID-19". EMBO Reports. 24 (4): e56055. doi:10.15252/embr.202256055. PMC 10074129. PMID 36876574.
- Temmam, Sarah; Vongphayloth, Khamsing; Baquero, Eduard; Munier, Sandie (February 16, 2022). "Bat coronaviruses related to SARS-CoV-2 and infectious for human cells". Nature. 604 (7905): 330–336. Bibcode:2022Natur.604..330T. doi:10.1038/s41586-022-04532-4. PMID 35172323. S2CID 246902858.
- Tobias, Jimmy (July 12, 2023). "Amid Partisan Politicking, Revelations on a Covid Origins Article". The Nation.
- Willman, David (September 7, 2023). "The US quietly terminates a controversial $125m wildlife virus hunting programme amid safety fears". BMJ. 382: 2002. doi:10.1136/bmj.p2002. PMID 37678902. S2CID 261557718.
- Willman, David; Warrick, Joby (April 10, 2023). "Research with exotic viruses risks a deadly outbreak, scientists warn". Washington Post.
- Wu, Yiran; Zhao, Suwen (January 2021). "Furin cleavage sites naturally occur in coronaviruses". Stem Cell Research. 50. doi:10.1016/j.scr.2020.102115. PMC 7836551. PMID 33340798.
- "An acrimonious debate about covid's origins will rumble on". The Economist. June 26, 2023. Archived from the original on November 7, 2023.
