| Revision as of 13:03, 2 May 2007 view sourceDanfass (talk | contribs)22 editsNo edit summary← Previous edit | Latest revision as of 12:48, 29 November 2024 view source Mondtaler (talk | contribs)181 edits Added a significant risk factor, see: https://www.uicc.org/what-we-do/thematic-areas/cancer-and-air-pollution - "Nearly half of lung cancer cases in people who have never smoked are estimated to be related to air pollution."Tag: 2017 wikitext editor | ||
| Line 1: | Line 1: | ||
| {{Short description|Malignant tumor characterized by uncontrolled cell growth in lung tissue}} | |||
| {{Infobox_Disease | | |||
| {{cs1 config|name-list-style=vanc}} | |||
| Name = LUNG cancer | | |||
| {{pp-semi-indef|small=yes}} | |||
| Image = cancerous_lung.jpg | | |||
| {{About|lung carcinomas|other lung tumors|Lung tumor}} | |||
| Caption = Cross section of a human lung. The white area in the upper lobe is cancer; the black areas indicate the patient was a smoker. | | |||
| {{featured article}} | |||
| DiseasesDB = 7616 | | |||
| {{Use dmy dates|date=January 2023}} | |||
| ICD10 = {{ICD10|C|33||c|30}}-{{ICD10|C|34||c|30}} | | |||
| {{Infobox medical condition (new) | |||
| ICD9 = {{ICD9|162}} | | |||
| |
| name = Lung cancer | ||
| |
| synonyms = Lung carcinoma | ||
| | image = LungCACXR.PNG | |||
| MedlinePlus = 007194 | | |||
| | caption = A ] showing a tumor in the lung (marked by arrow) | |||
| eMedicineSubj = med | | |||
| | alt = X-ray with an arrow pointing to a hazy circular mass in the chest | |||
| eMedicineTopic = 1333 | | |||
| | field = ], ] | |||
| eMedicine_mult = {{eMedicine2|med|1336}} {{eMedicine2|emerg|335}} {{eMedicine2|radio|807}} {{eMedicine2|radio|405}} {{eMedicine2|radio|406}} | | |||
| | symptoms = ] (including ]), ], ] | |||
| MeshID = D002283 | | |||
| | complications = | |||
| | onset = After age 40;{{sfn|Horn|Iams|2022|loc="Epidemiology"}} 70 years on average{{sfn|Bade|Dela Cruz|2020|loc="Age"}} | |||
| | duration = | |||
| | types = ] (SCLC), ] (NSCLC) | |||
| | causes = | |||
| | risks = {{hlist|]|]|]|]|Other environmental ]s}} | |||
| | diagnosis = ], ] | |||
| | differential = | |||
| | prevention = Avoid smoking and other environmental mutagens | |||
| | treatment = ], ], ], molecular therapies, ]s | |||
| | medication = | |||
| | prognosis = ]: 10 to 20% (most countries){{sfn|Sung|Ferlay|Siegel|Laversanne|2021|loc="Lung cancer"}} | |||
| | frequency = 2.2 million (2020){{sfn|Sung|Ferlay|Siegel|Laversanne|2021|loc="Lung cancer"}} | |||
| | deaths = 1.8 million (2020){{sfn|Sung|Ferlay|Siegel|Laversanne|2021|loc="Lung cancer"}} | |||
| }} | }} | ||
| '''Lung cancer''' is the malignant transformation and expansion of lung tissue, and is the most lethal of all cancers worldwide, responsible for 1.2 million ] | |||
| annually. It is caused predominantly by cigarette smoking, and predominantly affects men, with it being the leading cause of death of men between the ages of 40 and 65. With increased smoking among women, it is now occuring in women at an alarming rate.<ref></ref> While some people who have never smoked do still get lung cancer, this appears to be due to a combination of genetic factors<ref>Gorlova OY, Weng SF, Zhang Y, Amos CI, Spitz MR. Aggregation of cancer among relatives of never-smoking lung cancer patients. Int J Cancer. 2007 Feb 15;121(1):2865-2872 PMID 17304511</ref> and exposure to secondhand smoke.<ref>Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004 Aug;45 Suppl 2:S3-9. PMID 15552776</ref><ref>Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1997 Oct 18;315(7114):980-8. PMID 9365295 </ref> | |||
| '''Lung cancer''', also known as '''lung carcinoma''', is a malignant ] that begins in the ]. Lung cancer is caused by ] to the ] of ]s in the airways, often caused by ] or inhaling damaging chemicals. Damaged airway cells gain the ability to multiply unchecked, causing the growth of a tumor. Without treatment, tumors spread throughout the lung, damaging lung function. Eventually lung tumors ], spreading to other parts of the body. | |||
| Current research indicates that the ] with the greatest impact on risk of lung cancer is long-term exposure to inhaled ]s. The most common means of such exposure is air pollution and ]. | |||
| Early lung cancer often has no symptoms and can only be detected by ]. As the cancer progresses, most people experience nonspecific respiratory problems: ], ], or ]. Other symptoms depend on the location and size of the tumor. Those suspected of having lung cancer typically undergo a series of imaging tests to determine the location and extent of any tumors. Definitive diagnosis of lung cancer requires a ] of the suspected tumor be examined by a ] under a ]. In addition to recognizing cancerous cells, a pathologist can classify the tumor according to the type of cells it originates from. Around 15% of cases are ] (SCLC), and the remaining 85% (the ]s or NSCLC) are ]s, ]s, and ]s. After diagnosis, further imaging and biopsies are done to determine the cancer's ] based on how far it has spread. | |||
| Treatment and ] depend upon the ] type of cancer, the ] (degree of spread), and the patient's ]. Treatments include ], ], and ]. | |||
| Treatment for early stage lung cancer includes ] to remove the tumor, sometimes followed by ] and ] to kill any remaining cancer cells. Later stage cancer is treated with radiation therapy and chemotherapy alongside drug treatments that target specific cancer subtypes. Even with treatment, only around 20% of people survive five years on from their diagnosis.{{sfn|Rivera|Mody|Weiner|2022|loc="Introduction"}} Survival rates are higher in those diagnosed at an earlier stage, diagnosed at a younger age, and in women compared to men. | |||
| ==Signs and symptoms== | |||
| Symptoms that suggest lung cancer include: | |||
| * ] (shortness of breath) | |||
| * ] (coughing up blood) | |||
| * chronic ] or change in regular coughing pattern | |||
| * wheezing | |||
| * ] or pain in the abdomen | |||
| * ] (weight loss), ] and loss of ] | |||
| * ] (hoarse voice) | |||
| * ] of the fingernails (uncommon) | |||
| * ] | |||
| * rapid drop in sodium | |||
| Most lung cancer cases are caused by ]. The remainder are caused by exposure to hazardous substances like ] and ] gas, or by ]s that arise by chance. Consequently, lung cancer prevention efforts encourage people to avoid hazardous chemicals and quit smoking. Quitting smoking both reduces one's chance of developing lung cancer and improves treatment outcomes in those already diagnosed with lung cancer. | |||
| If the cancer grows into the lumen it may obstruct the ], causing ]. This can lead to accumulation of secretions behind the blockage, predisposing the patient to ]. | |||
| Lung cancer is the most diagnosed and deadliest cancer worldwide, with 2.2 million cases in 2020 resulting in 1.8 million deaths.{{sfn|Sung|Ferlay|Siegel|Laversanne|2021|loc="Lung cancer"}} Lung cancer is rare in those younger than 40; the average age at diagnosis is 70 years, and the average age at death 72.{{sfn|Bade|Dela Cruz|2020|loc="Age"}} Incidence and outcomes vary widely across the world, depending on patterns of tobacco use. Prior to the advent of cigarette smoking in the 20th century, lung cancer was a rare disease. In the 1950s and 1960s, increasing evidence linked lung cancer and tobacco use, culminating in declarations by most large national health bodies discouraging tobacco use. | |||
| Many lung cancers have a rich blood supply. The surface of the cancer may be fragile, leading to bleeding from the cancer into the airway. This blood may subsequently be coughed up. | |||
| {{TOC limit}} | |||
| ==Signs and symptoms== | |||
| Depending on the type of tumor, so-called ] may initially attract attention to the disease. In lung cancer, this may be ] (muscle weakness due to ]), ] and ]. Tumors in the top (apex) of the lung, known as ]s, may invade the local part of the ], leading to changed sweating patterns and eye muscle problems (a combination known as ]), as well as muscle weakness in the hands due to invasion of the ]. | |||
| Early lung cancer often has no symptoms. When symptoms do arise they are often ] respiratory problems – ]ing, ], or ] – that can differ from person to person.{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Presentation/Initial Evaluation"}} Those who experience coughing tend to report either a new cough, or an increase in the frequency or strength of a pre-existing cough.{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Presentation/Initial Evaluation"}} Around one in four ], ranging from small streaks in the ] to large amounts.{{sfn|Nasim|Sabath|Eapen|2019|loc="Clinical Manifestations"}}{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Presentation/Initial Evaluation"}} Around half of those diagnosed with lung cancer experience shortness of breath, while 25–50% experience a dull, persistent chest pain that remains in the same location over time.{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Presentation/Initial Evaluation"}} In addition to respiratory symptoms, some experience ] including ], ], general weakness, ], and ].{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Presentation/Initial Evaluation"}}{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} | |||
| Some less common symptoms suggest tumors in particular locations. Tumors in the ] can cause breathing problems by obstructing the ] or disrupting the nerve to the ]; ] by compressing the ]; ] by disrupting the ]s of the ]; and ] by disrupting the ].{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Presentation/Initial Evaluation"}}{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} Horner's syndrome is also common in tumors at the ], known as ]s, which also cause ] that radiates down the little-finger side of the arm as well as destruction of the topmost ]s.{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} ] ]s above the ] can indicate a tumor that has spread within the chest.{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Presentation/Initial Evaluation"}} Tumors obstructing bloodflow to the heart can cause ] (swelling of the upper body and shortness of breath), while tumors infiltrating the area around the heart can cause ], ] (irregular heartbeat), and ].{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} | |||
| In many patients, the cancer has already spread beyond the original site by the time they have symptoms and seek medical attention. Common sites of ] include the ], such as the ] (causing back pain and occasionally ]), the liver and the ]. | |||
| About one in three people diagnosed with lung cancer have symptoms caused by ] in sites other than the lungs.{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} Lung cancer can metastasize anywhere in the body, with different symptoms depending on the location. Brain metastases can cause ], ], ], ]s, and ]s. Bone metastases can cause pain, ]s, and compression of the ]. Metastasis into the ] can ] and cause ] (immature cells in the blood).{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} Liver metastases can cause ], pain in the ], fever, and weight loss.{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} | |||
| ==Diagnosis== | |||
| Performing a ] is the first step if a patient reports symptoms that may be suggestive of lung cancer. This may reveal an obvious mass, widening of the ] (suggestive of spread to ]s there), ] (collapse), consolidation (]) and ]. If there are no X-ray findings but the suspicion is high (e.g. a heavy smoker with blood-stained sputum), ] and/or a ] may provide the necessary information. In any case, bronchoscopy or CT-guided ] is often necessary to identify the tumor type. | |||
| Lung tumors often cause the release of body-altering ]s, which cause unusual symptoms, called ]s.{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} Inappropriate hormone release can cause dramatic shifts in concentrations of blood ]. Most common is ] (high blood calcium) caused by over-production of ] or ]. Hypercalcemia can manifest as nausea, vomiting, abdominal pain, constipation, ], ], and altered mental status.{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} Those with lung cancer also commonly experience ] (low potassium) due to inappropriate secretion of ], as well as ] (low sodium) due to overproduction of ] or ].{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} About one of three people with lung cancer develop ], while up to one in ten experience ] (nail clubbing, joint soreness, and skin thickening). A variety of ]s can arise as paraneoplastic syndromes in those with lung cancer, including ] (which causes muscle weakness), ], ], ], and autoimmune deterioration of ], ], or ].{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} Up to one in twelve people with lung cancer have paraneoplastic blood clotting, including ], clots in the heart, and ] (clots throughout the body).{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} Paraneoplastic syndromes involving the skin and kidneys are rare, each occurring in up to 1% of those with lung cancer.{{sfn|Horn|Iams|2022|loc="Clinical Manifestations"}} | |||
| If investigations have confirmed lung cancer, scan results and often ] (PET) are used to determine whether the disease is localised and amenable to surgery or whether it has spread to the point it cannot be cured surgically. PET is not useful as screening, as not all malignancies are positive on PET scan (such as ]), and lung infections may be positive on PET Scan. | |||
| ==Diagnosis== | |||
| ]s and ] (lung function testing) are also necessary to assess whether the patient is well enough to be operated on. If spirometry reveals a very poor respiratory reserve, as may occur in chronic smokers, surgery may be contraindicated. | |||
| ] showing a cancerous tumor in the left lung]] | |||
| A person suspected of having lung cancer will have imaging tests done to evaluate the presence, extent, and location of tumors. First, many ]s perform a ] to look for a mass inside the lung.<ref name=NHS>{{cite web|url=https://www.nhs.uk/conditions/lung-cancer/diagnosis/ |accessdate=30 November 2022 |title=Diagnosis – Lung Cancer |publisher= ] |date=1 November 2022}}</ref> The X-ray may reveal an obvious mass, the widening of the ] (suggestive of spread to ]s there), ] (lung collapse), consolidation (]), or ];<ref>{{cite web | title=Lung Carcinoma: Tumors of the Lungs | publisher=Merck Manual Professional|edition= online|url=http://www.merck.com/mmpe/sec05/ch062/ch062b.html#sec05-ch062-ch062b-1405 | access-date=21 July 2021 |date=July 2020 }}</ref> however, some lung tumors are not visible by X-ray.{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Presentation/Initial Evaluation"}} Next, many undergo ], which can reveal the sizes and locations of tumors.<ref name=NHS/>{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Noninvasive Staging"}} | |||
| ==Types== | |||
| There are two main types of lung cancer categorized by the size and appearance of the malignant cells seen by a ] under a ]: ''non-small cell'' (80%) and ''small-cell'' (roughly 20%) lung cancer. This classification although based on simple pathomorphological criteria has very important implications for clinical management and prognosis of the disease. | |||
| A definitive diagnosis of lung cancer requires a ] of the suspected tissue be ] examined for cancer cells.{{sfn|Horn|Iams|2022|loc="Diagnosing Lung Cancer"}} Given the location of lung cancer tumors, biopsies can often be obtained by minimally invasive techniques: a fiberoptic ] that can retrieve tissue (sometimes guided by ]), ], or other imaging-guided biopsy through the skin.{{sfn|Horn|Iams|2022|loc="Diagnosing Lung Cancer"}} Those who cannot undergo a typical biopsy procedure may instead have a ] taken (that is, a sample of some body fluid) which may contain ] that can be detected.{{sfn|Alexander|Kim|Cheng|2020|loc="Liquid Biopsy"}} | |||
| ===Non-small cell lung cancer=== | |||
| ]]] | |||
| For non-small cell lung cancer (NSCLC), prognosis is poor. For advanced NSCLC, average survival is 6 months for untreated patients, and 9 months for patients treated with chemotherapy. The 5-year survival rate of patients with NSCLC is 60 to 70% for patients with stage I disease and zero for patients with stage IV disease.<ref name="Merck">{{cite web | title=Lung Carcinoma: Tumors of the Lungs: Merck Manual Professional Edition, Online edition | url=http://www.merck.com/mmpe/sec05/ch062/ch062b.html#sec05-ch062-ch062b-1405 | |||
| Imaging is also used to assess the extent of cancer spread. ] (PET) scanning or combined ] scanning is often used to locate metastases in the body. Since PET scanning is less sensitive in the brain, the ] recommends ] (MRI) – or CT where MRI is unavailable – to scan the brain for metastases in those with NSCLC and large tumors, or tumors that have spread to the nearby lymph nodes.{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Suspected Metastatic Disease"}} When imaging suggests the tumor has spread, the suspected metastasis is often biopsied to confirm that it is cancerous.{{sfn|Horn|Iams|2022|loc="Diagnosing Lung Cancer"}} Lung cancer most commonly metastasizes to the brain, bones, liver, and ]s.{{sfn|Morgensztern|Boffa|Chen|Dhanasopon|2023|loc="Clinical manifestations"}} | |||
| |accessdate=March 19 | accessyear=2007}}</ref> | |||
| Lung cancer can often appear as a ] on a chest radiograph or CT scan. In lung cancer screening studies as many as 30% of those screened have a lung nodule, the majority of which turn out to be benign.{{sfn|Tanoue|Mazzone|Tanner|2022|loc="Evidence for Lung Cancer Screening"}} Besides lung cancer many other diseases can also give this appearance, including ]s, and infectious ]s caused by ], ], or ].{{sfn|Salahuddin|Ost|2023|loc="Table 110-1: Differential Diagnosis of Solitary Pulmonary Nodules"}} | |||
| The NSCLCs are grouped together because their prognosis and management is roughly identical. When it cannot be subtyped, it is frequently coded to 8046/3. The subtypes are: | |||
| * '']'', accounting for 29% of lung cancers,<ref name="Harrison">{{cite book | last = | first = | authorlink = | coauthors =John D Minna | title =Harrison's Principle's of Internal Medicine | publisher = | date =2005 | location = | pages =506 | url = | doi =10.1036/0071402357 | id = }}</ref> also starts in the larger bronchi but grows slower. This means that the size of these tumours varies on diagnosis. | |||
| * '']'' is the most common subtype of NSCLC, accounting for 32% of lung cancers.<ref name="Harrison"/> It is a form which starts near the gas-exchanging surface of the lung. Most cases of the adenocarcinoma are associated with smoking. However, among non-smokers and in particular female non-smokers, adenocarcinoma is the most common form of lung cancer. A subtype of adenocarcinoma, the ''bronchioalveolar carcinoma'', is more common in female non-smokers and may have different responses to treatment. | |||
| * '']'' is a fast-growing form, accounting for 9% of lung cancers,<ref name="Harrison"/> that grows near the surface of the lung. | |||
| === |
===Classification=== | ||
| ]ed samples from lung biopsies: (Top-left) Normal bronchiole surrounded by alveoli, (top-right) adenocarcinoma with papillary (finger-like) growth, (bottom-left) alveoli filled with mucin suggesting adenocarcinoma nearby, (bottom-right) squamous-cell carcinoma, with alveoli full of keratin.]] | |||
| ] | |||
| ] | |||
| At diagnosis, lung cancer is classified based on the type of cells the tumor is derived from; tumors derived from different cells progress and respond to treatment differently. There are two main types of lung cancer, categorized by the size and appearance of the malignant cells seen by a ] under a ]: ] (SCLC; 15% of cases) and ] (NSCLC; 85% of cases).{{sfn|Thai|Solomon|Sequist|Gainor|2021|loc="Histology"}} SCLC tumors are often found near the center of the lungs, in the major airways.{{sfn|Rudin|Brambilla|Faivre-Finn|Sage|2021|loc="Signs and Symptoms"}} Their cells appear small with ill-defined boundaries, not much ], many ], and have distinctive ] with granular-looking ] and no visible ].{{sfn|Horn|Iams|2022|loc="Pathology"}} NSCLCs comprise a group of three cancer types: ], ], and ].{{sfn|Horn|Iams|2022|loc="Pathology"}} Nearly 40% of lung cancers are adenocarcinomas.{{sfn|Morgensztern|Boffa|Chen|Dhanasopon|2023|loc="Precursor lesions"}} Their cells grow in three-dimensional clumps, resemble glandular cells, and may produce ].{{sfn|Horn|Iams|2022|loc="Pathology"}} About 30% of lung cancers are squamous-cell carcinomas. They typically occur close to large airways.{{sfn|Morgensztern|Boffa|Chen|Dhanasopon|2023|loc="Precursor lesions"}} The tumors consist of sheets of cells, with ].{{sfn|Horn|Iams|2022|loc="Pathology"}} A hollow cavity and associated ] are commonly found at the center of the tumor.{{sfn|Morgensztern|Boffa|Chen|Dhanasopon|2023|loc="Precursor lesions"}} Less than 10% of lung cancers are large-cell carcinomas,{{sfn|Horn|Iams|2022|loc="Pathology"}} so named because the cells are large, with excess cytoplasm, large nuclei, and conspicuous ].{{sfn|Morgensztern|Boffa|Chen|Dhanasopon|2023|loc="Precursor lesions"}} Around 10% of lung cancers are rarer types.{{sfn|Horn|Iams|2022|loc="Pathology"}} These include mixes of the above subtypes like ], and rare subtypes such as ], and ].{{sfn|Morgensztern|Boffa|Chen|Dhanasopon|2023|loc="Precursor lesions"}} | |||
| Several lung cancer types are subclassified based on the growth characteristics of the cancer cells. Adenocarcinomas are classified as lepidic (growing along the surface of intact ] walls),{{sfn|Jones|2013|loc="Conclusion"}} ] and ], or micropapillary and solid pattern. Lepidic adenocarcinomas tend to be least aggressive, while micropapillary and solid pattern adenocarcinomas are most aggressive.{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Histology and Prognosis"}} | |||
| For '']'' (SCLC), prognosis is also poor. The overall five-year survival for patients with SCLC is about 5%.<ref name="Harrison"/> Patients with extensive-stage SCLC have an average five-year survival rate of less than 1%. The ] survival time for limited-stage disease is 20 months, with a five-year survival rate of 20%.<ref name="Merck"/> | |||
| In addition to examining cell morphology, biopsies are often stained by ] to confirm lung cancer classification. SCLCs bear the markers of ]s, such as ], ], and ].{{sfn|Rudin|Brambilla|Faivre-Finn|Sage|2021|loc="Immunohistochemistry"}} Adenocarcinomas tend to express {{nowrap|]}} and {{nowrap|]}}; squamous cell carcinomas lack {{nowrap|Napsin-A}} and {{nowrap|TTF-1}}, but express ] and its cancer-specific isoform p40.{{sfn|Horn|Iams|2022|loc="Pathology"}} ] and ] are also commonly used to differentiate lung cancers. CK20 is found in several cancers, but typically absent from lung cancer. CK7 is present in many lung cancers, but absent from squamous cell carcinomas.{{sfn|Horn|Iams|2022|loc="Immunohistochemistry"}} | |||
| * '']'' (also called "]") is the less common form of lung cancer. It tends to start in the larger breathing tubes and grows rapidly becoming quite large. The ] most commonly involved is '']''. The "oat" cell contains dense neurosecretory granules which give this an endocrine/paraneoplastic syndrome association. It is initially more sensitive to chemotherapy, but ultimately carries a worse prognosis and is often metastatic at presentation. This type of lung cancer is strongly associated with smoking. | |||
| === |
===Staging=== | ||
| {{see also|Lung cancer staging}} | |||
| * ] | |||
| {| class="wikitable floatright" style="text-align:right;font-size:90%;margin-left:1em;background:#E5AFAA;" | |||
| * ] | |||
| |+ Stage group according to TNM classification in lung cancer{{sfn|Lim|Ridge|Nicholson|Mirsadraee|2018|loc="Table 5: Overall stage based on T, N, and M descriptors"}} | |||
| * ] | |||
| |- | |||
| * ] | |||
| ! TNM | |||
| ! Stage group | |||
| |- | |||
| | T1a N0 M0 | |||
| | IA1 | |||
| |- | |||
| | T1b N0 M0 | |||
| | IA2 | |||
| |- | |||
| | T1c N0 M0 | |||
| | IA3 | |||
| |- | |||
| | T2a N0 M0 | |||
| | IB | |||
| |- | |||
| | T2b N0 M0 | |||
| | IIA | |||
| |- | |||
| | T1–T2 N1 M0 | |||
| | rowspan="2" | IIB | |||
| |- | |||
| | T3 N0 M0 | |||
| |- | |||
| | T1–T2 N2 M0 | |||
| | rowspan="3" | IIIA | |||
| |- | |||
| | T3 N1 M0 | |||
| |- | |||
| | T4 N0–N1 M0 | |||
| |- | |||
| | T1–T2 N3 M0 | |||
| | rowspan="2" | IIIB | |||
| |- | |||
| | T3–T4 N2 M0 | |||
| |- | |||
| | T3–T4 N3 M0 | |||
| | IIIC | |||
| |- | |||
| | Any T, any N, M1a–M1b | |||
| | IVA | |||
| |- | |||
| | Any T, any N, M1c | |||
| | IVB | |||
| |} | |||
| Lung ] is an assessment of the degree of spread of the cancer from its original source. It is one of the factors affecting both the ] and the treatment of lung cancer.<ref name=ACS-SCLC-Stage>{{cite web|url=https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/staging-sclc.html |accessdate=2 December 2022 |title=Small Cell Lung Cancer Stages |publisher= ] |date=1 October 2019}}</ref> | |||
| ===Metastatic=== | |||
| The lung is a common place for ] from tumors in other parts of the body. These cancers, however, are identified by the site of origin, i.e., a breast cancer metastasis to the lung is still known as breast cancer. The adrenal glands, liver, brain, and bone are the most common sites of metastasis from primary lung cancer itself. | |||
| SCLC is typically staged with a relatively simple system: limited stage or extensive stage. Around a third of people are diagnosed at the limited stage, meaning cancer is confined to one side of the chest, within the scope of a single ] field.<ref name=ACS-SCLC-Stage/> The other two thirds are diagnosed at the "extensive stage", with cancer spread to both sides of the chest, or to other parts of the body.<ref name=ACS-SCLC-Stage/> | |||
| ==Causes== | |||
| Exposure to ]s, such as those present in air pollution and ], immediately causes cumulative changes to the tissue lining the ] of the lungs (the ''bronchial mucous membrane'') and more tissue gets damaged until a tumour develops. | |||
| NSCLC – and sometimes SCLC – is typically staged with the ]'s ].<ref name=ACS-NSCLC-Stage>{{cite web|url=https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/staging-nsclc.html |accessdate=2 December 2022 |title=Non-small Cell Lung Cancer Stages |publisher= ] |date=1 October 2019}}</ref> The size and extent of the tumor (T), spread to regional lymph nodes (N), and distant metastases (M) are scored individually, and combined to form stage groups.{{sfn|Horn|Iams|2022|loc="Staging System for Non-Small-Cell Lung Cancer"}} | |||
| There are four major causes of lung cancer (and cancer in general): | |||
| * ]s such as those in ] smoke | |||
| * ] exposure | |||
| * ] susceptibility | |||
| * ] infection | |||
| Relatively small tumors are designated T1, which are subdivided by size: tumors ≤ 1 ] (cm) across are T1a; 1–2 cm T1b; 2–3 cm T1c. Tumors up to 5 cm across, or those that have spread to the ] (tissue covering the lung) or ], are designated T2. T2a designates 3–4 cm tumors; T2b 4–5 cm tumors. T3 tumors are up to 7 cm across, have multiple nodules in the same ] of the lung, or invade the ], diaphragm (or the ]), or area around the heart.{{sfn|Horn|Iams|2022|loc="Staging System for Non-Small-Cell Lung Cancer"}}{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Eight Edition Lung Cancer Stage Classification"}} Tumors that are larger than 7 cm, have nodules spread in different lobes of a lung, or invade the ] (center of the chest cavity), heart, ] that supply the heart, ], ], or ] are designated T4.{{sfn|Horn|Iams|2022|loc="Staging System for Non-Small-Cell Lung Cancer"}}{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Eight Edition Lung Cancer Stage Classification"}} ] staging depends on the extent of local spread: with the cancer metastasized to no lymph nodes (N0), pulmonary or ] (along the bronchi) on the same side as the tumor (N1), ] or subcarinal lymph nodes (in the middle of the lungs, N2), or lymph nodes on the opposite side of the lung from the tumor (N3).{{sfn|Pastis|Gonzalez|Silvestri|2022|loc="Eight Edition Lung Cancer Stage Classification"}} Metastases are staged as no metastases (M0), nearby metastases (M1a; the space around the lung or the heart, or the opposite lung), a single distant metastasis (M1b), or multiple metastases (M1c).{{sfn|Horn|Iams|2022|loc="Staging System for Non-Small-Cell Lung Cancer"}} | |||
| ===The role of smoking=== | |||
| These T, N, and M scores are combined to designate a stage grouping for the cancer. Cancer limited to smaller tumors is designated stage I. Disease with larger tumors or spread to the nearest lymph nodes is stage II. Cancer with the largest tumors or extensive lymph node spread is stage III. Cancer that has metastasized is stage IV. Each stage is further subdivided based on the combination of T, N, and M scores.{{sfn|Horn|Iams|2022|loc="Table 78–6 TNM Stage Groupings, Eighth Edition"}} | |||
| ] | |||
| {| class="wikitable" style="text-align:center;font-size:90%;margin-left:1em;background:#E5AFAA;" | |||
| ], particularly of ]s, is by far the main contributor to lung cancer, which at least in theory makes it one of the easiest diseases to prevent. In the United States, smoking is estimated to account for 87% of lung cancer cases (90% in men and 79% in women), and in the ] for 90%. Cigarette smoke contains 19 known carcinogens<ref>{{cite web|author=Dr. ]|title=Smoking and smokeless tobacco|url=http://www.drkoop.com/ency/93/002032.html|accessdate=July 15|accessyear=2006}}</ref> including ] from the ] decay sequence, ], and ]. Additionally, nicotine appears to depress the immune response to malignant growths in exposed tissue. The length of time a person continues to smoke as well as the amount smoked increases the person's chances of developing lung cancer. If a person stops smoking, these chances steadily decrease as damage to the lungs is repaired and contaminant particles are gradually vacated. More recent work has shown that, across the developed world, almost 90% of lung cancer deaths are caused by smoking.<ref>{{cite book | |||
| |+ TNM classification in lung cancer<ref>{{cite web | title=Lung Cancer TNM staging summary|edition=8th | publisher=International Association for the Study of Lung Cancer | url=https://www.iaslc.org/sites/default/files/wysiwyg-assets/iaslc_8th_posters_24x36_2018_final_version_1.pdf | access-date=30 May 2018 | archive-url=https://web.archive.org/web/20180617220133/https://www.iaslc.org/sites/default/files/wysiwyg-assets/iaslc_8th_posters_24x36_2018_final_version_1.pdf | archive-date=17 June 2018 | url-status=dead }}</ref> | |||
| | last = Peto R | first = R | authorlink = | coauthors = Lopez AD, Boreham J, Thun M, Heath Jr C. | title = Mortality from smoking in developed countries 1950–2000: Indirect estimates from National Vital Statistics. | publisher = Oxford University Press | date = 1994 | location = Oxford | url = | doi = | id = ISBN 0-19-262535-7 }}</ref> In addition, there is evidence that lung cancer in never-smokers has a better prognosis than in smokers,<ref>Nordquist LT, Simon GR, Cantor A, Alberts WM, Bepler G. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest. 2004 Aug;126(2):347-51. PMID 15302716</ref> and that patients who smoke at the time of diagnosis have shorter survival than those who have quit.<ref>Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest. 2004 Jan;125(1):27-37. PMID 14718417</ref> | |||
| ]—the inhalation of smoke from another's smoking— is claimed to be a cause of lung cancer in non-smokers. Studies from the USA (1986,<ref>US Department of Health and Human Services., The health consequences of involuntary smoking: report of the Surgeon General (DHHS Pub No (PHS) 87–8398), DHHS, Washington, DC (1986). PMID 3097495</ref><ref>National Research Council., Environmental tobacco smoke: measuring exposures and assessing health effects, NRC, Washington, DC (1986).</ref> 1992,<ref>US Environmental Protection Agency., Respiratory health effects of passive smoking: lung cancer and other disorders, EPA, Washington, DC (1992).</ref> 1997,<ref>California Environmental Protection Agency., Health effects of exposure to environmental tobacco smoke, California EPA, Sacramento (1997). PMID 9583639.</ref> 2001,<ref>Centers for Disease Control and Prevention (CDC). State-specific prevalence of current cigarette smoking among adults, and policies and attitudes about secondhand smoke--United States, 2000. MMWR Morb Mortal Wkly Rep. 2001 Dec 14;50(49):1101-6. PMID 11794619</ref> 2003<ref>Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003 Jan;123(1 Suppl):21S-49S. PMID 12527563 </ref>), Europe (1998<ref>In: P Boffetta, A Agudo and W Ahrens et al., Editors, Multicenter case-control study of exposure to environmental tobacco smoke and lung cancer in Europe, J Natl Cancer Inst 90 (1998), pp. 1440–1450.</ref>), the UK (1998,<ref>Scientific Committee on Tobacco and Health., Report of the Scientific Committee on Tobacco and Health, Department of Health, London (1998) </ref><ref>Hackshaw AK. Lung cancer and passive smoking. Stat Methods Med Res. 1998 Jun;7(2):119-36. PMID 9654638</ref>), and Australia (1997<ref>National Health and Medical Research Council., The health effects of passive smoking, Australian Government Publishing Service, Canberra (1997).</ref>) have consistently shown a significant increase in ] among those exposed to passive smoke. | |||
| The ] in 1993 claimed that about 3,000 lung cancer-related deaths a year were caused by passive smoking. However, since this report was based on a study that was alleged to be heavily biased and was ruled by a federal judge to be "unscientific", the EPA report was declared null and void by a federal judge in 1998(,<ref></ref><ref></ref>). | |||
| {| border="1" class="wikitable" | |||
| |+ Percentage of lung cancer deaths attributable to smoking in the developed world | |||
| ! !! 35-69 years !! 70 years+ !! All ages | |||
| |- | |- | ||
| | | |||
| ! Men | |||
| {| class="wikitable" | |||
| | 93.9% || 90.3% || 92.5% | |||
| |- | |- | ||
| ! colspan="3" | T: Primary tumor | |||
| ! Women | |||
| | 68.8% || 68.9% || 68.8% | |||
| |- | |- | ||
| | T0 | |||
| ! Both | |||
| | colspan="2" | No primary tumor | |||
| | 88.7% || 84.3% || 86.6% | |||
| |- | |||
| | Tis | |||
| | colspan="2" | ] | |||
| |- | |||
| | T1 | |||
| | colspan="2" | Tumor ≤ 3 cm across, surrounded by lung or visceral pleura | |||
| |- | |||
| | rowspan="4" | | |||
| | T1mi | |||
| | Minimally invasive adenocarcinoma | |||
| |- | |||
| | T1a | |||
| | Tumor ≤ 1 cm across | |||
| |- | |||
| | T1b | |||
| | Tumor > 1 cm but ≤ 2 cm across | |||
| |- | |||
| | T1c | |||
| | Tumor > 2 cm but ≤ 3 cm across | |||
| |- | |||
| | rowspan="4" | T2 | |||
| | rowspan="4" | Any of: | |||
| | Tumor size > 3 cm but ≤ 5 cm across | |||
| |- | |||
| | Involvement of the main bronchus but not the carina | |||
| |- | |||
| | Invasion of visceral pleura | |||
| |- | |||
| | Atelectasis/] extending to the ] | |||
| |- | |||
| | rowspan="2" | | |||
| | T2a | |||
| | Tumor > 3 cm but ≤ 4 cm across | |||
| |- | |||
| | T2b | |||
| | Tumor > 4 cm but ≤ 5 cm across | |||
| |- | |||
| | rowspan="3" | T3 | |||
| | rowspan="3" | Any of: | |||
| | Tumor size > 5 cm but ≤ 7 cm across | |||
| |- | |||
| | Invasion into the chest wall, ], or parietal ] | |||
| |- | |||
| | Separate tumor nodule in the same lobe | |||
| |- | |||
| | rowspan="3" | T4 | |||
| | rowspan="3" | Any of: | |||
| | Tumor size > 7 cm | |||
| |- | |||
| | Invasion of the diaphragm, mediastinum, heart, ], ], ], ], ], or ] | |||
| |- | |||
| | Separate tumor nodule in a different lobe of the same lung | |||
| |} | |||
| | style="vertical-align:top;" | | |||
| {| class="wikitable" | |||
| |- | |||
| ! colspan="3" | N: Lymph nodes | |||
| |- | |||
| | N0 | |||
| | colspan="2" | No lymph node metastasis | |||
| |- | |||
| | N1 | |||
| | colspan="2" | Metastasis to ] peribronchial or hilar lymph nodes | |||
| |- | |||
| | N2 | |||
| | colspan="2" | Metastasis to ipsilateral mediastinal or subcarinal lymph nodes | |||
| |- | |||
| | rowspan="2" | N3 | |||
| | rowspan="2" | Any of: | |||
| | Metastasis to scalene or supraclavicular lymph nodes | |||
| |- | |||
| | Metastasis to contralateral hilar or mediastinal lymph nodes | |||
| |} | |||
| | style="vertical-align:top;" | | |||
| {| class="wikitable" | |||
| |- | |||
| ! colspan="3" | M: Metastasis | |||
| |- | |||
| | M0 | |||
| | colspan="2" | No distant metastasis | |||
| |- | |||
| | rowspan="3" | M1a | |||
| | rowspan="3" | Any of: | |||
| | Separate tumor nodule in the other lung | |||
| |- | |||
| | Tumor with pleural or pericardial nodules | |||
| |- | |||
| | Malignant ] or ] | |||
| |- | |||
| | M1b | |||
| | colspan="2" | A single metastasis outside the chest | |||
| |- | |||
| | M1c | |||
| | colspan="2" | Two or more metastases outside the chest | |||
| |} | |||
| |} | |} | ||
| ===Screening=== | |||
| The extensive attempts made by ] to delay the release of the 1997 IARC study, to affect the wording of its conclusions, to neutralise its negative results for their business, and to counteract its impact on public and policymakers' opinion have been documented by Ong & Glantz in '']'' journal.<ref>{{cite journal|last=Ong|first=E.K.|coauthors=S.A. Glantz|title=Tobacco industry efforts subverting International Agency for Research on Cancer's second-hand smoke study|journal=Lancet|publisher=2000|issue=355(9211)|pages=1253-9|id=PMID 10770318.}}</ref> Their work was based on 32 million pages of documents made public as part of the settlement of the 1998 legal case of State of Minnesota and Blue Cross/Blue Shield of Minnesota vs Philip Morris Inc, et al. and available at Philip Morris' own website.<ref></ref> | |||
| {{main|Lung cancer screening}} | |||
| Some countries recommend that people who are at a high risk of developing lung cancer be screened at different intervals using low-dose CT lung scans. Screening programs may result in early detection of lung tumors in people who are not yet experiencing symptoms of lung cancer, ideally, early enough that the tumors can be successfully treated and result in decreased mortality.<ref name=Jonas2021>{{Cite web|url=https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/detection.html |accessdate=30 April 2023 |title=Can Lung Cancer Be Found Early? |publisher=American Cancer Society |date=18 January 2023}}</ref> There is evidence that regular low-dose CT scans in people at high risk of developing lung cancer reduces total lung cancer deaths by as much as 20%.{{sfn|Tanoue|Mazzone|Tanner|2022|loc="Evidence for Lung Cancer Screening"}} Despite evidence of benefit in these populations, potential harms of screening include the potential for a person to have a 'false positive' screening result that may lead to unnecessary testing, invasive procedures, and distress.{{sfn|Jonas|Reuland|Reddy|Nagle|2021|loc=Abstract – "Conclusions and Relevance"}} Although rare, there is also a risk of ].{{sfn|Jonas|Reuland|Reddy|Nagle|2021|loc=Abstract – "Conclusions and Relevance"}} The ] recommends yearly screening using low-dose CT in people between 55 and 80 who have a smoking history of at least 30 ]s.{{sfn|Alexander|Kim|Cheng|2020|loc="Lung Cancer Screening"}} The ] recommends that cancer screening programs across the ] be extended to include low-dose CT lung scans for current or previous smokers.{{sfn|Cancer screening in the European Union|2022|p= 27}} Similarly, The Canadian Task Force for Preventative Health recommends that people who are current or former smokers (smoking history of more than 30 pack years) and who are between the ages of 55–74 years be screened for lung cancer.{{sfn|Canadian Task Force|2016|loc= "Recommendations" }} | |||
| Recent investigation of ] suggests it is more dangerous than direct smoke inhalation.<ref>Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob Control. 2005 Dec;14(6):396-404. PMID 16319363</ref> | |||
| == |
==Treatment== | ||
| {{main|Treatment of lung cancer}} | |||
| ] can cause a variety of lung diseases. It increases the risk of developing lung cancer. There is a ] effect between tobacco smoking and asbestos in the formation of lung cancer.<ref>{{cite journal|last=Hammond|first=E.C.|coauthors=I.J. Selikoff|title=Asbestos exposure, cigarette smoking and death rates|journal=Ann N Y Acad Sci|publisher=1979|issue=330|pages=473|id=PMID 294198.}}</ref> | |||
| Treatment for lung cancer depends on the cancer's specific cell type, how far it has ], and the person's health. Common treatments for early stage cancer includes ] of the tumor, ], and ]. For later-stage cancer, chemotherapy and radiation therapy are combined with newer ] and ]s.{{sfn|Rivera|Mody|Weiner|2022|loc="Introduction"}} All lung cancer treatment regimens are combined with lifestyle changes and ] to improve quality of life.{{sfn|Rivera|Mody|Weiner|2022|loc="Palliative Care"}} | |||
| ===Small-cell lung cancer=== | |||
| Asbestos can also cause cancer of the ], called ] (which is distinct from lung cancer). | |||
| ] | |||
| Limited-stage SCLC is typically treated with a combination of chemotherapy and radiotherapy.{{sfn|Horn|Iams|2022|loc="Treatment – Small-Cell Lung Cancer"}} For chemotherapy, the ] and ] guidelines recommend four to six cycles of a ] – ] or ] – combined with either ] or ].{{sfn|Rivera|Mody|Weiner|2022|loc="Treatment of Small Cell Lung Cancer"}} This is typically combined with thoracic radiation therapy – 45 ] (Gy) twice-daily – alongside the first two chemotherapy cycles.{{sfn|Horn|Iams|2022|loc="Treatment – Small-Cell Lung Cancer"}} First-line therapy causes remission in up to 80% of those who receive it; however most people relapse with chemotherapy-resistant disease. Those who relapse are given second-line chemotherapies. ] and ] are approved by the US ] for this purpose.{{sfn|Horn|Iams|2022|loc="Treatment – Small-Cell Lung Cancer"}} Irinotecan, ], ], ], etoposide, and ] are also sometimes used, and are similarly efficacious.{{sfn|Horn|Iams|2022|loc="Treatment – Small-Cell Lung Cancer"}} ] can reduce the risk of brain metastases and improve survival in those with limited-stage disease.{{sfn|Rudin|Brambilla|Faivre-Finn|Sage|2021|loc="Locally advanced SCLC"}}{{sfn|Horn|Iams|2022|loc="Treatment – Small-Cell Lung Cancer"}} | |||
| Extensive-stage SCLC is treated first with etoposide along with either cisplatin or carboplatin. Radiotherapy is used only to shrink tumors that are causing particularly severe symptoms. Combining standard chemotherapy with an ] can improve survival for a minority of those affected, extending the average person's lifespan by around 2 months.{{sfn|Rudin|Brambilla|Faivre-Finn|Sage|2021|loc="Metastatic Disease"}} | |||
| ===Radon gas=== | |||
| ] is a colorless and odourless ] generated by the breakdown of radioactive ], which in turn is the decay product of ], found in the earth's ]. Radon exposure is the second major cause of lung cancer after smoking. The radiation decay products ]ize genetic material, causing mutations that sometimes turn cancerous. Radon gas levels vary by locality and the composition of the underlying ] and ]s. For example, in areas such as ] in the UK (which has ] as substrata), radon gas is a major problem, and buildings have to be force-ventilated with fans to lower radon gas concentrations. In the US, the EPA estimates that one in 15 homes has radon levels above the recommended guideline of 4 pCi/L (150 Bq/m<sup>3</sup>). Iowa has the highest average radon concentrations in the United States. Studies performed by ], ], ], Brian J. Smith and colleagues at the ] have demonstrated a 50% increased lung cancer risk with prolonged radon exposure at the EPA's action level of 4 pCi/L () . Recent pooled epidemiologic radon studies by ] et al. (2005; 2006) and ] et al. (2005) have also shown an increased lung cancer risk from radon below the U.S. EPA's action level of 4 pCi/L. | |||
| ===Non-small-cell lung cancer=== | |||
| Radon causes lung cancer because it causes arbitrary damage to the ]s and ] molecules contained in the ] of the ]. | |||
| ] | |||
| <!--Add something about wait-and-see for certain small nodules. A bit in Harrison's-->For stage I and stage II NSCLC the first line of treatment is often surgical removal of the affected lobe of the lung.{{sfn|Horn|Iams|2022|loc="Management of Stages I and II NSCLC"}} For those not well enough to tolerate full lobe removal<!--expand on this?-->, a smaller chunk of lung tissue can be removed by ] or ] surgery.{{sfn|Horn|Iams|2022|loc="Management of Stages I and II NSCLC"}} Those with centrally located tumors and otherwise-healthy respiratory systems may have more extreme surgery to remove an entire lung (]).{{sfn|Horn|Iams|2022|loc="Management of Stages I and II NSCLC"}} Experienced ]s, and a high-volume surgery clinic improve chances of survival.{{sfn|Horn|Iams|2022|loc="Management of Stages I and II NSCLC"}} Those who are unable or unwilling to undergo surgery can instead receive radiation therapy. <!--Would be nice to have a clinical recommendation statement here-->] is best practice, typically administered several times over 1–2 weeks.{{sfn|Horn|Iams|2022|loc="Management of Stages I and II NSCLC"}} Chemotherapy has little effect in those with stage I NSCLC, and may worsen disease outcomes in those with the earliest disease. In those with stage II disease, chemotherapy is usually initiated six to twelve weeks after surgery, with up to four cycles of cisplatin – or ] in those with kidney problems, ], or ] – combined with ], ], gemcitabine, or ].{{sfn|Horn|Iams|2022|loc="Management of Stages I and II NSCLC"}} | |||
| Treatment for those with stage III NSCLC depends on the nature of their disease. Those with more limited spread may undergo surgery to have the tumor and affected lymph nodes removed, followed by chemotherapy and potentially radiotherapy. Those with particularly large tumors (T4) and those for whom surgery is impractical are treated with combination chemotherapy and radiotherapy along with the ] ].{{sfn|Horn|Iams|2022|loc="Management of Stage III NSCLC"}} Combined chemotherapy and radiation enhances survival compared to chemotherapy followed by radiation, though the combination therapy comes with harsher side effects.{{sfn|Horn|Iams|2022|loc="Management of Stage III NSCLC"}} | |||
| ===Genetics and viruses=== | |||
| ]s are genes that are believed make people more susceptible to cancer. ]s are believed to turn into oncogenes when exposed to particular carcinogens. ]es are also suspected of causing cancer in humans, as this link has already been proven in animals. Genetic susceptibility and viral infection are not of major importance in lung cancer, but they may influence pathogenesis. | |||
| Those with stage IV disease are treated with combinations of pain medication, radiotherapy, immunotherapy, and chemotherapy.{{sfn|Horn|Iams|2022|loc="Management of Metastatic NSCLC"}} Many cases of advanced disease can be treated with targeted therapies depending on the genetic makeup of the cancerous cells. Up to 30% of tumors have mutations in the '']'' gene that result in an overactive EGFR protein;{{sfn|Alexander|Kim|Cheng|2020|loc="Basis of Molecularly Targeted Therapy in Lung Cancer"}} these can be treated with EGFR inhibitors ], ], ], ], or ] – with osimertinib known to be superior to erlotinib and gefitinib, and all superior to chemotherapy alone.{{sfn|Horn|Iams|2022|loc="Management of Metastatic NSCLC"}} Up to 7% of those with NSCLC harbor mutations that result in hyperactive ] protein, which can be treated with ]s ], or its successors ], ], and ].{{sfn|Horn|Iams|2022|loc="Management of Metastatic NSCLC"}} Those treated with ALK inhibitors who relapse can then be treated with the third-generation ALK inhibitor ].{{sfn|Horn|Iams|2022|loc="Management of Metastatic NSCLC"}} Up to 5% with NSCLC have overactive ], which can be inhibited with ] ] or ].{{sfn|Horn|Iams|2022|loc="Management of Metastatic NSCLC"}} Targeted therapies are also available for some cancers with rare mutations. Cancers with hyperactive ] (around 2% of NSCLC) can be treated by ] combined with the ] ]; those with activated ] (around 1% of NSCLC) can be inhibited by crizotinib, lorlatinib, or ]; overactive ] (<1% of NSCLC) by entrectinib or ]; active ] (around 1% of NSCLC) by ].{{sfn|Horn|Iams|2022|loc="Management of Metastatic NSCLC"}} | |||
| == Lung cancer staging == | |||
| People whose NSCLC is not targetable by current molecular therapies instead can be treated with combination chemotherapy plus immune checkpoint inhibitors, which prevent cancer cells from inactivating immune ]s. The chemotherapeutic agent of choice depends on the NSCLC subtype: cisplatin plus gemcitabine for squamous cell carcinoma, cisplatin plus pemetrexed for non-squamous cell carcinoma.{{sfn|Horn|Iams|2022|loc="Cytotoxic Chemotherapy for Metastatic or Recurrent NSCLC"}} Immune checkpoint inhibitors are most effective against tumors that express the protein ], but are sometimes effective in those that do not.{{sfn|Horn|Iams|2022|loc="Immunotherapy"}} Treatment with ], ], or combination ] plus ] are all superior to chemotherapy alone against tumors expressing PD-L1.{{sfn|Horn|Iams|2022|loc="Immunotherapy"}} Those who relapse on the above are treated with second-line chemotherapeutics ] and ].{{sfn|Horn|Iams|2022|loc="Second-Line Therapy and Beyond"}} | |||
| Lung ] is an important part of the assessment of ] and potential treatment for lung cancer. | |||
| ===Palliative care=== | |||
| ''See ]''. | |||
| ] (internal radiotherapy) for lung cancer given via the airway]] | |||
| Integrating palliative care (medical care focused on improving symptoms and lessening discomfort) into lung cancer treatment from the time of diagnosis improves the survival time and quality of life of those with lung cancer.{{sfn|Aragon|2020|loc="Integrating palliative care into lung cancer care"}} Particularly common symptoms of lung cancer are shortness of breath and pain. Supplemental oxygen, improved airflow, re-orienting an affected person in bed, and low-dose ] can all improve shortness of breath.{{sfn|Aragon|2020|loc="Dyspnea"}} <ref name="Dy-2020" />In around 20 to 30% of those with lung cancer – particularly those with late-stage disease – growth of the tumor can ], causing coughing and difficulty breathing.{{sfn|Obeng|Folch|Fernando Santacruz|2018|loc="Introduction", "Prevalence", and "Clinical presentation"}} Obstructing tumors can be surgically removed where possible, though typically those with airway obstruction are not well enough for surgery. In such cases the American College of Chest Physicians recommends opening the airway by inserting a ], attempting to shrink the tumor with localized radiation (]), or physically removing the blocking tissue by bronchoscopy, sometimes aided by thermal or ].{{sfn|Obeng|Folch|Fernando Santacruz|2018|loc="Management"}} Other causes of lung cancer-associated shortness of breath can be treated directly, such as ]s for a lung infection, ]s for ], ]s for anxiety, and ]s for airway obstruction.{{sfn|Aragon|2020|loc="Dyspnea"}} | |||
| Up to 92% of those with lung cancer report pain, either from tissue damage at the tumor site(s) or nerve damage.{{sfn|Aragon|2020|loc="Cancer-related pain"}} The ] (WHO) has developed a three-tiered system for managing cancer pain. For those with mild pain (tier one), the WHO recommends ] or a ].{{sfn|Aragon|2020|loc="Cancer-related pain"}} Around a third of people experience moderate (tier two) or severe (tier three) pain, for which the WHO recommends opioid painkillers.{{sfn|Aragon|2020|loc="Cancer-related pain"}} Opioids are typically effective at easing ] (pain caused by damage to various body tissues). Opioids are occasionally effective at easing ] (pain caused by nerve damage). Neuropathic agents such as ]s, ]s, and ]s, are often used to ease neuropathic pain, either alone or in combination with opioids.{{sfn|Aragon|2020|loc="Cancer-related pain"}} In many cases, targeted radiotherapy can be used to shrink tumors, reducing pain and other symptoms caused by tumor growth.{{sfn|Spencer|Parrish|Barton|Henry|2018|loc="What are the indications for using palliative radiotherapy?"}} | |||
| == Treatment == | |||
| Treatment for lung cancer depends on the cancer's specific cell type, how far it has ], and the patient's ]. Common treatments include ], ], and ]. The 5-year overall survival rate is 14%.<ref>John D. Minna, "Neoplasms of the Lung," in ''Harrison's Principles of Internal Medicine'', 16th ed. (2005), p. 506</ref> | |||
| Individuals who have advanced disease and are approaching end-of-life can benefit from dedicated ] to manage symptoms and ease suffering. As in earlier disease, pain and difficulty breathing are common, and can be managed with opioid pain medications, transitioning from oral medication to injected medication if the affected individual loses the ability to swallow.{{sfn|Lim|2016|loc="Key area three: providing symptom management in the last days"}}<ref name="Dy-2020">{{Cite report |title=Interventions for Breathlessness in Patients With Advanced Cancer |last1=Dy |first1=Sydney M. |last2=Gupta |first2=Arjun |date=2020-11-19 |publisher=Agency for Healthcare Research and Quality (AHRQ) |doi=10.23970/ahrqepccer232 |language=en |last3=Waldfogel |first3=Julie M. |last4=Sharma |first4=Ritu |last5=Zhang |first5=Allen |last6=Feliciano |first6=Josephine L. |last7=Sedhom |first7=Ramy |last8=Day |first8=Jeff |last9=Gersten |first9=Rebecca A.|doi-access=free }}</ref> Coughing is also common, and can be managed with opioids or ]s. Some experience terminal delirium – confused behavior, unexplained movements, or a reversal of the sleep-wake cycle – which can be managed by antipsychotic drugs, low-dose sedatives, and investigating other causes of discomfort such as ], ], and ].{{sfn|Lim|2016|loc="Key area three: providing symptom management in the last days"}} In the last few days of life, many develop ] – pooled fluid in the airways that can cause a rattling sound while breathing. This is thought not to cause respiratory problems, but can distress family members and caregivers. Terminal secretions can be reduced by ]s.{{sfn|Lim|2016|loc="Key area three: providing symptom management in the last days"}} Even those who are non-communicative or have reduced consciousness may be able to experience cancer-related pain, so pain medications are typically continued until the time of death.{{sfn|Lim|2016|loc="Key area three: providing symptom management in the last days"}} | |||
| See also '']''. | |||
| == |
==Prognosis== | ||
| ] program]] | |||
| Surgery itself has an overall operative death rate of 5%, depending on the patient's lung function and other risk factors.<ref> New York Times, March 13, 2007</ref> Surgery is usually only an option in NSCLC limited to one lung. This is assessed with medical imaging (], ]). A sufficient pre-operative respiratory reserve must be present to allow adequate lung function after the tissue is removed. Procedures include wedge excision or segmentectomy (removal of part of a lobe), ] (one lobe), bilobectomy (two lobes) or ] (whole lung). | |||
| {| class="wikitable floatright" style="text-align:center;font-size:90%;width:25%;margin-left:1em" | |||
| Extended wedge resection is controversial. Overall survival is equivalent to lobectomy, but local recurrence rate is 3 times as high (19% compared to 5%, respectively). Accordingly, sub lobar resection has been used as a "compromise resection" for the management of small (less than 3 centimeters diameter) stage I peripheral NSCLC in patients with impaired cardiopulmonary reserve. Radioactive iodine brachytherapy at the margins of sublobar resection may reduce recurrence to that of lobectomy.{{Fact|date=March 2007}} | |||
| |+ style="background:#E5AFAA;"|Five-year survival in those diagnosed with lung cancer, by stage{{sfn|Goldstraw|Chansky|Crowley|Rami-Porta|2016|loc="Figure 2"}} | |||
| |- style="background: #E5AFAA;text-align:center;font-size:90%;" | |||
| ! abbr="Type" | Clinical stage | |||
| !Five-year survival (%) | |||
| |- | |||
| | IA1 | |||
| | 92 | |||
| |- | |||
| |IA2 | |||
| |83 | |||
| |- | |||
| |IA3 | |||
| |77 | |||
| |- | |||
| | IB | |||
| | 68 | |||
| |- | |||
| | IIA | |||
| | 60 | |||
| |- | |||
| | IIB | |||
| | 53 | |||
| |- | |||
| | IIIA | |||
| | 36 | |||
| |- | |||
| | IIIB | |||
| | 26 | |||
| |- | |||
| |IIIC | |||
| |13 | |||
| |- | |||
| | IVA | |||
| | 10 | |||
| |- | |||
| |IVB | |||
| |0 | |||
| |} | |||
| Around 19% of people diagnosed with lung cancer survive ], though prognosis varies based on the stage of the disease at diagnosis and the type of lung cancer.{{sfn|Rivera|Mody|Weiner|2022|loc="Introduction"}} Prognosis is better for people with lung cancer diagnosed at an earlier stage; those diagnosed at the earliest TNM stage, IA1 (small tumor, no spread), have a two-year survival of 97% and five-year survival of 92%.{{sfn|Goldstraw|Chansky|Crowley|Rami-Porta|2016|loc="Figure 2"}} Those diagnosed at the most-advanced stage, IVB, have a two-year survival of 10% and a five-year survival of 0%.{{sfn|Goldstraw|Chansky|Crowley|Rami-Porta|2016|loc="Figure 2"}} Five-year survival is higher in women (22%) than men (16%).{{sfn|Rivera|Mody|Weiner|2022|loc="Introduction"}} Women tend to be diagnosed with less-advanced disease, and have better outcomes than men diagnosed at the same stage.{{sfn|Rivera|Mody|Weiner|2022|loc="Prognostic and Predictive Factors in Lung Cancer"}} Average five-year survival also varies across the world, with particularly high five-year survival in Japan (33%), and five-year survival above 20% in 12 other countries: Mauritius, Canada, the US, China, South Korea, Taiwan, Israel, Latvia, Iceland, Sweden, Austria, and Switzerland.{{sfn|Allemani|Matsuda|Di Carlo|Harewood|2018|loc="Lung"}} | |||
| Anatomic segmentectomy (a larger sublobar resection) with complete lymph node staging has survival benefits similar to lobectomy, in peripheral small (less than 2 cm diameter) stage I NSCLC where a margin of resection equivalent to the diameter of the tumor can be achieved. | |||
| SCLC is particularly aggressive. 10–15% of people survive five years after a SCLC diagnosis.{{sfn|Horn|Iams|2022|loc="Treatment – Small-Cell Lung Cancer"}} As with other types of lung cancer, the extent of disease at diagnosis also influences prognosis. The average person diagnosed with limited-stage SCLC survives 12–20 months from diagnosis; with extensive-stage SCLC around 12 months.{{sfn|Horn|Iams|2022|loc="Treatment – Small-Cell Lung Cancer"}} While SCLC often responds initially to treatment, most people eventually relapse with chemotherapy-resistant cancer, surviving an average 3–4 months from the time of relapse.{{sfn|Horn|Iams|2022|loc="Treatment – Small-Cell Lung Cancer"}} Those with limited stage SCLC that go into complete remission after chemotherapy and radiotherapy have a 50% chance of brain metastases developing within the next two years – a chance reduced by prophylactic cranial irradiation.{{sfn|Rivera|Mody|Weiner|2022|loc="Treatment of Small Cell Lung Cancer"}} | |||
| Five-year prognosis is up to 70% following complete resection of limited (stage I) disease.{{Fact|date=March 2007}} | |||
| Several other personal and disease factors are associated with improved outcomes. Those diagnosed at a younger age tend to have better outcomes. Those who smoke or experience weight loss as a symptom tend to have worse outcomes. Tumor mutations in ] are associated with reduced survival.{{sfn|Rivera|Mody|Weiner|2022|loc="Prognostic and Predictive Factors in Lung Cancer"}} | |||
| After surgery, if lymph nodes are positive in the resected lung tissues (stage II) or the mediastinum (peri-tracheal region, stage III), ] chemotherapy may improve survival by up to 15%. The role of adjuvant chemotherapy for patients with large stage I NSCLC (tumor diameter greater than 3 cm without lymph node involvement, stage IB) is controversial.{{Fact|date=March 2007}} | |||
| ===Experience=== | |||
| Trials of preoperative chemotherapy in resectable NSCLC have been inconclusive.<ref name="Clinical evidence">, BMJ Publishing Group, London. 2006. ISBN 1-90554501206 ISSN 1465-9225</ref> | |||
| The uncertainty of lung cancer prognosis often causes stress, and makes future planning difficult, for those with lung cancer and their families.{{sfn|Temel|Petrillo|Greer|2022|loc="Coping with Prognostic Uncertainty"}} Those whose cancer goes into remission often experience fear of their cancer returning or progressing, associated with poor quality of life, negative mood, and functional impairment. This fear is exacerbated by frequent or prolonged surveillance imaging, and other reminders of cancer risks.{{sfn|Temel|Petrillo|Greer|2022|loc="Coping with Prognostic Uncertainty"}} | |||
| ==Causes== | |||
| The NCI Canada study JBR.10 treated patients with stage IB to IIB NSCLC with vinorelbine and cisplatin chemotherapy and showed a significant survival benefit of 15% over 5 years. However subgroup analysis of patients in stage IB showed that chemotherapy did not result in any survival gain in them.{{Fact|date=March 2007}} Similarly, while the Italian ANITA study showed a survival benefit of 8% over 5 years with vinorelbine and cisplatin chemotherapy in stages IB to IIIA, subgroup analysis also showed no benefit in the IB stage.{{Fact|date=March 2007}} | |||
| Lung cancer is caused by ] to the ] of lung cells. These changes are sometimes random, but are typically induced by breathing in toxic substances such as cigarette smoke.<ref>{{cite web|url=https://www.cancer.org/cancer/lung-cancer/causes-risks-prevention/what-causes.html |title=What Causes Lung Cancer |publisher=American Cancer Society |date=1 October 2019 |accessdate=31 January 2023}}</ref><ref>{{cite web|url=https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/basics/what-causes-lung-cancer |accessdate=31 January 2023 |title=What Causes Lung Cancer? |publisher=American Lung Association |date=17 November 2022}}</ref> Cancer-causing genetic changes affect the ], including ], programmed cell death (]), and ].{{sfn|Massion|Lehman|2022|loc=Table 73.1: Hallmarks of Cancer}} Eventually, cells gain enough genetic changes to grow uncontrollably, forming a tumor, and eventually spreading within and then beyond the lung.<!--Cite--> Rampant tumor growth and spread causes the symptoms of lung cancer. If unstopped, the spreading tumor will eventually cause the death of affected individuals.<!--Cite--> | |||
| The Cancer and Leukemia Group B (CALGB) study,a randomized trial of carboplatin and paclitaxel in stage IB, reported no survival advantage at the June 2006 American Society of Clinical Oncology meeting.{{Fact|date=March 2007}} However, subgroup analysis suggested benefit for tumors greater than 4 centimeters. | |||
| ===Smoking=== | |||
| For patients with resected stage II-IIIA NSCLC, standard practice is to offer adjuvant third generation platinum-based chemotherapy (e.g. cisplatin and vinorelbine).{{Fact|date=March 2007}} | |||
| ] | |||
| ] is by far the major contributor to lung cancer, causing 80% to 90% of cases.{{sfn|Schabath|Cote|2019|loc="Introduction"}} Lung cancer risk increases with quantity of cigarettes consumed.{{sfn|Bade|Dela Cruz|2020|loc="Tobacco Smoke Carcinogens"}} Tobacco smoking's carcinogenic effect is due to ] that cause DNA mutations, increasing the chance of cells becoming cancerous.<ref>{{cite web|url=https://www.cdc.gov/cancer/tobacco/index.htm |accessdate=29 December 2022 |title=Tobacco and Cancer |date=18 November 2021 |publisher= ]}}</ref> The ] identifies at least 50 chemicals in tobacco smoke as ]ic, and the most potent is ].{{sfn|Bade|Dela Cruz|2020|loc="Tobacco Smoke Carcinogens"}} Exposure to these chemicals causes several kinds of DNA damage: ]s, ], and breaks in the DNA strands.{{sfn|Massion|Lehman|2022|loc="DNA Damage Response"}} Being around tobacco smoke – called ] – can also cause lung cancer. Living with a tobacco smoker increases one's risk of developing lung cancer by 24%. An estimated 17% of lung cancer cases in those who do not smoke are caused by high levels of environmental tobacco smoke.{{sfn|Bade|Dela Cruz|2020|loc="Environmental Tobacco Smoke"}} | |||
| Adjuvant chemotherapy for patients with stage IB is controversial as clinical trials have not clearly demonstrated a survival benefit.{{Fact|date=March 2007}} | |||
| ] may be a risk factor for lung cancer, but less than that of cigarettes, and further research as of 2021 is necessary due to the length of time it can take for lung cancer to develop following an exposure to carcinogens.{{sfn|Bracken-Clarke|Kapoor|Baird|Buchanan|2021|loc=Abstract – "Conclusion"}} | |||
| ===Chemotherapy=== | |||
| Small-cell lung cancer is treated primarily with chemotherapy, as surgery has no demonstrable influence on survival. Primary chemotherapy is also given in metastatic NSCLC. | |||
| The smoking of non-tobacco products is not known to be associated with lung cancer development. Marijuana smoking does not seem to independently cause lung cancer – despite the relatively high levels of ] and known carcinogens in marijuana smoke. The relationship between smoking cocaine and developing lung cancer has not been studied as of 2020.{{sfn|Bade|Dela Cruz|2020|loc="Marijuana and Other Recreational Drugs"}} | |||
| The combination regimen depends on the tumour type: | |||
| * NSCLC: ] or ], in combination with ], ], ], ] or ]. In metastatic lung cancer, the addition of ] when added to carboplatin and paclitaxel was found to improve survival (though in this study, patients with squamous cell lung cancer were excluded because of problems with pulmonary hemorrhage in this group in the past). | |||
| * SCLC: ] or ], in combination ] or ]; combinations with ], ], ], ] and ] are being studied. | |||
| === |
===Environmental exposures=== | ||
| ] | |||
| In recent years, various molecular targeted therapies have been developed for the treatment of advanced lung cancer. ] (Iressa) is one such drug, which targets the ] (EGF-R) which is expressed in many cases of NSCLC. However despite an exciting start it was not shown to increase survival, although females, Asians, non-smokers and those with the adenocarcinoma cell type appear to be deriving most benefit from gefitinib. | |||
| Exposure to a variety of other toxic chemicals – typically encountered in certain occupations – is associated with an increased risk of lung cancer.{{sfn|Christiani|Amos|2022|loc="Occupational Exposures"}} Occupational exposures to carcinogens cause 9–15% of lung cancer.{{sfn|Christiani|Amos|2022|loc="Occupational Exposures"}} A prominent example is ], which causes lung cancer either directly or indirectly by inflaming the lung.{{sfn|Christiani|Amos|2022|loc="Occupational Exposures"}} Exposure to all commercially available forms of asbestos increases cancer risk, and cancer risk increases with time of exposure.{{sfn|Christiani|Amos|2022|loc="Occupational Exposures"}} Asbestos and cigarette smoking increase risk synergistically – that is, the risk of someone who smokes and has asbestos exposure dying from lung cancer is much higher than would be expected from adding the two risks together.{{sfn|Christiani|Amos|2022|loc="Occupational Exposures"}} Similarly, exposure to ], a naturally occurring breakdown product of the Earth's ]s, is associated with increased lung cancer risk. Radon levels vary with geography.{{sfn|Schabath|Cote|2019|loc="Radon"}} Underground miners have the greatest exposure; however even the lower levels of radon that seep into residential spaces can increase occupants' risk of lung cancer. Like asbestos, cigarette smoking and radon exposure increase risk synergistically.{{sfn|Christiani|Amos|2022|loc="Occupational Exposures"}} Radon exposure is responsible for between 3% and 14% of lung cancer cases.{{sfn|Schabath|Cote|2019|loc="Radon"}} | |||
| Several other chemicals encountered in various occupations are also associated with increased lung cancer risk including ] used in ], ] application, and some ore ]; ] encountered during ]; ] in ]; ] in ]s, ]s workers, missile technicians, and ] workers; ] in ] production, ], and ]; ] in ]rs, glass workers, metal workers, welders, and those who make batteries, ceramics, and jewelry; and ] encountered by miners.{{sfn|Christiani|Amos|2022|loc="Occupational Exposures"}} | |||
| A newer drug called ] (Tarceva), another EGF-R inhibitor, has been shown to increase survival in lung cancer patients and has recently been approved by the FDA for second-line treatment of advanced non-small cell lung cancer. | |||
| Exposure to ], especially ] released by motor vehicle exhaust and ]-burning power plants, increases the risk of lung cancer.{{sfn|Christiani|Amos|2022|loc="Air Pollution"}}{{sfn|Balmes|Holm|2022|loc=Table 102.2: Major Pollutants Associated with Adverse Pulmonary Effects}} ] from burning ], ], or crop residue for cooking and heating has also been linked to an increased risk of developing lung cancer.{{sfn|Bade|Dela Cruz|2020|loc="Biomass Burning"}} The International Agency for Research on Cancer has classified emission from household burning of coal and biomass as "carcinogenic" and "probably carcinogenic" respectively.{{sfn|Bade|Dela Cruz|2020|loc="Biomass Burning"}} | |||
| A number of targeted agents are at the early stages of clinical research, such as cyclo-oxygenase-2 (COX-2) inhibitors, the pre-apoptic inhibitor ], proteasome inhibitors, ] (Targretin) and vaccines<ref> {{cite web | author=H-H Hansen | year=2006 | title=Non-Small Cell Cancer - An Update for 2006 | url=http://www.touchoncologicaldisease.com/articles.cfm?article_id=6109&level=2}}</ref> | |||
| ===Other diseases=== | |||
| Treatment of non-small cell lung cancer is evolving. | |||
| Several other diseases that cause inflammation of the lung increase one's risk of lung cancer. This association is strongest for ] – the risk is highest in those with the most inflammation, and reduced in those whose inflammation is treated with ]s.{{sfn|Bade|Dela Cruz|2020|loc="Chronic Lung Diseases"}} Other inflammatory lung and immune system diseases such as ], ], ], '']'' infection, ], and ] are associated with increased risk of developing lung cancer.{{sfn|Bade|Dela Cruz|2020|loc="Chronic Lung Diseases"}} ] is associated with the development of the rare lung cancer ] in people from Asia, but not in people from ].{{sfn|Bade|Dela Cruz|2020|loc="Infections"}} A role for several other infectious agents – namely ]es, ], ], ], ], ], and ] – in lung cancer development has been studied but remains inconclusive as of 2020.{{sfn|Bade|Dela Cruz|2020|loc="Infections"}} | |||
| === |
===Genetics=== | ||
| Particular gene combinations may make some people more susceptible to lung cancer. Close family members of those with lung cancer have around twice the risk of developing lung cancer as an average person, even after controlling for occupational exposure and smoking habits.{{sfn|Christiani|Amos|2022|loc="Genetic Susceptibility to Lung Cancer"}} ] have identified many gene variants associated with lung cancer risk, each of which contributes a small risk increase.{{sfn|Bade|Dela Cruz|2020|loc="Genetic Predisposition and History of Cancer"}} Many of these genes participate in pathways known to be involved in carcinogenesis, namely ], ], the ], ]s, and ].{{sfn|Bade|Dela Cruz|2020|loc="Genetic Predisposition and History of Cancer"}} Some rare genetic disorders that increase the risk of various cancers also increase the risk of lung cancer, namely ] and ].{{sfn|Christiani|Amos|2022|loc="High-Risk Syndromes Conferring an Increased Risk of Lung Cancer"}} | |||
| ] is often given together with chemotherapy, and may be used with curative intent in patients who are not eligible for surgery. A radiation dose of 40 or more ] in many fractions is commonly used with curative intent in non-small cell lung cancer; typically in North America, the dose prescribed is 60 or 66 Gy in 30 to 33 fractions given once daily, 5 days a week, for 6 to 6½ weeks. For small cell lung cancer cases that are potentially curable, in addition to chemotherapy, chest radiation is often recommended. For these small cell lung cancer cases, chest radiation doses of 40 Gy or more in many fractions are commonly given; typically in North America, the dose prescribed is 45 to 50 Gy and can be given in either once daily treatments for 5 weeks or twice daily treatments for 3 weeks. New research using higher doses of radiation may improve the results of treatment. The use of ] scans for treatment planning and novel radiation therapy delivery systems such as ] may also be of benifit. | |||
| ==Pathogenesis== | |||
| For both non-small cell lung cancer and small cell lung cancer patients, radiation of disease in the chest to smaller doses (typically 20 Gy in 5 fractions) may be used for symptom control. | |||
| As with all cancers, lung cancer is triggered by mutations that allow tumor cells to endlessly multiply, stimulate ], avoid ] (programmed cell death), generate pro-growth signalling molecules, ignore anti-growth signalling molecules, and eventually spread into surrounding tissue or metastasize throughout the body.{{sfn|Horn|Iams|2022|loc="Molecular Pathogenesis"}} Different tumors can acquire these abilities through different mutations, though generally cancer-contributing mutations activate ]s and inactivate ]s.{{sfn|Horn|Iams|2022|loc="Molecular Pathogenesis"}} Some mutations – called "driver mutations" – are particularly common in adenocarcinomas, and contribute disproportionately to tumor development. These typically occur in the ]s EGFR, BRAF, MET, ], and ].{{sfn|Horn|Iams|2022|loc="Molecular Pathogenesis"}} Similarly, some adenocarcinomas are driven by chromosomal rearrangements that result in overexpression of ] ALK, ROS1, NTRK, and RET. A given tumor will typically have just one driver mutation.{{sfn|Horn|Iams|2022|loc="Molecular Pathogenesis"}} In contrast, SCLCs rarely have these driver mutations, and instead often have mutations that have inactivated the tumor suppressors ] and ].{{sfn|Rudin|Brambilla|Faivre-Finn|Sage|2021|loc="Mechanisms/Pathophysiology"}} A cluster of tumor suppressor genes on the short arm of ] are often lost early in the development of all lung cancers.{{sfn|Horn|Iams|2022|loc="Molecular Pathogenesis"}} | |||
| ==Prevention== | |||
| ===Interventional radiology=== | |||
| ===Smoking cessation=== | |||
| ] is increasing in popularity for this condition as it is nontoxic and causes very little pain. It seems especially effective when combined with chemotherapy as it catches the cells inside a tumor—the ones difficult to get with chemotherapy due to reduced blood supply to the inside of the tumor. It is done by inserting a small heat probe into the tumor to cook the tumor cells. The body then disposes of the cooked cells through its normal eliminative processes. | |||
| Those who smoke can reduce their lung cancer risk by quitting smoking – the risk reduction is greater the longer a person goes without smoking.{{sfn|Horn|Iams|2022|loc="Risk Factors"}} Self-help programs tend to have little influence on success of smoking cessation, whereas combined counseling and pharmacotherapy improve cessation rates.{{sfn|Horn|Iams|2022|loc="Risk Factors"}} The US FDA has approved ] therapies and the nicotine replacement ] as first-line therapies to aid in smoking cessation. ] and ] are recommended second-line therapies<!--recommended by whom?-->.{{sfn|Horn|Iams|2022|loc="Risk Factors"}} The majority of those diagnosed with lung cancer attempt to quit smoking; around half succeed.{{sfn|Jassem|2019|loc="Prevalence and determinants of continued tobacco use after diagnosis of cancer"}} Even after lung cancer diagnosis, smoking cessation improves treatment outcomes, reducing cancer treatment toxicity and failure rates, and lengthening survival time.{{sfn|Jassem|2019|loc="Consequences of continued smoking after diagnosis of cancer"}} | |||
| {{Multiple image|total_width=300 | |||
| |image2=Belgian cigarette pack (generic).jpg | |||
| |caption2=Graphic cigarette packaging in Belgium labelled "open wound following lung surgery" | |||
| |alt2=A cigarette package features warning text and a large photograph of a person with a large side wound. | |||
| |image1=RTD, No Smoking on Platform sign, FCS.jpg | |||
| |caption1=No smoking sign at a train station in Colorado | |||
| |alt1=A sign reads "No smoking on platform" | |||
| }} | |||
| At a societal level, smoking cessation can be promoted by ] policies that make tobacco products more difficult to obtain or use. Many such policies are mandated or recommended by the ], ratified by 182 countries, representing over 90% of the world's population.{{sfn|Peruga|López|Martinez|Fernández|2021|loc="2.1. Galvanizing global political will around international law"}} The WHO groups these policies into six intervention categories, each of which has been shown to be effective in reducing the cost of tobacco-induced disease burden on a population: | |||
| #increasing the price of tobacco by raising taxes; | |||
| #banning tobacco use in public places to reduce exposure; | |||
| # banning tobacco advertisements; | |||
| #publicizing the dangers of tobacco products; | |||
| # instituting help programs for those attempting to quit smoking; and | |||
| # monitoring population-level tobacco use and the effectiveness of tobacco control policies.{{sfn|Peruga|López|Martinez|Fernández|2021|loc="2.2. Quadrupling the number of people benefiting from at least one cost-effective tobacco control policy since 2007"}} | |||
| Policies implementing each intervention are associated with decreases in tobacco smoking prevalence. The more policies implemented, the greater the reduction.{{sfn|Arnott|Lindorff|Goddard|2022|p=427}} Reducing access to tobacco for adolescents is particularly effective at decreasing uptake of habitual smoking, and adolescent demand for tobacco products is particularly sensitive to increases in cost.{{sfn|Christiani|Amos|2022|loc="Smoking Behavior and Risk for Lung Cancer"}} | |||
| ===Diet and lifestyle=== | |||
| Several foods and dietary supplements have been associated with lung cancer risk. High consumption of some animal products – ] (but not other meats or fish), ]s, as well as ]s and ]s (found in salted and smoked meats) – is associated with an increased risk of developing lung cancer.{{sfn|Bade|Dela Cruz|2020|loc="Diet"}} In contrast, high consumption of fruits and vegetables is associated with a reduced risk of lung cancer, particularly consumption of ] and raw fruits and vegetables.{{sfn|Bade|Dela Cruz|2020|loc="Diet"}} Based on the beneficial effects of fruits and vegetables, supplementation of several individual vitamins have been studied. Supplementation with ] or ] had no effect on lung cancer, and instead slightly increased mortality.{{sfn|Bade|Dela Cruz|2020|loc="Diet"}} Dietary supplementation with ] or ]s similarly had no effect.{{sfn|Bade|Dela Cruz|2020|loc="Chemopreventive Agents"}} Consumption of ]s, tea, alcoholic beverages, and coffee are all associated with reduced risk of developing lung cancer.{{sfn|Bade|Dela Cruz|2020|loc="Diet"}} | |||
| Along with diet, body weight and exercise habits are also associated with lung cancer risk. Being ] is associated with a lower risk of developing lung cancer, possibly due to the tendency of those who smoke cigarettes to have a lower body weight.{{sfn|Bade|Dela Cruz|2020|loc="Obesity and Exercise"}} However, being ] is also associated with a reduced lung cancer risk.{{sfn|Bade|Dela Cruz|2020|loc="Obesity and Exercise"}} Some studies have shown those who exercise regularly or have better cardiovascular fitness to have a lower risk of developing lung cancer.{{sfn|Bade|Dela Cruz|2020|loc="Obesity and Exercise"}} | |||
| ==Epidemiology== | ==Epidemiology== | ||
| ] lung cancer incidence in 2020 per 100,000 people:<ref>{{cite web|url=https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=15&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=0&include_nmsc_other=0&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&show_ranking=0&rotate=%255B10%252C0%255D |accessdate=28 April 2023 |title=Estimated age-standardized incidence rates (World) in 2020, lung, both sexes, all ages |publisher=World Health Organization, International Agency for Research on Cancer}}</ref> {{Div col|small=yes|colwidth=10em}}{{legend|#045a8d|>40}}{{legend|#2b8cbe|30–40}}{{legend|#74a9cf|20–30}}{{legend|#bdc9e1|10–20}}{{legend|#f1eef6|<10}}{{div col end}}]] | |||
| ].]] | |||
| Worldwide, lung cancer is the most diagnosed type of cancer, and the leading cause of cancer death.{{sfn|Schabath|Cote|2019|loc="Descriptive Epidemiology"}}{{sfn|Christiani|Amos|2022|loc="Introduction"}} In 2020, 2.2 million new cases were diagnosed, and 1.8 million people died from lung cancer, representing 18% of all cancer deaths.{{sfn|Sung|Ferlay|Siegel|Laversanne|2021|loc="Lung cancer"}} Lung cancer deaths are expected to rise globally to nearly 3 million annual deaths by 2035, due to high rates of tobacco use and aging populations.{{sfn|Christiani|Amos|2022|loc="Introduction"}} Lung cancer is rare among those younger than 40; after that, cancer rates increase with age, stabilizing around age 80.{{sfn|Horn|Iams|2022|loc="Epidemiology"}} The median age of a person diagnosed with lung cancer is 70; the median age of death is 72.{{sfn|Bade|Dela Cruz|2020|loc="Age"}} | |||
| The population segment most likely to develop lung cancer is the over-fifties who also have a history of smoking. Lung cancer is the second most commonly occurring form of cancer in most western countries, and it is the leading cancer-related cause of death for men and women. In the US, 175,000 new cases are expected in 2006:<ref> from National Lung Cancer Partnership | |||
| new cases of lung cancer in the US</ref> 90,700 in men and 80,000 in women. Although the rate of men dying from lung cancer is declining in western countries, it is actually increasing for women due to the increased takeup of smoking by this group. Among lifetime non-smokers, men who have never smoked have higher age-standardized lung cancer death rates than women. Of the 80,000 women who are diagnosed with lung cancer in 2006, approximately 70,000 are expected to die from it.<ref> from National Lung Cancer Partnership News </ref> | |||
| Lung cancer incidence varies by geography and sex, with the highest rates in Micronesia, Polynesia, Europe, Asia, and North America; and lowest rates in Africa and Central America.{{sfn|Sung|Ferlay|Siegel|Laversanne|2021|loc="Figure 9"}} Globally, around 8% of men and 6% of women develop lung cancer in their lifetimes.{{sfn|Horn|Iams|2022|loc="Epidemiology"}} The ratio of lung cancer cases in men to women varies considerably by geography, from as high as nearly 12:1 in Belarus, to 1:1 in Brazil, likely due to differences in smoking patterns.{{sfn|Christiani|Amos|2022|loc="Geographic, Gender, and Ethnic Variability"}} | |||
| Lung cancer was extremely rare prior to the advent of cigarette smoking. In 1878, malignant lung tumors made up only 1% of all cancers seen at autopsy; this had risen to 10-15% by the early 1900s.<ref>Witschi H. . Toxicol Sci. 2001 Nov;64(1):4-6. PMID 11606795</ref> Case reports in the medical literature numbered only 374 worldwide in 1912.<ref>Adler I. Primary malignant growths of the lungs and bronchi. New York: Longmans, Green, and Company; 1912., cited in Spiro SG, Silvestri GA. Am J Respir Crit Care Med. 2005 Sep 1;172(5):523-9. PMID 15961694</ref> The ], published in the ], first offered solid ] evidence on the link between lung cancer and smoking. | |||
| Lung cancer risk is influenced by environmental exposure, namely cigarette smoking, as well as occupational risks in mining, shipbuilding, petroleum refining, and occupations that involve asbestos exposure.{{sfn|Christiani|Amos|2022|loc="Geographic, Gender, and Ethnic Variability"}} People who have smoked cigarettes account for 85–90% of lung cancer cases, and 15% of smokers develop lung cancer.{{sfn|Christiani|Amos|2022|loc="Geographic, Gender, and Ethnic Variability"}} Non-smokers' risk of developing lung cancer is also influenced by tobacco smoking; ] (that is, being around tobacco smoke) increases risk of developing lung cancer around 30%, with risk correlated to duration of exposure.{{sfn|Christiani|Amos|2022|loc="Geographic, Gender, and Ethnic Variability"}} As the global incidence of lung cancer decreases in parallel with declining smoking rates in developed countries, the incidence of lung cancer in individuals who have never smoked is stable or increasing.<ref>{{Cite journal |last1=LoPiccolo |first1=Jaclyn |last2=Gusev |first2=Alexander |last3=Christiani |first3=David C. |last4=Jänne |first4=Pasi A. |date=January 9, 2024 |title=Lung cancer in patients who have never smoked — an emerging disease |journal=Nature Reviews Clinical Oncology |language=en |volume=21 |issue=2 |pages=121–146 |doi=10.1038/s41571-023-00844-0 |issn=1759-4782 |pmc=11014425 |pmid=38195910}}</ref> | |||
| Not all cases of lung cancer are due to smoking, but the role of ] is increasingly being recognized as a risk factor for lung cancer, leading to policy interventions to decrease undesired exposure of non-smokers to others' tobacco smoke. Emissions from automobiles, factories and power plants also pose potential risks.{{fact|date=April 2007}} | |||
| ==History== | |||
| In the ] and ], smoking-related lung cancer is rising rapidly in incidence. Countries such as ] are expected to see a marked increase in lung cancer cases as smoking is exceedingly common and other causes of death (such as ]s) are becoming ''less'' common, revealing an "iceberg" of pulmonary neoplasms. Cheap tobacco products and heavy advertising are seen by health campaigners as a major problem in these countries. | |||
| Lung cancer was uncommon before the advent of cigarette smoking. Surgeon ] recalled that as a ] medical student in 1919, his entire medical school class was summoned to witness an autopsy of a man who had died from lung cancer, and told they may never see such a case again.{{sfn|Spiro|Silvestri|2005|loc="Introduction"}}<!--Spiro says the year was 1910, but that must be a mistake as Ochsner would've been 14 years old at the time-->{{sfn|Blum|1999|p=102}} In ]'s 1912 ''Primary Malignant Growths of the Lungs and Bronchi'', he called lung cancer "among the rarest forms of disease";{{sfn|Adler|1912|p=3}} Adler tabulated the 374 cases of lung cancer that had been published to that time, concluding the disease was increasing in incidence.{{sfn|Proctor|2012|loc="Introduction"}} By the 1920s, several theories had been put forward linking the increase in lung cancer to various chemical exposures that had increased including tobacco smoke, asphalt dust, industrial air pollution, and poisonous gasses from World War I.{{sfn|Proctor|2012|loc="Introduction"}} | |||
| Over the following decades, growing scientific evidence linked lung cancer to cigarette consumption. Through the 1940s and early 1950s, several ] showed that those with lung cancer were more likely to have smoked cigarettes compared to those without lung cancer.{{sfn|Proctor|2012|loc="Population studies"}} These were followed by several ] in the 1950s – including the first report of the ] in 1954 – all of which showed that those who smoked tobacco were at dramatically increased risk of developing lung cancer.{{sfn|Proctor|2012|loc="Population studies"}} | |||
| ==Prevention== | |||
| ]", an advertisement run in newspapers nationwide in January 1954 as part of Hill & Knowlton's campaign to cast doubt on the link between cigarettes and cancer]] | |||
| ===Primary prevention=== | |||
| A 1953 study showing that tar from cigarette smoke could cause tumors in mice attracted attention in the popular press, with features in '']'' and '']'' magazines. Facing public concern and falling stock prices, the ]s of six of the largest American tobacco companies gathered in December 1953.{{sfn|Proctor|2012|loc="Animal experimentation"}} They enlisted the help of public relations firm ] to craft a multi-pronged strategy aiming to distract from accumulating evidence by funding tobacco-friendly research, declaring the link to lung cancer "controversial", and demanding ever-more research to settle this purported controversy.{{sfn|Proctor|2012|loc="Animal experimentation"}}{{sfn|Brandt|2012|loc="Industry response to emerging tobacco science"}} At the same time, internal research at the major tobacco companies supported the link between tobacco and lung cancer; though these results were kept secret from the public.{{sfn|Proctor|2012|loc="Cancer-causing chemicals in cigarette smoke"}} | |||
| Prevention is the most cost-effective means of fighting lung cancer on the national and global scales. While in most countries industrial and domestic carcinogens have been identified and banned, tobacco smoking is still widespread. Eliminating tobacco smoking is a primary goal in the fight to prevent lung cancer, and ] is the most important preventative tool in this process. | |||
| As evidence linking tobacco use with lung cancer mounted, various health bodies announced official positions linking the two. In 1962, the United Kingdom's ] officially concluded that cigarette smoking causes lung cancer, prompting the ] to empanel (enroll or enlist) an advisory committee, which deliberated in secret over nine sessions between November 1962 and December 1963.{{sfn|Hall|2022|loc="Establishing the advisory committee to the US Surgeon General"}} ], published in January 1964, firmly concluded that cigarette smoking "far outweighs all other factors" in causing lung cancer.{{sfn|Hall|2022|loc="Cigarette smoking and lung cancer"}} The report received substantial coverage in the popular press, and is widely seen as a turning point for public recognition that tobacco smoking causes lung cancer.{{sfn|Hall|2022|loc="Establishing the advisory committee to the US Surgeon General"}}{{sfn|Parascandola|2020|loc="Introduction"}} | |||
| Policy interventions to decrease ] (e.g. in restaurants and workplaces) have become more common in various Western countries, with ] taking a lead in banning smoking in public establishments in ], ] playing a similar role in ] in ], followed by ] and ] in 2005 and ] as well as several others in 2006. ] has also recently banned smoking in public places. (See ]). | |||
| The connection with ] gas was first recognized among miners in Germany's ]. As early as 1500, miners were noted to develop a deadly disease called "mountain sickness" ("Bergkrankheit"), identified as lung cancer by the late 19th century.{{sfn|Witschi|2001|p=2}}{{sfn|Mc Laughlin|2012|loc="Miner epidemiological studies"}} By 1938, up to 80% of miners in affected regions died from the disease.{{sfn|Witschi|2001|p=2}} In the 1950s radon and its breakdown products became established as causes of lung cancer in miners. Based largely on studies of miners, the International Agency for Research on Cancer classified radon as "carcinogenic to humans" in 1988.{{sfn|Mc Laughlin|2012|loc="Miner epidemiological studies"}} In 1956, a study revealed radon in Swedish residences. Over the following decades, high radon concentrations were found in residences across the world; by the 1980s many countries had established national radon programs to catalog and mitigate residential radon.{{sfn|Mc Laughlin|2012|loc="Residential radon epidemiology"}} | |||
| Only the Asian state of ] has a complete ] (since 2005). In many countries pressure groups are campaigning for similar bans. Arguments cited against such bans is ] of smoking, increased risk of ] and the risk that such a ban cannot be enforced. | |||
| The first successful ] for lung cancer was performed in 1933 by ] at ] in St. Louis, Missouri.{{sfn|Horn|Johnson|2008|loc="Introduction"}} Over the following decades, surgical development focused on sparing as much healthy lung tissue as possible, with the ] surpassing the pneumectomy in frequency by the 1960s, and the wedge resection appearing in the early 1970s.{{sfn|Walcott-Sapp|Sukumar|2016|loc="Evolution of Indications and Operative Technique"}}{{sfn|Spiro|Silvestri|2005|loc="Surgery"}} This trend continued with the development of ] in the 1980s, now widely performed for many lung cancer surgeries.{{sfn|Walcott-Sapp|Sukumar|2016|loc="A Delayed Entrance to the Modern Era of Minimally Invasive Lung Resection"}} | |||
| ===Screening and secondary prevention=== | |||
| Regular chest radiography and sputum examination programs were not effective in reducing mortality from lung cancer.<ref> Screening for lung cancer, The Cochrane Database of Systematic Reviews 2007</ref> Earlier studies (Mayo Lung Project and Czechoslovakia lung cancer screening study, combining over 17,000 smokers) showed earlier detection of lung cancer was possible but mortality was not improved. Simply detecting a tumor at an earlier stage may not necessarily yield improved mortality. For example, plain radiography resulted in increased time from diagnosis of cancer until death and those cancers being detected by screening tended to be earlier stages. However, these patients continued to die at the same rate as those who are not screened. At present, no professional or specialty organization advocates screening for lung cancer outside of clinical trials. | |||
| ==Research== | |||
| A ] (CT) scan can uncover tumors not yet visible on an X-ray. CT scanning is now being actively evaluated as a screening tool for lung cancer in high risk patients, and it is showing promising results. The USA-based ] is currently completing a randomized trial comparing CT scans with chest radiographs. Several single-institution trials are ongoing around the world. | |||
| While lung cancer is the deadliest type of cancer, it receives the third-most funding from the US ] (NCI, the world's largest cancer research funder) behind ]s and ].<ref>{{cite web|url=https://www.cancer.gov/about-nci/budget/fact-book/data/research-funding |accessdate=22 April 2023 |title=Funding for Research Areas |date=10 May 2022 |publisher=National Cancer Institute}}</ref> Despite high levels of gross research funding, lung cancer funding per death lags behind many other cancers, with around $3,200 spent on lung cancer research in 2022 per US death, considerably lower than that for brain cancer ($22,000 per death), breast cancer ($14,000 per death), and cancer as a whole ($11,000 per death).<ref>{{cite web|url=https://report.nih.gov/funding/categorical-spending#/ |accessdate=30 April 2023 |title=Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC) |date=31 March 2023 |publisher=US ]}}</ref> A similar trend holds for private ]s. Annual revenues of lung cancer-focused nonprofits rank fifth among cancer types, but lung cancer nonprofits have lower revenue than would be expected for the number of lung cancer cases, deaths, and potential years of life lost.{{sfn|Kamath|Kircher|Benson|2019|loc="Results"}} | |||
| The International Early Lung Cancer Action Project published the results of CT screening on over 31,000 high-risk patients in late 2006 in the New England Journal of Medicine.<ref>Henschke CI, et al, Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006 Oct 26;355(17):1763-71. PMID 17065637</ref> In this study 85% of the 484 detected lung cancers were stage I and thus highly treatable. Mathematically these stage I patients would have an expected 10-year survival of 88%. However, there was no randomization of patients (all received CT scans and there was no comparison group receiving only x-rays) and the patients were not actually followed out to 10 years post detection (the median followup was 40 months). | |||
| Despite this, many investigational lung cancer treatments are undergoing ]s – with nearly 2,250 active clinical trials registered as of 2021.{{sfn|Batra|Pawar|Bahl|2021|loc="Practice Points"}} Of these, a large plurality are testing radiotherapy regimens (26% of trials) and surgical techniques (22%). Many others are testing targeted anticancer drugs, with targets including EGFR (17% of trials), ]s (12%), VEGF (12%), immune pathways (10%), mTOR (1%), and ]s (<1%).{{sfn|Batra|Pawar|Bahl|2021|loc="Figure 2: Types of treatment for lung cancer in clinical trials, Phase I-IV"}} | |||
| In contrast, a March 2007 study in JAMA found no benefit. 3,200 current or former smokers were screened for 4 years and offered 3 or 4 CT scans. Lung cancer diagnoses were 3 times as high, and surgeries were 10 times as high, as predicted by a model, but there were no significant differences between observed and expected numbers of advanced cancers or deaths.<ref> Peter B. Bach, et al., Computed Tomography Screening and Lung Cancer Outcomes, JAMA. 2007;297:953-961. (March 7, 2007)</ref> | |||
| == References == | |||
| Randomized controlled studies are underway in this area to see if decreased long-term mortality can be directly observed from CT screening.{{Fact|date=March 2007}} | |||
| {{Reflist}} | |||
| ===Cited=== | |||
| It should be noted that screening studies have only been done in high risk populations, such as smokers and workers with occupational exposure to certain substances. This is important when one considers that repeated radiation exposure from screening could actually induce carcinogenesis in a small percentage of screened subjects, so this risk should be mitigated by a (relatively) high prevalence of lung cancer in the population being screened. | |||
| {{refbegin|32em}} | |||
| '''Books''' | |||
| * {{Cite book | vauthors = Adler I | year=1912 | title=Primary Malignant Growths of the Lungs and Bronchi | place= New York | publisher=Longmans, Green, and Company | oclc=14783544 | ol=24396062M }} | |||
| * {{cite book |title= Murray & Nadel's Textbook of Respiratory Medicine|edition=7th |date=2022 |publisher=Elsevier |veditors= Broaddus C, Ernst JD, King TE, ''et al''|isbn=978-0323655873|ref=none}} | |||
| **{{cite book|vauthors=Balmes JR, Holm SM |chapter=Indoor and Outdoor Air Pollution |title=Murray & Nadel's Textbook of Respiratory Medicine |edition=7th |date=2022 |publisher=Elsevier |pages=1423–1434 |veditors= Broaddus C, Ernst JD, King TE, ''et al''}} | |||
| ** {{cite book |vauthors=Christiani DC, Amos CI |chapter=Lung Cancer: Epidemiology |title=Murray & Nadel's Textbook of Respiratory Medicine |edition=7th |date=2022 |publisher=Elsevier |pages=1018–1028 |veditors= Broaddus C, Ernst JD, King TE, ''et al''}} | |||
| ** {{cite book|vauthors=Massion PP, Lehman JM |chapter=Lung Cancer: Molecular Biology and Targets |title=Murray & Nadel's Textbook of Respiratory Medicine |edition=7th |date=2022 |publisher=Elsevier |pages=1005–1017 |veditors= Broaddus C, Ernst JD, King TE, ''et al''}} | |||
| ** {{cite book|vauthors=Pastis NJ, Gonzalez AV, Silvestri GA |chapter=Lung Cancer: Diagnosis and Staging |title=Murray & Nadel's Textbook of Respiratory Medicine |edition=7th |date=2022 |publisher=Elsevier |pages=1039–1051 |veditors= Broaddus C, Ernst JD, King TE, ''et al''}} | |||
| ** {{cite book|vauthors=Rivera P, Mody GN, Weiner AA |chapter=Lung Cancer: Treatment |title=Murray & Nadel's Textbook of Respiratory Medicine |edition=7 |date=2022 |publisher=Elsevier |pages=1052–1065 |veditors= Broaddus C, Ernst JD, King TE, ''et al''}} | |||
| ** {{cite book|vauthors=Tanoue L, Mazzone PJ, Tanner NT |chapter=Lung Cancer: Screening |title=Murray & Nadel's Textbook of Respiratory Medicine |edition=7th |date=2022 |publisher=Elsevier |pages=1029–1038 |veditors= Broaddus C, Ernst JD, King TE, ''et al''}} | |||
| * {{Cite book |url=https://data.europa.eu/doi/10.2777/867180 |title=Cancer screening in the European Union |date=2022 |publisher=Publications Office of the European Union |isbn=978-92-76-45603-2 |doi=10.2777/867180 |ref={{harvid|Cancer screening in the European Union|2022}} |author1=European Commission. Directorate General for Research and Innovation. |author2=European Commission Group of Chief Scientific Advisors. }} | |||
| * {{cite book|vauthors=Horn L, Iams WT |chapter=78: Neoplasms of the Lung |title=] |edition=21st |publisher=McGraw Hill |date=2022|veditors= Loscalzo J, Fauci A, Kasper D, ''et al'' |isbn= 978-1264268504}} | |||
| * {{cite book|veditors=Bast RC, Byrd JC, Croce CM, ''et al'' |title=Holland-Frei Cancer Medicine |edition=10th |isbn=978-1-119-75068-0 |date=April 2023 |publisher=Wiley |chapter=80: Cancer of the Lung |vauthors=Morgensztern D, Boffa D, Chen A, Dhanasopon A, Goldberg SB, Decker RH, Devarakonda S, Ko JP, Solis Soto LM, Waqar SN, Wistuba II, Herbst RS}} | |||
| * {{cite book|vauthors=Salahuddin M, Ost DE |chapter=110: Approach to the Patient with Pulmonary Nodules |publisher=McGraw Hill |title=Fishman's Pulmonary Diseases and Disorders |edition=6th |veditors=Grippi MA, Antin-Ozerkis DE, Dela Cruz CS, ''et al''|isbn=978-1260473988 |year=2023}} | |||
| '''Journal articles''' | |||
| ==References== | |||
| * {{cite journal |vauthors=Alexander M, Kim SY, Cheng H |title=Update 2020: Management of Non-Small Cell Lung Cancer |journal=Lung |volume=198 |issue=6 |pages=897–907 |date=December 2020 |pmid=33175991 |pmc=7656891 |doi=10.1007/s00408-020-00407-5 }} | |||
| <!--See http://en.wikipedia.org/Wikipedia:Footnotes for an explanation of how to generate footnotes using the <ref(erences/)> tags--> | |||
| * {{cite journal |vauthors=Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, ((Azevedo e Silva G)), Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP |display-authors=6|title=Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries |journal=Lancet |volume=391 |issue=10125 |pages=1023–1075 |date=March 2018 |pmid=29395269 |pmc=5879496 |doi=10.1016/S0140-6736(17)33326-3 |url=}} | |||
| <div class="references-small"><references/></div> | |||
| * {{cite journal |vauthors=Aragon KN |title=Palliative Care in Lung Cancer |journal=Clin Chest Med |volume=41 |issue=2 |pages=281–293 |date=June 2020 |pmid=32402363 |doi=10.1016/j.ccm.2020.02.005 |s2cid=218633948 }} | |||
| * {{cite journal |vauthors=Arnott D, Lindorff K, Goddard A |title=Tobacco control: the FCTC provides the route to the finish line |journal=Lancet |volume=400 |issue=10350 |pages=427 |date=August 2022 |pmid=35878621 |doi=10.1016/S0140-6736(22)01334-4 |s2cid=250960604 |url=|doi-access=free }} | |||
| * {{cite journal |vauthors=Bade BC, Dela Cruz CS |title=Lung Cancer 2020: Epidemiology, Etiology, and Prevention |journal=Clin Chest Med |volume=41 |issue=1 |pages=1–24 |date=March 2020 |pmid=32008623 |doi=10.1016/j.ccm.2019.10.001|s2cid=211015015 }} | |||
| * {{cite journal | vauthors=Batra H, Pawar S, Bahl D | title=Current clinical trials and patent update on lung cancer: a retrospective review | journal=Lung Cancer Management | volume=10 | issue=5 | pages=LMT45 | date=February 2021 | pmid=34084211 | pmc=8162165 | doi=10.2217/lmt-2020-0029 }} | |||
| * {{cite journal |vauthors=Blum A |title=Alton ochsner, MD, 1896–1981 anti-smoking pioneer |journal=Ochsner J |volume=1 |issue=3 |pages=102–105 |date=July 1999 |pmid=21845126 |pmc=3145444 |doi= |url=}} | |||
| * {{cite journal | vauthors = Bracken-Clarke D, Kapoor D, Baird AM, Buchanan PJ, Gately K, Cuffe S, Finn SP | title = Vaping and lung cancer – A review of current data and recommendations | journal = Lung Cancer | volume = 153 | pages = 11–20 | date = March 2021 | pmid = 33429159 | doi = 10.1016/j.lungcan.2020.12.030 | s2cid = 231586192 | doi-access = }} | |||
| * {{cite journal |vauthors=Brandt AM |title=Inventing conflicts of interest: a history of tobacco industry tactics |journal=Am J Public Health |volume=102 |issue=1 |pages=63–71 |date=January 2012 |pmid=22095331 |pmc=3490543 |doi=10.2105/AJPH.2011.300292 }} | |||
| * {{Cite journal |last=Canadian Task Force on Preventive Health Care |date=April 2016 |title=Recommendations on screening for lung cancer |journal=CMAJ |volume=188 |issue=6 |pages=425–432 |doi=10.1503/cmaj.151421 |issn=0820-3946 |pmc=4818132 |pmid=26952527 |ref={{harvid|Canadian Task Force|2016}} }} | |||
| * {{cite journal |vauthors=Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V |display-authors=6|title=The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (8th) ed. of the TNM Classification for Lung Cancer |journal=J Thorac Oncol |volume=11 |issue=1 |pages=39–51 |date=January 2016 |pmid=26762738 |doi=10.1016/j.jtho.2015.09.009 |s2cid=5368645 |hdl=10044/1/31538 |hdl-access=free }} | |||
| * {{cite journal |vauthors=Hall W |title=The 1964 US Surgeon General's report on smoking and health |journal=Addiction |volume=117 |issue=12 |pages=3170–3175 |date=December 2022 |pmid=35852022 |doi=10.1111/add.16007 |s2cid=250642397 |doi-access=free }} | |||
| * {{cite journal | vauthors = Horn L, Johnson DH | title = Evarts A. Graham and the first pneumonectomy for lung cancer | journal = Journal of Clinical Oncology | volume = 26 | issue = 19 | pages = 3268–3275 | date = July 2008 | pmid = 18591561 | doi = 10.1200/JCO.2008.16.8260 | url = http://jco.ascopubs.org/cgi/pdf_extract/26/19/3268 | access-date = 20 March 2009 | archive-date = 17 March 2020 | archive-url = https://web.archive.org/web/20200317080747/https://ascopubs.org/cgi/pdf_extract/26/19/3268 | url-status = dead }} | |||
| * {{cite journal |vauthors=Jassem J |title=Tobacco smoking after diagnosis of cancer: clinical aspects |journal=Translational Lung Cancer Research |volume=8 |date=May 2019 |issue=Suppl 1 |pages=S50–S58 |doi=10.21037/tlcr.2019.04.01|pmid=31211105 |pmc=6546630 |doi-access=free }} | |||
| * {{cite journal |vauthors=Jonas DE, Reuland DS, Reddy SM, Nagle M, Clark SD, Weber RP, Enyioha C, Malo TL, Brenner AT, Armstrong C, Coker-Schwimmer M, Middleton JC, Voisin C, Harris RP |title=Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force |journal=JAMA |volume=325 |issue=10 |pages=971–987 |date=March 2021 |pmid=33687468 |doi=10.1001/jama.2021.0377 |s2cid=232159404 |url=|doi-access=free }} | |||
| * {{cite journal |vauthors=Jones KD |title=Whence lepidic?: the history of a Canadian neologism |journal=Arch Pathol Lab Med |volume=137 |issue=12 |pages=1822–1824 |date=December 2013 |pmid=23937575 |doi=10.5858/arpa.2013-0144-HP }} | |||
| * {{cite journal |vauthors=Kamath SD, Kircher SM, Benson AB |title=Comparison of Cancer Burden and Nonprofit Organization Funding Reveals Disparities in Funding Across Cancer Types |journal=J Natl Compr Canc Netw |volume=17 |issue=7 |pages=849–854 |date=July 2019 |pmid=31319386 |doi=10.6004/jnccn.2018.7280 |s2cid=197666475 |url=|doi-access=free }} | |||
| * {{cite journal |vauthors=Lim RB |title=End-of-life care in patients with advanced lung cancer |journal=Ther Adv Respir Dis |volume=10 |issue=5 |pages=455–467 |date=October 2016 |pmid=27585597 |pmc=5933619 |doi=10.1177/1753465816660925}} | |||
| * {{cite journal | vauthors=Lim W, Ridge CA, Nicholson AG, Mirsadraee S | title=The 8th lung cancer TNM classification and clinical staging system: review of the changes and clinical implications | journal=Quantitative Imaging in Medicine and Surgery | volume=8 | issue=7 | pages=709–718 | date =August 2018 | pmc=6127520 | pmid=30211037 | doi=10.21037/qims.2018.08.02 | doi-access=free }} | |||
| * {{cite journal |vauthors=Mc Laughlin J |title=An historical overview of radon and its progeny: applications and health effects |journal=Radiat Prot Dosimetry |volume=152 |issue=1–3 |pages=2–8 |date=November 2012 |pmid=22914338 |doi=10.1093/rpd/ncs189 |url=}} | |||
| * {{cite journal |vauthors=Nasim F, Sabath BF, Eapen GA |title=Lung Cancer |journal=Med Clin North Am |volume=103 |issue=3 |pages=463–473 |date=May 2019 |pmid=30955514 |doi=10.1016/j.mcna.2018.12.006|s2cid=102349766 }} | |||
| * {{cite journal|vauthors=Obeng C, Folch E, Fernando Santacruz J |title=Management of malignant airway obstruction |date=December 2018 |volume=3 |doi=10.21037/amj.2018.11.06 |journal=AME Medical Journal|page=115 |s2cid=80791599 |doi-access=free }} | |||
| * {{cite journal|vauthors=Parascandola M |title=The other Surgeon General's report: history of the U.S. public health response to air pollution, cigarette smoking, and lung cancer |journal=Annals of Cancer Epidemiology |volume=4 |date=March 2020 |page=3 |doi=10.21037/ace.2020.03.01|s2cid=216205576 |doi-access=free }} | |||
| * {{cite journal |vauthors=Peruga A, López MJ, Martinez C, Fernández E |title=Tobacco control policies in the 21st century: achievements and open challenges |journal=Mol Oncol |volume=15 |issue=3 |pages=744–752 |date=March 2021 |pmid=33533185 |pmc=7931122 |doi=10.1002/1878-0261.12918 }} | |||
| * {{cite journal |vauthors=Proctor RN |title=The history of the discovery of the cigarette-lung cancer link: evidentiary traditions, corporate denial, global toll |journal=Tob Control |volume=21 |issue=2 |pages=87–91 |date=March 2012 |pmid=22345227 |doi=10.1136/tobaccocontrol-2011-050338 |s2cid=2734836 |url=|doi-access=free }} | |||
| * {{cite journal |vauthors=Rudin CM, Brambilla E, Faivre-Finn C, Sage J |title=Small-cell lung cancer |journal=Nat Rev Dis Primers |volume=7 |issue=1 |page=3 |date=January 2021 |pmid=33446664 |pmc=8177722 |doi=10.1038/s41572-020-00235-0 }} | |||
| * {{cite journal |vauthors=Schabath MB, Cote ML |title=Cancer Progress and Priorities: Lung Cancer |journal=Cancer Epidemiol Biomarkers Prev |volume=28 |issue=10 |pages=1563–1579 |date=October 2019 |pmid=31575553 |pmc=6777859 |doi=10.1158/1055-9965.EPI-19-0221 }} | |||
| * {{cite journal |vauthors=Spencer K, Parrish R, Barton R, Henry A |title=Palliative radiotherapy |journal=BMJ |volume=360 |issue= |page=k821 |date=March 2018 |pmid=29572337 |pmc=5865075 |doi=10.1136/bmj.k821 }} | |||
| * {{cite journal | vauthors = Spiro SG, Silvestri GA | title = One hundred years of lung cancer | journal = American Journal of Respiratory and Critical Care Medicine | volume = 172 | issue = 5 | pages = 523–529 | date = September 2005 | pmid = 15961694 | doi = 10.1164/rccm.200504-531OE }} | |||
| * {{cite journal | vauthors = Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F | title = Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries | journal = CA: A Cancer Journal for Clinicians | volume = 71 | issue = 3 | pages = 209–249 | date = May 2021 | pmid = 33538338 | doi = 10.3322/caac.21660 | doi-access = free }} | |||
| * {{cite journal |vauthors=Temel JS, Petrillo LA, Greer JA |title=Patient-Centered Palliative Care for Patients With Advanced Lung Cancer |journal=J Clin Oncol |volume=40 |issue=6 |pages=626–634 |date=February 2022 |pmid=34985932 |doi=10.1200/JCO.21.01710 |s2cid=245772225 }} | |||
| * {{cite journal |vauthors=Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS |title=Lung cancer |journal=Lancet |volume=398 |issue=10299 |pages=535–554 |date=August 2021 |pmid=34273294 |doi=10.1016/S0140-6736(21)00312-3|s2cid=236034814 }} | |||
| * {{cite journal |vauthors=Walcott-Sapp S, Sukumar M |title=The history of pulmonary lobectomy: Two phases of innovation |journal=CTSNet |date=8 December 2016 |url=https://www.ctsnet.org/article/history-pulmonary-lobectomy-two-phases-innovation |accessdate=28 April 2023 }} | |||
| * {{cite journal | vauthors = Witschi H | title = A short history of lung cancer | journal = Toxicological Sciences | volume = 64 | issue = 1 | pages = 4–6 | date = November 2001 | pmid = 11606795 | doi = 10.1093/toxsci/64.1.4 | doi-access = }} | |||
| {{refend}} | |||
| == External links == | == External links == | ||
| {{commons category|Cancers of bronchus and lung}} | |||
| * | |||
| {{wikiquote}} | |||
| * at the National Cancer Institute. | |||
| * by the ]. | |||
| * Stop Smoking Articles & Information | |||
| * - The radiology information resource for patients with lung cancer | |||
| {{Medical resources | |||
| {{Tumors}} | |||
| |DiseasesDB = 7616 | |||
| |ICD11 = {{ICD11|2C24}}, {{ICD11|2C25}} | |||
| |ICD10 = {{ICD10|C33}}, {{ICD10|C34}} | |||
| |ICD9 = {{ICD9|162}} | |||
| |ICDO = | |||
| |OMIM =211980 | |||
| |MedlinePlus = 007194 | |||
| |eMedicineSubj = med | |||
| |eMedicineTopic = 1333 | |||
| |eMedicine_mult = {{eMedicine2|med|1336}} {{eMedicine2|emerg|335}} {{eMedicine2|radio|807}} {{eMedicine2|radio|405}} {{eMedicine2|radio|406}} | |||
| |MeshID = D002283 | |||
| }} | |||
| {{Respiratory tract neoplasia}} | |||
| {{Authority control}} | |||
| {{DEFAULTSORT:Lung cancer}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 12:48, 29 November 2024
Malignant tumor characterized by uncontrolled cell growth in lung tissueThis article is about lung carcinomas. For other lung tumors, see Lung tumor.
Medical condition
| Lung cancer | |
|---|---|
| Other names | Lung carcinoma |
 | |
| A chest X-ray showing a tumor in the lung (marked by arrow) | |
| Specialty | Oncology, pulmonology |
| Symptoms | Coughing (including coughing up blood), shortness of breath, chest pain |
| Usual onset | After age 40; 70 years on average |
| Types | Small-cell lung carcinoma (SCLC), non-small-cell lung carcinoma (NSCLC) |
| Risk factors |
|
| Diagnostic method | Medical imaging, tissue biopsy |
| Prevention | Avoid smoking and other environmental mutagens |
| Treatment | Surgery, chemotherapy, radiotherapy, molecular therapies, immune checkpoint inhibitors |
| Prognosis | Five-year survival rate: 10 to 20% (most countries) |
| Frequency | 2.2 million (2020) |
| Deaths | 1.8 million (2020) |
Lung cancer, also known as lung carcinoma, is a malignant tumor that begins in the lung. Lung cancer is caused by genetic damage to the DNA of cells in the airways, often caused by cigarette smoking or inhaling damaging chemicals. Damaged airway cells gain the ability to multiply unchecked, causing the growth of a tumor. Without treatment, tumors spread throughout the lung, damaging lung function. Eventually lung tumors metastasize, spreading to other parts of the body.
Early lung cancer often has no symptoms and can only be detected by medical imaging. As the cancer progresses, most people experience nonspecific respiratory problems: coughing, shortness of breath, or chest pain. Other symptoms depend on the location and size of the tumor. Those suspected of having lung cancer typically undergo a series of imaging tests to determine the location and extent of any tumors. Definitive diagnosis of lung cancer requires a biopsy of the suspected tumor be examined by a pathologist under a microscope. In addition to recognizing cancerous cells, a pathologist can classify the tumor according to the type of cells it originates from. Around 15% of cases are small-cell lung cancer (SCLC), and the remaining 85% (the non-small-cell lung cancers or NSCLC) are adenocarcinomas, squamous-cell carcinomas, and large-cell carcinomas. After diagnosis, further imaging and biopsies are done to determine the cancer's stage based on how far it has spread.
Treatment for early stage lung cancer includes surgery to remove the tumor, sometimes followed by radiation therapy and chemotherapy to kill any remaining cancer cells. Later stage cancer is treated with radiation therapy and chemotherapy alongside drug treatments that target specific cancer subtypes. Even with treatment, only around 20% of people survive five years on from their diagnosis. Survival rates are higher in those diagnosed at an earlier stage, diagnosed at a younger age, and in women compared to men.
Most lung cancer cases are caused by tobacco smoking. The remainder are caused by exposure to hazardous substances like asbestos and radon gas, or by genetic mutations that arise by chance. Consequently, lung cancer prevention efforts encourage people to avoid hazardous chemicals and quit smoking. Quitting smoking both reduces one's chance of developing lung cancer and improves treatment outcomes in those already diagnosed with lung cancer.
Lung cancer is the most diagnosed and deadliest cancer worldwide, with 2.2 million cases in 2020 resulting in 1.8 million deaths. Lung cancer is rare in those younger than 40; the average age at diagnosis is 70 years, and the average age at death 72. Incidence and outcomes vary widely across the world, depending on patterns of tobacco use. Prior to the advent of cigarette smoking in the 20th century, lung cancer was a rare disease. In the 1950s and 1960s, increasing evidence linked lung cancer and tobacco use, culminating in declarations by most large national health bodies discouraging tobacco use.
Signs and symptoms
Early lung cancer often has no symptoms. When symptoms do arise they are often nonspecific respiratory problems – coughing, shortness of breath, or chest pain – that can differ from person to person. Those who experience coughing tend to report either a new cough, or an increase in the frequency or strength of a pre-existing cough. Around one in four cough up blood, ranging from small streaks in the sputum to large amounts. Around half of those diagnosed with lung cancer experience shortness of breath, while 25–50% experience a dull, persistent chest pain that remains in the same location over time. In addition to respiratory symptoms, some experience systemic symptoms including loss of appetite, weight loss, general weakness, fever, and night sweats.
Some less common symptoms suggest tumors in particular locations. Tumors in the thorax can cause breathing problems by obstructing the trachea or disrupting the nerve to the diaphragm; difficulty swallowing by compressing the esophagus; hoarseness by disrupting the nerves of the larynx; and Horner's syndrome by disrupting the sympathetic nervous system. Horner's syndrome is also common in tumors at the top of the lung, known as Pancoast tumors, which also cause shoulder pain that radiates down the little-finger side of the arm as well as destruction of the topmost ribs. Swollen lymph nodes above the collarbone can indicate a tumor that has spread within the chest. Tumors obstructing bloodflow to the heart can cause superior vena cava syndrome (swelling of the upper body and shortness of breath), while tumors infiltrating the area around the heart can cause fluid buildup around the heart, arrhythmia (irregular heartbeat), and heart failure.
About one in three people diagnosed with lung cancer have symptoms caused by metastases in sites other than the lungs. Lung cancer can metastasize anywhere in the body, with different symptoms depending on the location. Brain metastases can cause headache, nausea, vomiting, seizures, and neurological deficits. Bone metastases can cause pain, bone fractures, and compression of the spinal cord. Metastasis into the bone marrow can deplete blood cells and cause leukoerythroblastosis (immature cells in the blood). Liver metastases can cause liver enlargement, pain in the right upper quadrant of the abdomen, fever, and weight loss.
Lung tumors often cause the release of body-altering hormones, which cause unusual symptoms, called paraneoplastic syndromes. Inappropriate hormone release can cause dramatic shifts in concentrations of blood minerals. Most common is hypercalcemia (high blood calcium) caused by over-production of parathyroid hormone-related protein or parathyroid hormone. Hypercalcemia can manifest as nausea, vomiting, abdominal pain, constipation, increased thirst, frequent urination, and altered mental status. Those with lung cancer also commonly experience hypokalemia (low potassium) due to inappropriate secretion of adrenocorticotropic hormone, as well as hyponatremia (low sodium) due to overproduction of antidiuretic hormone or atrial natriuretic peptide. About one of three people with lung cancer develop nail clubbing, while up to one in ten experience hypertrophic pulmonary osteoarthropathy (nail clubbing, joint soreness, and skin thickening). A variety of autoimmune disorders can arise as paraneoplastic syndromes in those with lung cancer, including Lambert–Eaton myasthenic syndrome (which causes muscle weakness), sensory neuropathies, muscle inflammation, brain swelling, and autoimmune deterioration of cerebellum, limbic system, or brainstem. Up to one in twelve people with lung cancer have paraneoplastic blood clotting, including migratory venous thrombophlebitis, clots in the heart, and disseminated intravascular coagulation (clots throughout the body). Paraneoplastic syndromes involving the skin and kidneys are rare, each occurring in up to 1% of those with lung cancer.
Diagnosis

A person suspected of having lung cancer will have imaging tests done to evaluate the presence, extent, and location of tumors. First, many primary care providers perform a chest X-ray to look for a mass inside the lung. The X-ray may reveal an obvious mass, the widening of the mediastinum (suggestive of spread to lymph nodes there), atelectasis (lung collapse), consolidation (pneumonia), or pleural effusion; however, some lung tumors are not visible by X-ray. Next, many undergo computed tomography (CT) scanning, which can reveal the sizes and locations of tumors.
A definitive diagnosis of lung cancer requires a biopsy of the suspected tissue be histologically examined for cancer cells. Given the location of lung cancer tumors, biopsies can often be obtained by minimally invasive techniques: a fiberoptic bronchoscope that can retrieve tissue (sometimes guided by endobronchial ultrasound), fine needle aspiration, or other imaging-guided biopsy through the skin. Those who cannot undergo a typical biopsy procedure may instead have a liquid biopsy taken (that is, a sample of some body fluid) which may contain circulating tumor DNA that can be detected.
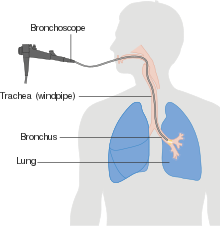
Imaging is also used to assess the extent of cancer spread. Positron emission tomography (PET) scanning or combined PET-CT scanning is often used to locate metastases in the body. Since PET scanning is less sensitive in the brain, the National Comprehensive Cancer Network recommends magnetic resonance imaging (MRI) – or CT where MRI is unavailable – to scan the brain for metastases in those with NSCLC and large tumors, or tumors that have spread to the nearby lymph nodes. When imaging suggests the tumor has spread, the suspected metastasis is often biopsied to confirm that it is cancerous. Lung cancer most commonly metastasizes to the brain, bones, liver, and adrenal glands.
Lung cancer can often appear as a solitary pulmonary nodule on a chest radiograph or CT scan. In lung cancer screening studies as many as 30% of those screened have a lung nodule, the majority of which turn out to be benign. Besides lung cancer many other diseases can also give this appearance, including hamartomas, and infectious granulomas caused by tuberculosis, histoplasmosis, or coccidioidomycosis.
Classification


At diagnosis, lung cancer is classified based on the type of cells the tumor is derived from; tumors derived from different cells progress and respond to treatment differently. There are two main types of lung cancer, categorized by the size and appearance of the malignant cells seen by a histopathologist under a microscope: small cell lung cancer (SCLC; 15% of cases) and non-small-cell lung cancer (NSCLC; 85% of cases). SCLC tumors are often found near the center of the lungs, in the major airways. Their cells appear small with ill-defined boundaries, not much cytoplasm, many mitochondria, and have distinctive nuclei with granular-looking chromatin and no visible nucleoli. NSCLCs comprise a group of three cancer types: adenocarcinoma, squamous-cell carcinoma, and large-cell carcinoma. Nearly 40% of lung cancers are adenocarcinomas. Their cells grow in three-dimensional clumps, resemble glandular cells, and may produce mucin. About 30% of lung cancers are squamous-cell carcinomas. They typically occur close to large airways. The tumors consist of sheets of cells, with layers of keratin. A hollow cavity and associated cell death are commonly found at the center of the tumor. Less than 10% of lung cancers are large-cell carcinomas, so named because the cells are large, with excess cytoplasm, large nuclei, and conspicuous nucleoli. Around 10% of lung cancers are rarer types. These include mixes of the above subtypes like adenosquamous carcinoma, and rare subtypes such as carcinoid tumors, and sarcomatoid carcinomas.
Several lung cancer types are subclassified based on the growth characteristics of the cancer cells. Adenocarcinomas are classified as lepidic (growing along the surface of intact alveolar walls), acinar and papillary, or micropapillary and solid pattern. Lepidic adenocarcinomas tend to be least aggressive, while micropapillary and solid pattern adenocarcinomas are most aggressive.
In addition to examining cell morphology, biopsies are often stained by immunohistochemistry to confirm lung cancer classification. SCLCs bear the markers of neuroendocrine cells, such as chromogranin, synaptophysin, and CD56. Adenocarcinomas tend to express Napsin-A and TTF-1; squamous cell carcinomas lack Napsin-A and TTF-1, but express p63 and its cancer-specific isoform p40. CK7 and CK20 are also commonly used to differentiate lung cancers. CK20 is found in several cancers, but typically absent from lung cancer. CK7 is present in many lung cancers, but absent from squamous cell carcinomas.
Staging
See also: Lung cancer staging| TNM | Stage group |
|---|---|
| T1a N0 M0 | IA1 |
| T1b N0 M0 | IA2 |
| T1c N0 M0 | IA3 |
| T2a N0 M0 | IB |
| T2b N0 M0 | IIA |
| T1–T2 N1 M0 | IIB |
| T3 N0 M0 | |
| T1–T2 N2 M0 | IIIA |
| T3 N1 M0 | |
| T4 N0–N1 M0 | |
| T1–T2 N3 M0 | IIIB |
| T3–T4 N2 M0 | |
| T3–T4 N3 M0 | IIIC |
| Any T, any N, M1a–M1b | IVA |
| Any T, any N, M1c | IVB |
Lung cancer staging is an assessment of the degree of spread of the cancer from its original source. It is one of the factors affecting both the prognosis and the treatment of lung cancer.
SCLC is typically staged with a relatively simple system: limited stage or extensive stage. Around a third of people are diagnosed at the limited stage, meaning cancer is confined to one side of the chest, within the scope of a single radiotherapy field. The other two thirds are diagnosed at the "extensive stage", with cancer spread to both sides of the chest, or to other parts of the body.
NSCLC – and sometimes SCLC – is typically staged with the American Joint Committee on Cancer's Tumor, Node, Metastasis (TNM) staging system. The size and extent of the tumor (T), spread to regional lymph nodes (N), and distant metastases (M) are scored individually, and combined to form stage groups.
Relatively small tumors are designated T1, which are subdivided by size: tumors ≤ 1 centimeter (cm) across are T1a; 1–2 cm T1b; 2–3 cm T1c. Tumors up to 5 cm across, or those that have spread to the visceral pleura (tissue covering the lung) or main bronchi, are designated T2. T2a designates 3–4 cm tumors; T2b 4–5 cm tumors. T3 tumors are up to 7 cm across, have multiple nodules in the same lobe of the lung, or invade the chest wall, diaphragm (or the nerve that controls it), or area around the heart. Tumors that are larger than 7 cm, have nodules spread in different lobes of a lung, or invade the mediastinum (center of the chest cavity), heart, largest blood vessels that supply the heart, trachea, esophagus, or spine are designated T4. Lymph node staging depends on the extent of local spread: with the cancer metastasized to no lymph nodes (N0), pulmonary or hilar nodes (along the bronchi) on the same side as the tumor (N1), mediastinal or subcarinal lymph nodes (in the middle of the lungs, N2), or lymph nodes on the opposite side of the lung from the tumor (N3). Metastases are staged as no metastases (M0), nearby metastases (M1a; the space around the lung or the heart, or the opposite lung), a single distant metastasis (M1b), or multiple metastases (M1c).
These T, N, and M scores are combined to designate a stage grouping for the cancer. Cancer limited to smaller tumors is designated stage I. Disease with larger tumors or spread to the nearest lymph nodes is stage II. Cancer with the largest tumors or extensive lymph node spread is stage III. Cancer that has metastasized is stage IV. Each stage is further subdivided based on the combination of T, N, and M scores.
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Screening
Main article: Lung cancer screeningSome countries recommend that people who are at a high risk of developing lung cancer be screened at different intervals using low-dose CT lung scans. Screening programs may result in early detection of lung tumors in people who are not yet experiencing symptoms of lung cancer, ideally, early enough that the tumors can be successfully treated and result in decreased mortality. There is evidence that regular low-dose CT scans in people at high risk of developing lung cancer reduces total lung cancer deaths by as much as 20%. Despite evidence of benefit in these populations, potential harms of screening include the potential for a person to have a 'false positive' screening result that may lead to unnecessary testing, invasive procedures, and distress. Although rare, there is also a risk of radiation-induced cancer. The United States Preventive Services Task Force recommends yearly screening using low-dose CT in people between 55 and 80 who have a smoking history of at least 30 pack-years. The European Commission recommends that cancer screening programs across the European Union be extended to include low-dose CT lung scans for current or previous smokers. Similarly, The Canadian Task Force for Preventative Health recommends that people who are current or former smokers (smoking history of more than 30 pack years) and who are between the ages of 55–74 years be screened for lung cancer.
Treatment
Main article: Treatment of lung cancerTreatment for lung cancer depends on the cancer's specific cell type, how far it has spread, and the person's health. Common treatments for early stage cancer includes surgical removal of the tumor, chemotherapy, and radiation therapy. For later-stage cancer, chemotherapy and radiation therapy are combined with newer targeted molecular therapies and immune checkpoint inhibitors. All lung cancer treatment regimens are combined with lifestyle changes and palliative care to improve quality of life.
Small-cell lung cancer

Limited-stage SCLC is typically treated with a combination of chemotherapy and radiotherapy. For chemotherapy, the National Comprehensive Cancer Network and American College of Chest Physicians guidelines recommend four to six cycles of a platinum-based chemotherapeutic – cisplatin or carboplatin – combined with either etoposide or irinotecan. This is typically combined with thoracic radiation therapy – 45 Gray (Gy) twice-daily – alongside the first two chemotherapy cycles. First-line therapy causes remission in up to 80% of those who receive it; however most people relapse with chemotherapy-resistant disease. Those who relapse are given second-line chemotherapies. Topotecan and lurbinectedin are approved by the US FDA for this purpose. Irinotecan, paclitaxel, docetaxel, vinorelbine, etoposide, and gemcitabine are also sometimes used, and are similarly efficacious. Prophylactic cranial irradiation can reduce the risk of brain metastases and improve survival in those with limited-stage disease.
Extensive-stage SCLC is treated first with etoposide along with either cisplatin or carboplatin. Radiotherapy is used only to shrink tumors that are causing particularly severe symptoms. Combining standard chemotherapy with an immune checkpoint inhibitor can improve survival for a minority of those affected, extending the average person's lifespan by around 2 months.
Non-small-cell lung cancer

For stage I and stage II NSCLC the first line of treatment is often surgical removal of the affected lobe of the lung. For those not well enough to tolerate full lobe removal, a smaller chunk of lung tissue can be removed by wedge resection or segmentectomy surgery. Those with centrally located tumors and otherwise-healthy respiratory systems may have more extreme surgery to remove an entire lung (pneumonectomy). Experienced thoracic surgeons, and a high-volume surgery clinic improve chances of survival. Those who are unable or unwilling to undergo surgery can instead receive radiation therapy. Stereotactic body radiation therapy is best practice, typically administered several times over 1–2 weeks. Chemotherapy has little effect in those with stage I NSCLC, and may worsen disease outcomes in those with the earliest disease. In those with stage II disease, chemotherapy is usually initiated six to twelve weeks after surgery, with up to four cycles of cisplatin – or carboplatin in those with kidney problems, neuropathy, or hearing impairment – combined with vinorelbine, pemetrexed, gemcitabine, or docetaxel.
Treatment for those with stage III NSCLC depends on the nature of their disease. Those with more limited spread may undergo surgery to have the tumor and affected lymph nodes removed, followed by chemotherapy and potentially radiotherapy. Those with particularly large tumors (T4) and those for whom surgery is impractical are treated with combination chemotherapy and radiotherapy along with the immunotherapy durvalumab. Combined chemotherapy and radiation enhances survival compared to chemotherapy followed by radiation, though the combination therapy comes with harsher side effects.
Those with stage IV disease are treated with combinations of pain medication, radiotherapy, immunotherapy, and chemotherapy. Many cases of advanced disease can be treated with targeted therapies depending on the genetic makeup of the cancerous cells. Up to 30% of tumors have mutations in the EGFR gene that result in an overactive EGFR protein; these can be treated with EGFR inhibitors osimertinib, erlotinib, gefitinib, afatinib, or dacomitinib – with osimertinib known to be superior to erlotinib and gefitinib, and all superior to chemotherapy alone. Up to 7% of those with NSCLC harbor mutations that result in hyperactive ALK protein, which can be treated with ALK inhibitors crizotinib, or its successors alectinib, brigatinib, and ceritinib. Those treated with ALK inhibitors who relapse can then be treated with the third-generation ALK inhibitor lorlatinib. Up to 5% with NSCLC have overactive MET, which can be inhibited with MET inhibitors capmatinib or tepotinib. Targeted therapies are also available for some cancers with rare mutations. Cancers with hyperactive BRAF (around 2% of NSCLC) can be treated by dabrafenib combined with the MEK inhibitor trametinib; those with activated ROS1 (around 1% of NSCLC) can be inhibited by crizotinib, lorlatinib, or entrectinib; overactive NTRK (<1% of NSCLC) by entrectinib or larotrectinib; active RET (around 1% of NSCLC) by selpercatinib.
People whose NSCLC is not targetable by current molecular therapies instead can be treated with combination chemotherapy plus immune checkpoint inhibitors, which prevent cancer cells from inactivating immune T cells. The chemotherapeutic agent of choice depends on the NSCLC subtype: cisplatin plus gemcitabine for squamous cell carcinoma, cisplatin plus pemetrexed for non-squamous cell carcinoma. Immune checkpoint inhibitors are most effective against tumors that express the protein PD-L1, but are sometimes effective in those that do not. Treatment with pembrolizumab, atezolizumab, or combination nivolumab plus ipilimumab are all superior to chemotherapy alone against tumors expressing PD-L1. Those who relapse on the above are treated with second-line chemotherapeutics docetaxel and ramucirumab.
Palliative care

Integrating palliative care (medical care focused on improving symptoms and lessening discomfort) into lung cancer treatment from the time of diagnosis improves the survival time and quality of life of those with lung cancer. Particularly common symptoms of lung cancer are shortness of breath and pain. Supplemental oxygen, improved airflow, re-orienting an affected person in bed, and low-dose morphine can all improve shortness of breath. In around 20 to 30% of those with lung cancer – particularly those with late-stage disease – growth of the tumor can narrow or block the airway, causing coughing and difficulty breathing. Obstructing tumors can be surgically removed where possible, though typically those with airway obstruction are not well enough for surgery. In such cases the American College of Chest Physicians recommends opening the airway by inserting a stent, attempting to shrink the tumor with localized radiation (brachytherapy), or physically removing the blocking tissue by bronchoscopy, sometimes aided by thermal or laser ablation. Other causes of lung cancer-associated shortness of breath can be treated directly, such as antibiotics for a lung infection, diuretics for pulmonary edema, benzodiazepines for anxiety, and steroids for airway obstruction.
Up to 92% of those with lung cancer report pain, either from tissue damage at the tumor site(s) or nerve damage. The World Health Organization (WHO) has developed a three-tiered system for managing cancer pain. For those with mild pain (tier one), the WHO recommends acetominophen or a nonsteroidal anti-inflammatory drug. Around a third of people experience moderate (tier two) or severe (tier three) pain, for which the WHO recommends opioid painkillers. Opioids are typically effective at easing nociceptive pain (pain caused by damage to various body tissues). Opioids are occasionally effective at easing neuropathic pain (pain caused by nerve damage). Neuropathic agents such as anticonvulsants, tricyclic antidepressants, and serotonin–norepinephrine reuptake inhibitors, are often used to ease neuropathic pain, either alone or in combination with opioids. In many cases, targeted radiotherapy can be used to shrink tumors, reducing pain and other symptoms caused by tumor growth.
Individuals who have advanced disease and are approaching end-of-life can benefit from dedicated end-of-life care to manage symptoms and ease suffering. As in earlier disease, pain and difficulty breathing are common, and can be managed with opioid pain medications, transitioning from oral medication to injected medication if the affected individual loses the ability to swallow. Coughing is also common, and can be managed with opioids or cough suppressants. Some experience terminal delirium – confused behavior, unexplained movements, or a reversal of the sleep-wake cycle – which can be managed by antipsychotic drugs, low-dose sedatives, and investigating other causes of discomfort such as low blood sugar, constipation, and sepsis. In the last few days of life, many develop terminal secretions – pooled fluid in the airways that can cause a rattling sound while breathing. This is thought not to cause respiratory problems, but can distress family members and caregivers. Terminal secretions can be reduced by anticholinergic medications. Even those who are non-communicative or have reduced consciousness may be able to experience cancer-related pain, so pain medications are typically continued until the time of death.
Prognosis

| Clinical stage | Five-year survival (%) |
|---|---|
| IA1 | 92 |
| IA2 | 83 |
| IA3 | 77 |
| IB | 68 |
| IIA | 60 |
| IIB | 53 |
| IIIA | 36 |
| IIIB | 26 |
| IIIC | 13 |
| IVA | 10 |
| IVB | 0 |
Around 19% of people diagnosed with lung cancer survive five years from diagnosis, though prognosis varies based on the stage of the disease at diagnosis and the type of lung cancer. Prognosis is better for people with lung cancer diagnosed at an earlier stage; those diagnosed at the earliest TNM stage, IA1 (small tumor, no spread), have a two-year survival of 97% and five-year survival of 92%. Those diagnosed at the most-advanced stage, IVB, have a two-year survival of 10% and a five-year survival of 0%. Five-year survival is higher in women (22%) than men (16%). Women tend to be diagnosed with less-advanced disease, and have better outcomes than men diagnosed at the same stage. Average five-year survival also varies across the world, with particularly high five-year survival in Japan (33%), and five-year survival above 20% in 12 other countries: Mauritius, Canada, the US, China, South Korea, Taiwan, Israel, Latvia, Iceland, Sweden, Austria, and Switzerland.
SCLC is particularly aggressive. 10–15% of people survive five years after a SCLC diagnosis. As with other types of lung cancer, the extent of disease at diagnosis also influences prognosis. The average person diagnosed with limited-stage SCLC survives 12–20 months from diagnosis; with extensive-stage SCLC around 12 months. While SCLC often responds initially to treatment, most people eventually relapse with chemotherapy-resistant cancer, surviving an average 3–4 months from the time of relapse. Those with limited stage SCLC that go into complete remission after chemotherapy and radiotherapy have a 50% chance of brain metastases developing within the next two years – a chance reduced by prophylactic cranial irradiation.
Several other personal and disease factors are associated with improved outcomes. Those diagnosed at a younger age tend to have better outcomes. Those who smoke or experience weight loss as a symptom tend to have worse outcomes. Tumor mutations in KRAS are associated with reduced survival.
Experience
The uncertainty of lung cancer prognosis often causes stress, and makes future planning difficult, for those with lung cancer and their families. Those whose cancer goes into remission often experience fear of their cancer returning or progressing, associated with poor quality of life, negative mood, and functional impairment. This fear is exacerbated by frequent or prolonged surveillance imaging, and other reminders of cancer risks.
Causes
Lung cancer is caused by genetic damage to the DNA of lung cells. These changes are sometimes random, but are typically induced by breathing in toxic substances such as cigarette smoke. Cancer-causing genetic changes affect the cell's normal functions, including cell proliferation, programmed cell death (apoptosis), and DNA repair. Eventually, cells gain enough genetic changes to grow uncontrollably, forming a tumor, and eventually spreading within and then beyond the lung. Rampant tumor growth and spread causes the symptoms of lung cancer. If unstopped, the spreading tumor will eventually cause the death of affected individuals.
Smoking

Tobacco smoking is by far the major contributor to lung cancer, causing 80% to 90% of cases. Lung cancer risk increases with quantity of cigarettes consumed. Tobacco smoking's carcinogenic effect is due to various chemicals in tobacco smoke that cause DNA mutations, increasing the chance of cells becoming cancerous. The International Agency for Research on Cancer identifies at least 50 chemicals in tobacco smoke as carcinogenic, and the most potent is tobacco-specific nitrosamines. Exposure to these chemicals causes several kinds of DNA damage: DNA adducts, oxidative stress, and breaks in the DNA strands. Being around tobacco smoke – called passive smoking – can also cause lung cancer. Living with a tobacco smoker increases one's risk of developing lung cancer by 24%. An estimated 17% of lung cancer cases in those who do not smoke are caused by high levels of environmental tobacco smoke.
Vaping may be a risk factor for lung cancer, but less than that of cigarettes, and further research as of 2021 is necessary due to the length of time it can take for lung cancer to develop following an exposure to carcinogens.
The smoking of non-tobacco products is not known to be associated with lung cancer development. Marijuana smoking does not seem to independently cause lung cancer – despite the relatively high levels of tar and known carcinogens in marijuana smoke. The relationship between smoking cocaine and developing lung cancer has not been studied as of 2020.
Environmental exposures

Exposure to a variety of other toxic chemicals – typically encountered in certain occupations – is associated with an increased risk of lung cancer. Occupational exposures to carcinogens cause 9–15% of lung cancer. A prominent example is asbestos, which causes lung cancer either directly or indirectly by inflaming the lung. Exposure to all commercially available forms of asbestos increases cancer risk, and cancer risk increases with time of exposure. Asbestos and cigarette smoking increase risk synergistically – that is, the risk of someone who smokes and has asbestos exposure dying from lung cancer is much higher than would be expected from adding the two risks together. Similarly, exposure to radon, a naturally occurring breakdown product of the Earth's radioactive elements, is associated with increased lung cancer risk. Radon levels vary with geography. Underground miners have the greatest exposure; however even the lower levels of radon that seep into residential spaces can increase occupants' risk of lung cancer. Like asbestos, cigarette smoking and radon exposure increase risk synergistically. Radon exposure is responsible for between 3% and 14% of lung cancer cases.
Several other chemicals encountered in various occupations are also associated with increased lung cancer risk including arsenic used in wood preservation, pesticide application, and some ore smelting; ionizing radiation encountered during uranium mining; vinyl chloride in papermaking; beryllium in jewelers, ceramics workers, missile technicians, and nuclear reactor workers; chromium in stainless steel production, welding, and hide tanning; nickel in electroplaters, glass workers, metal workers, welders, and those who make batteries, ceramics, and jewelry; and diesel exhaust encountered by miners.
Exposure to air pollution, especially particulate matter released by motor vehicle exhaust and fossil fuel-burning power plants, increases the risk of lung cancer. Indoor air pollution from burning wood, charcoal, or crop residue for cooking and heating has also been linked to an increased risk of developing lung cancer. The International Agency for Research on Cancer has classified emission from household burning of coal and biomass as "carcinogenic" and "probably carcinogenic" respectively.
Other diseases
Several other diseases that cause inflammation of the lung increase one's risk of lung cancer. This association is strongest for chronic obstructive pulmonary disorder – the risk is highest in those with the most inflammation, and reduced in those whose inflammation is treated with inhaled corticosteroids. Other inflammatory lung and immune system diseases such as alpha-1 antitrypsin deficiency, interstitial fibrosis, scleroderma, Chlamydia pneumoniae infection, tuberculosis, and HIV infection are associated with increased risk of developing lung cancer. Epstein–Barr virus is associated with the development of the rare lung cancer lymphoepithelioma-like carcinoma in people from Asia, but not in people from Western nations. A role for several other infectious agents – namely human papillomaviruses, BK virus, JC virus, human cytomegalovirus, SV40, measles virus, and Torque teno virus – in lung cancer development has been studied but remains inconclusive as of 2020.
Genetics
Particular gene combinations may make some people more susceptible to lung cancer. Close family members of those with lung cancer have around twice the risk of developing lung cancer as an average person, even after controlling for occupational exposure and smoking habits. Genome-wide association studies have identified many gene variants associated with lung cancer risk, each of which contributes a small risk increase. Many of these genes participate in pathways known to be involved in carcinogenesis, namely DNA repair, inflammation, the cell division cycle, cellular stress responses, and chromatin remodeling. Some rare genetic disorders that increase the risk of various cancers also increase the risk of lung cancer, namely retinoblastoma and Li–Fraumeni syndrome.
Pathogenesis
As with all cancers, lung cancer is triggered by mutations that allow tumor cells to endlessly multiply, stimulate blood vessel growth, avoid apoptosis (programmed cell death), generate pro-growth signalling molecules, ignore anti-growth signalling molecules, and eventually spread into surrounding tissue or metastasize throughout the body. Different tumors can acquire these abilities through different mutations, though generally cancer-contributing mutations activate oncogenes and inactivate tumor suppressors. Some mutations – called "driver mutations" – are particularly common in adenocarcinomas, and contribute disproportionately to tumor development. These typically occur in the receptor tyrosine kinases EGFR, BRAF, MET, KRAS, and PIK3CA. Similarly, some adenocarcinomas are driven by chromosomal rearrangements that result in overexpression of tyrosine kinases ALK, ROS1, NTRK, and RET. A given tumor will typically have just one driver mutation. In contrast, SCLCs rarely have these driver mutations, and instead often have mutations that have inactivated the tumor suppressors p53 and RB. A cluster of tumor suppressor genes on the short arm of chromosome 3 are often lost early in the development of all lung cancers.
Prevention
Smoking cessation
Those who smoke can reduce their lung cancer risk by quitting smoking – the risk reduction is greater the longer a person goes without smoking. Self-help programs tend to have little influence on success of smoking cessation, whereas combined counseling and pharmacotherapy improve cessation rates. The US FDA has approved antidepressant therapies and the nicotine replacement varenicline as first-line therapies to aid in smoking cessation. Clonidine and nortriptyline are recommended second-line therapies. The majority of those diagnosed with lung cancer attempt to quit smoking; around half succeed. Even after lung cancer diagnosis, smoking cessation improves treatment outcomes, reducing cancer treatment toxicity and failure rates, and lengthening survival time.
 No smoking sign at a train station in Colorado
No smoking sign at a train station in Colorado Graphic cigarette packaging in Belgium labelled "open wound following lung surgery"
Graphic cigarette packaging in Belgium labelled "open wound following lung surgery"
At a societal level, smoking cessation can be promoted by tobacco control policies that make tobacco products more difficult to obtain or use. Many such policies are mandated or recommended by the WHO Framework Convention on Tobacco Control, ratified by 182 countries, representing over 90% of the world's population. The WHO groups these policies into six intervention categories, each of which has been shown to be effective in reducing the cost of tobacco-induced disease burden on a population:
- increasing the price of tobacco by raising taxes;
- banning tobacco use in public places to reduce exposure;
- banning tobacco advertisements;
- publicizing the dangers of tobacco products;
- instituting help programs for those attempting to quit smoking; and
- monitoring population-level tobacco use and the effectiveness of tobacco control policies.
Policies implementing each intervention are associated with decreases in tobacco smoking prevalence. The more policies implemented, the greater the reduction. Reducing access to tobacco for adolescents is particularly effective at decreasing uptake of habitual smoking, and adolescent demand for tobacco products is particularly sensitive to increases in cost.
Diet and lifestyle
Several foods and dietary supplements have been associated with lung cancer risk. High consumption of some animal products – red meat (but not other meats or fish), saturated fats, as well as nitrosamines and nitrites (found in salted and smoked meats) – is associated with an increased risk of developing lung cancer. In contrast, high consumption of fruits and vegetables is associated with a reduced risk of lung cancer, particularly consumption of cruciferous vegetables and raw fruits and vegetables. Based on the beneficial effects of fruits and vegetables, supplementation of several individual vitamins have been studied. Supplementation with vitamin A or beta-carotene had no effect on lung cancer, and instead slightly increased mortality. Dietary supplementation with vitamin E or retinoids similarly had no effect. Consumption of polyunsaturated fats, tea, alcoholic beverages, and coffee are all associated with reduced risk of developing lung cancer.
Along with diet, body weight and exercise habits are also associated with lung cancer risk. Being overweight is associated with a lower risk of developing lung cancer, possibly due to the tendency of those who smoke cigarettes to have a lower body weight. However, being underweight is also associated with a reduced lung cancer risk. Some studies have shown those who exercise regularly or have better cardiovascular fitness to have a lower risk of developing lung cancer.
Epidemiology

Worldwide, lung cancer is the most diagnosed type of cancer, and the leading cause of cancer death. In 2020, 2.2 million new cases were diagnosed, and 1.8 million people died from lung cancer, representing 18% of all cancer deaths. Lung cancer deaths are expected to rise globally to nearly 3 million annual deaths by 2035, due to high rates of tobacco use and aging populations. Lung cancer is rare among those younger than 40; after that, cancer rates increase with age, stabilizing around age 80. The median age of a person diagnosed with lung cancer is 70; the median age of death is 72.
Lung cancer incidence varies by geography and sex, with the highest rates in Micronesia, Polynesia, Europe, Asia, and North America; and lowest rates in Africa and Central America. Globally, around 8% of men and 6% of women develop lung cancer in their lifetimes. The ratio of lung cancer cases in men to women varies considerably by geography, from as high as nearly 12:1 in Belarus, to 1:1 in Brazil, likely due to differences in smoking patterns.
Lung cancer risk is influenced by environmental exposure, namely cigarette smoking, as well as occupational risks in mining, shipbuilding, petroleum refining, and occupations that involve asbestos exposure. People who have smoked cigarettes account for 85–90% of lung cancer cases, and 15% of smokers develop lung cancer. Non-smokers' risk of developing lung cancer is also influenced by tobacco smoking; secondhand smoke (that is, being around tobacco smoke) increases risk of developing lung cancer around 30%, with risk correlated to duration of exposure. As the global incidence of lung cancer decreases in parallel with declining smoking rates in developed countries, the incidence of lung cancer in individuals who have never smoked is stable or increasing.
History
Lung cancer was uncommon before the advent of cigarette smoking. Surgeon Alton Ochsner recalled that as a Washington University medical student in 1919, his entire medical school class was summoned to witness an autopsy of a man who had died from lung cancer, and told they may never see such a case again. In Isaac Adler's 1912 Primary Malignant Growths of the Lungs and Bronchi, he called lung cancer "among the rarest forms of disease"; Adler tabulated the 374 cases of lung cancer that had been published to that time, concluding the disease was increasing in incidence. By the 1920s, several theories had been put forward linking the increase in lung cancer to various chemical exposures that had increased including tobacco smoke, asphalt dust, industrial air pollution, and poisonous gasses from World War I.
Over the following decades, growing scientific evidence linked lung cancer to cigarette consumption. Through the 1940s and early 1950s, several case-control studies showed that those with lung cancer were more likely to have smoked cigarettes compared to those without lung cancer. These were followed by several prospective cohort studies in the 1950s – including the first report of the British Doctors Study in 1954 – all of which showed that those who smoked tobacco were at dramatically increased risk of developing lung cancer.

A 1953 study showing that tar from cigarette smoke could cause tumors in mice attracted attention in the popular press, with features in Life and Time magazines. Facing public concern and falling stock prices, the CEOs of six of the largest American tobacco companies gathered in December 1953. They enlisted the help of public relations firm Hill & Knowlton to craft a multi-pronged strategy aiming to distract from accumulating evidence by funding tobacco-friendly research, declaring the link to lung cancer "controversial", and demanding ever-more research to settle this purported controversy. At the same time, internal research at the major tobacco companies supported the link between tobacco and lung cancer; though these results were kept secret from the public.
As evidence linking tobacco use with lung cancer mounted, various health bodies announced official positions linking the two. In 1962, the United Kingdom's Royal College of Physicians officially concluded that cigarette smoking causes lung cancer, prompting the United States Surgeon General to empanel (enroll or enlist) an advisory committee, which deliberated in secret over nine sessions between November 1962 and December 1963. The committee's report, published in January 1964, firmly concluded that cigarette smoking "far outweighs all other factors" in causing lung cancer. The report received substantial coverage in the popular press, and is widely seen as a turning point for public recognition that tobacco smoking causes lung cancer.
The connection with radon gas was first recognized among miners in Germany's Ore Mountains. As early as 1500, miners were noted to develop a deadly disease called "mountain sickness" ("Bergkrankheit"), identified as lung cancer by the late 19th century. By 1938, up to 80% of miners in affected regions died from the disease. In the 1950s radon and its breakdown products became established as causes of lung cancer in miners. Based largely on studies of miners, the International Agency for Research on Cancer classified radon as "carcinogenic to humans" in 1988. In 1956, a study revealed radon in Swedish residences. Over the following decades, high radon concentrations were found in residences across the world; by the 1980s many countries had established national radon programs to catalog and mitigate residential radon.
The first successful pneumonectomy for lung cancer was performed in 1933 by Evarts Graham at Barnes Hospital in St. Louis, Missouri. Over the following decades, surgical development focused on sparing as much healthy lung tissue as possible, with the lobectomy surpassing the pneumectomy in frequency by the 1960s, and the wedge resection appearing in the early 1970s. This trend continued with the development of video-assisted thoracoscopic surgery in the 1980s, now widely performed for many lung cancer surgeries.
Research
While lung cancer is the deadliest type of cancer, it receives the third-most funding from the US National Cancer Institute (NCI, the world's largest cancer research funder) behind brain cancers and breast cancer. Despite high levels of gross research funding, lung cancer funding per death lags behind many other cancers, with around $3,200 spent on lung cancer research in 2022 per US death, considerably lower than that for brain cancer ($22,000 per death), breast cancer ($14,000 per death), and cancer as a whole ($11,000 per death). A similar trend holds for private nonprofit organizations. Annual revenues of lung cancer-focused nonprofits rank fifth among cancer types, but lung cancer nonprofits have lower revenue than would be expected for the number of lung cancer cases, deaths, and potential years of life lost.
Despite this, many investigational lung cancer treatments are undergoing clinical trials – with nearly 2,250 active clinical trials registered as of 2021. Of these, a large plurality are testing radiotherapy regimens (26% of trials) and surgical techniques (22%). Many others are testing targeted anticancer drugs, with targets including EGFR (17% of trials), microtubules (12%), VEGF (12%), immune pathways (10%), mTOR (1%), and histone deacetylases (<1%).
References
- ^ Horn & Iams 2022, "Epidemiology".
- ^ Bade & Dela Cruz 2020, "Age".
- ^ Sung et al. 2021, "Lung cancer".
- ^ Rivera, Mody & Weiner 2022, "Introduction".
- ^ Pastis, Gonzalez & Silvestri 2022, "Presentation/Initial Evaluation".
- Nasim, Sabath & Eapen 2019, "Clinical Manifestations".
- ^ Horn & Iams 2022, "Clinical Manifestations".
- ^ "Diagnosis – Lung Cancer". National Health Service. 1 November 2022. Retrieved 30 November 2022.
- "Lung Carcinoma: Tumors of the Lungs" (online ed.). Merck Manual Professional. July 2020. Retrieved 21 July 2021.
- Pastis, Gonzalez & Silvestri 2022, "Noninvasive Staging".
- ^ Horn & Iams 2022, "Diagnosing Lung Cancer".
- Alexander, Kim & Cheng 2020, "Liquid Biopsy".
- Pastis, Gonzalez & Silvestri 2022, "Suspected Metastatic Disease".
- Morgensztern et al. 2023, "Clinical manifestations".
- ^ Tanoue, Mazzone & Tanner 2022, "Evidence for Lung Cancer Screening".
- Salahuddin & Ost 2023, "Table 110-1: Differential Diagnosis of Solitary Pulmonary Nodules".
- Image by Mikael Häggström, MD. Source for findings: Caroline I.M. Underwood, M.D., Carolyn Glass, M.D., Ph.D. "Lung - Small cell carcinoma". Pathology Outlines.
{{cite web}}: CS1 maint: multiple names: authors list (link) Last author update: 20 September 2022 - Thai et al. 2021, "Histology".
- Rudin et al. 2021, "Signs and Symptoms".
- ^ Horn & Iams 2022, "Pathology".
- ^ Morgensztern et al. 2023, "Precursor lesions".
- Jones 2013, "Conclusion".
- Pastis, Gonzalez & Silvestri 2022, "Histology and Prognosis".
- Rudin et al. 2021, "Immunohistochemistry".
- Horn & Iams 2022, "Immunohistochemistry".
- Lim et al. 2018, "Table 5: Overall stage based on T, N, and M descriptors".
- ^ "Small Cell Lung Cancer Stages". American Cancer Society. 1 October 2019. Retrieved 2 December 2022.
- "Non-small Cell Lung Cancer Stages". American Cancer Society. 1 October 2019. Retrieved 2 December 2022.
- ^ Horn & Iams 2022, "Staging System for Non-Small-Cell Lung Cancer".
- ^ Pastis, Gonzalez & Silvestri 2022, "Eight Edition Lung Cancer Stage Classification".
- Horn & Iams 2022, "Table 78–6 TNM Stage Groupings, Eighth Edition".
- "Lung Cancer TNM staging summary" (PDF) (8th ed.). International Association for the Study of Lung Cancer. Archived from the original (PDF) on 17 June 2018. Retrieved 30 May 2018.
- "Can Lung Cancer Be Found Early?". American Cancer Society. 18 January 2023. Retrieved 30 April 2023.
- ^ Jonas et al. 2021, Abstract – "Conclusions and Relevance".
- Alexander, Kim & Cheng 2020, "Lung Cancer Screening".
- Cancer screening in the European Union 2022, p. 27.
- Canadian Task Force 2016, "Recommendations".
- Rivera, Mody & Weiner 2022, "Palliative Care".
- ^ Horn & Iams 2022, "Treatment – Small-Cell Lung Cancer".
- ^ Rivera, Mody & Weiner 2022, "Treatment of Small Cell Lung Cancer".
- Rudin et al. 2021, "Locally advanced SCLC".
- Rudin et al. 2021, "Metastatic Disease".
- ^ Horn & Iams 2022, "Management of Stages I and II NSCLC".
- ^ Horn & Iams 2022, "Management of Stage III NSCLC".
- ^ Horn & Iams 2022, "Management of Metastatic NSCLC".
- Alexander, Kim & Cheng 2020, "Basis of Molecularly Targeted Therapy in Lung Cancer".
- Horn & Iams 2022, "Cytotoxic Chemotherapy for Metastatic or Recurrent NSCLC".
- ^ Horn & Iams 2022, "Immunotherapy".
- Horn & Iams 2022, "Second-Line Therapy and Beyond".
- Aragon 2020, "Integrating palliative care into lung cancer care".
- ^ Aragon 2020, "Dyspnea".
- ^ Dy SM, Gupta A, Waldfogel JM, Sharma R, Zhang A, Feliciano JL, Sedhom R, Day J, Gersten RA (19 November 2020). Interventions for Breathlessness in Patients With Advanced Cancer (Report). Agency for Healthcare Research and Quality (AHRQ). doi:10.23970/ahrqepccer232.
- Obeng, Folch & Fernando Santacruz 2018, "Introduction", "Prevalence", and "Clinical presentation".
- Obeng, Folch & Fernando Santacruz 2018, "Management".
- ^ Aragon 2020, "Cancer-related pain".
- Spencer et al. 2018, "What are the indications for using palliative radiotherapy?".
- ^ Lim 2016, "Key area three: providing symptom management in the last days".
- ^ Goldstraw et al. 2016, "Figure 2".
- ^ Rivera, Mody & Weiner 2022, "Prognostic and Predictive Factors in Lung Cancer".
- Allemani et al. 2018, "Lung".
- ^ Temel, Petrillo & Greer 2022, "Coping with Prognostic Uncertainty".
- "What Causes Lung Cancer". American Cancer Society. 1 October 2019. Retrieved 31 January 2023.
- "What Causes Lung Cancer?". American Lung Association. 17 November 2022. Retrieved 31 January 2023.
- Massion & Lehman 2022, Table 73.1: Hallmarks of Cancer.
- Schabath & Cote 2019, "Introduction".
- ^ Bade & Dela Cruz 2020, "Tobacco Smoke Carcinogens".
- "Tobacco and Cancer". Centers for Disease Control and Prevention. 18 November 2021. Retrieved 29 December 2022.
- Massion & Lehman 2022, "DNA Damage Response".
- Bade & Dela Cruz 2020, "Environmental Tobacco Smoke".
- Bracken-Clarke et al. 2021, Abstract – "Conclusion".
- Bade & Dela Cruz 2020, "Marijuana and Other Recreational Drugs".
- ^ Christiani & Amos 2022, "Occupational Exposures".
- ^ Schabath & Cote 2019, "Radon".
- Christiani & Amos 2022, "Air Pollution".
- Balmes & Holm 2022, Table 102.2: Major Pollutants Associated with Adverse Pulmonary Effects.
- ^ Bade & Dela Cruz 2020, "Biomass Burning".
- ^ Bade & Dela Cruz 2020, "Chronic Lung Diseases".
- ^ Bade & Dela Cruz 2020, "Infections".
- Christiani & Amos 2022, "Genetic Susceptibility to Lung Cancer".
- ^ Bade & Dela Cruz 2020, "Genetic Predisposition and History of Cancer".
- Christiani & Amos 2022, "High-Risk Syndromes Conferring an Increased Risk of Lung Cancer".
- ^ Horn & Iams 2022, "Molecular Pathogenesis".
- Rudin et al. 2021, "Mechanisms/Pathophysiology".
- ^ Horn & Iams 2022, "Risk Factors".
- Jassem 2019, "Prevalence and determinants of continued tobacco use after diagnosis of cancer".
- Jassem 2019, "Consequences of continued smoking after diagnosis of cancer".
- Peruga et al. 2021, "2.1. Galvanizing global political will around international law".
- Peruga et al. 2021, "2.2. Quadrupling the number of people benefiting from at least one cost-effective tobacco control policy since 2007".
- Arnott, Lindorff & Goddard 2022, p. 427.
- Christiani & Amos 2022, "Smoking Behavior and Risk for Lung Cancer".
- ^ Bade & Dela Cruz 2020, "Diet".
- Bade & Dela Cruz 2020, "Chemopreventive Agents".
- ^ Bade & Dela Cruz 2020, "Obesity and Exercise".
- "Estimated age-standardized incidence rates (World) in 2020, lung, both sexes, all ages". World Health Organization, International Agency for Research on Cancer. Retrieved 28 April 2023.
- Schabath & Cote 2019, "Descriptive Epidemiology".
- ^ Christiani & Amos 2022, "Introduction".
- Sung et al. 2021, "Figure 9".
- ^ Christiani & Amos 2022, "Geographic, Gender, and Ethnic Variability".
- LoPiccolo J, Gusev A, Christiani DC, Jänne PA (9 January 2024). "Lung cancer in patients who have never smoked — an emerging disease". Nature Reviews Clinical Oncology. 21 (2): 121–146. doi:10.1038/s41571-023-00844-0. ISSN 1759-4782. PMC 11014425. PMID 38195910.
- Spiro & Silvestri 2005, "Introduction".
- Blum 1999, p. 102.
- Adler 1912, p. 3.
- ^ Proctor 2012, "Introduction".
- ^ Proctor 2012, "Population studies".
- ^ Proctor 2012, "Animal experimentation".
- Brandt 2012, "Industry response to emerging tobacco science".
- Proctor 2012, "Cancer-causing chemicals in cigarette smoke".
- ^ Hall 2022, "Establishing the advisory committee to the US Surgeon General".
- Hall 2022, "Cigarette smoking and lung cancer".
- Parascandola 2020, "Introduction".
- ^ Witschi 2001, p. 2.
- ^ Mc Laughlin 2012, "Miner epidemiological studies".
- Mc Laughlin 2012, "Residential radon epidemiology".
- Horn & Johnson 2008, "Introduction".
- Walcott-Sapp & Sukumar 2016, "Evolution of Indications and Operative Technique".
- Spiro & Silvestri 2005, "Surgery".
- Walcott-Sapp & Sukumar 2016, "A Delayed Entrance to the Modern Era of Minimally Invasive Lung Resection".
- "Funding for Research Areas". National Cancer Institute. 10 May 2022. Retrieved 22 April 2023.
- "Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC)". US National Institutes of Health. 31 March 2023. Retrieved 30 April 2023.
- Kamath, Kircher & Benson 2019, "Results".
- Batra, Pawar & Bahl 2021, "Practice Points".
- Batra, Pawar & Bahl 2021, "Figure 2: Types of treatment for lung cancer in clinical trials, Phase I-IV".
Cited
Books
- Adler I (1912). Primary Malignant Growths of the Lungs and Bronchi. New York: Longmans, Green, and Company. OCLC 14783544. OL 24396062M.
- Broaddus C, Ernst JD, King TE, et al., eds. (2022). Murray & Nadel's Textbook of Respiratory Medicine (7th ed.). Elsevier. ISBN 978-0323655873.
- Balmes JR, Holm SM (2022). "Indoor and Outdoor Air Pollution". In Broaddus C, Ernst JD, King TE, et al. (eds.). Murray & Nadel's Textbook of Respiratory Medicine (7th ed.). Elsevier. pp. 1423–1434.
- Christiani DC, Amos CI (2022). "Lung Cancer: Epidemiology". In Broaddus C, Ernst JD, King TE, et al. (eds.). Murray & Nadel's Textbook of Respiratory Medicine (7th ed.). Elsevier. pp. 1018–1028.
- Massion PP, Lehman JM (2022). "Lung Cancer: Molecular Biology and Targets". In Broaddus C, Ernst JD, King TE, et al. (eds.). Murray & Nadel's Textbook of Respiratory Medicine (7th ed.). Elsevier. pp. 1005–1017.
- Pastis NJ, Gonzalez AV, Silvestri GA (2022). "Lung Cancer: Diagnosis and Staging". In Broaddus C, Ernst JD, King TE, et al. (eds.). Murray & Nadel's Textbook of Respiratory Medicine (7th ed.). Elsevier. pp. 1039–1051.
- Rivera P, Mody GN, Weiner AA (2022). "Lung Cancer: Treatment". In Broaddus C, Ernst JD, King TE, et al. (eds.). Murray & Nadel's Textbook of Respiratory Medicine (7 ed.). Elsevier. pp. 1052–1065.
- Tanoue L, Mazzone PJ, Tanner NT (2022). "Lung Cancer: Screening". In Broaddus C, Ernst JD, King TE, et al. (eds.). Murray & Nadel's Textbook of Respiratory Medicine (7th ed.). Elsevier. pp. 1029–1038.
- European Commission. Directorate General for Research and Innovation., European Commission Group of Chief Scientific Advisors. (2022). Cancer screening in the European Union. Publications Office of the European Union. doi:10.2777/867180. ISBN 978-92-76-45603-2.
- Horn L, Iams WT (2022). "78: Neoplasms of the Lung". In Loscalzo J, Fauci A, Kasper D, et al. (eds.). Harrison's Principles of Internal Medicine (21st ed.). McGraw Hill. ISBN 978-1264268504.
- Morgensztern D, Boffa D, Chen A, Dhanasopon A, Goldberg SB, Decker RH, Devarakonda S, Ko JP, Solis Soto LM, Waqar SN, Wistuba II, Herbst RS (April 2023). "80: Cancer of the Lung". In Bast RC, Byrd JC, Croce CM, et al. (eds.). Holland-Frei Cancer Medicine (10th ed.). Wiley. ISBN 978-1-119-75068-0.
- Salahuddin M, Ost DE (2023). "110: Approach to the Patient with Pulmonary Nodules". In Grippi MA, Antin-Ozerkis DE, Dela Cruz CS, et al. (eds.). Fishman's Pulmonary Diseases and Disorders (6th ed.). McGraw Hill. ISBN 978-1260473988.
Journal articles
- Alexander M, Kim SY, Cheng H (December 2020). "Update 2020: Management of Non-Small Cell Lung Cancer". Lung. 198 (6): 897–907. doi:10.1007/s00408-020-00407-5. PMC 7656891. PMID 33175991.
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. (March 2018). "Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries". Lancet. 391 (10125): 1023–1075. doi:10.1016/S0140-6736(17)33326-3. PMC 5879496. PMID 29395269.
- Aragon KN (June 2020). "Palliative Care in Lung Cancer". Clin Chest Med. 41 (2): 281–293. doi:10.1016/j.ccm.2020.02.005. PMID 32402363. S2CID 218633948.
- Arnott D, Lindorff K, Goddard A (August 2022). "Tobacco control: the FCTC provides the route to the finish line". Lancet. 400 (10350): 427. doi:10.1016/S0140-6736(22)01334-4. PMID 35878621. S2CID 250960604.
- Bade BC, Dela Cruz CS (March 2020). "Lung Cancer 2020: Epidemiology, Etiology, and Prevention". Clin Chest Med. 41 (1): 1–24. doi:10.1016/j.ccm.2019.10.001. PMID 32008623. S2CID 211015015.
- Batra H, Pawar S, Bahl D (February 2021). "Current clinical trials and patent update on lung cancer: a retrospective review". Lung Cancer Management. 10 (5): LMT45. doi:10.2217/lmt-2020-0029. PMC 8162165. PMID 34084211.
- Blum A (July 1999). "Alton ochsner, MD, 1896–1981 anti-smoking pioneer". Ochsner J. 1 (3): 102–105. PMC 3145444. PMID 21845126.
- Bracken-Clarke D, Kapoor D, Baird AM, Buchanan PJ, Gately K, Cuffe S, Finn SP (March 2021). "Vaping and lung cancer – A review of current data and recommendations". Lung Cancer. 153: 11–20. doi:10.1016/j.lungcan.2020.12.030. PMID 33429159. S2CID 231586192.
- Brandt AM (January 2012). "Inventing conflicts of interest: a history of tobacco industry tactics". Am J Public Health. 102 (1): 63–71. doi:10.2105/AJPH.2011.300292. PMC 3490543. PMID 22095331.
- Canadian Task Force on Preventive Health Care (April 2016). "Recommendations on screening for lung cancer". CMAJ. 188 (6): 425–432. doi:10.1503/cmaj.151421. ISSN 0820-3946. PMC 4818132. PMID 26952527.
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. (January 2016). "The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (8th) ed. of the TNM Classification for Lung Cancer". J Thorac Oncol. 11 (1): 39–51. doi:10.1016/j.jtho.2015.09.009. hdl:10044/1/31538. PMID 26762738. S2CID 5368645.
- Hall W (December 2022). "The 1964 US Surgeon General's report on smoking and health". Addiction. 117 (12): 3170–3175. doi:10.1111/add.16007. PMID 35852022. S2CID 250642397.
- Horn L, Johnson DH (July 2008). "Evarts A. Graham and the first pneumonectomy for lung cancer". Journal of Clinical Oncology. 26 (19): 3268–3275. doi:10.1200/JCO.2008.16.8260. PMID 18591561. Archived from the original on 17 March 2020. Retrieved 20 March 2009.
- Jassem J (May 2019). "Tobacco smoking after diagnosis of cancer: clinical aspects". Translational Lung Cancer Research. 8 (Suppl 1): S50 – S58. doi:10.21037/tlcr.2019.04.01. PMC 6546630. PMID 31211105.
- Jonas DE, Reuland DS, Reddy SM, Nagle M, Clark SD, Weber RP, Enyioha C, Malo TL, Brenner AT, Armstrong C, Coker-Schwimmer M, Middleton JC, Voisin C, Harris RP (March 2021). "Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force". JAMA. 325 (10): 971–987. doi:10.1001/jama.2021.0377. PMID 33687468. S2CID 232159404.
- Jones KD (December 2013). "Whence lepidic?: the history of a Canadian neologism". Arch Pathol Lab Med. 137 (12): 1822–1824. doi:10.5858/arpa.2013-0144-HP. PMID 23937575.
- Kamath SD, Kircher SM, Benson AB (July 2019). "Comparison of Cancer Burden and Nonprofit Organization Funding Reveals Disparities in Funding Across Cancer Types". J Natl Compr Canc Netw. 17 (7): 849–854. doi:10.6004/jnccn.2018.7280. PMID 31319386. S2CID 197666475.
- Lim RB (October 2016). "End-of-life care in patients with advanced lung cancer". Ther Adv Respir Dis. 10 (5): 455–467. doi:10.1177/1753465816660925. PMC 5933619. PMID 27585597.
- Lim W, Ridge CA, Nicholson AG, Mirsadraee S (August 2018). "The 8th lung cancer TNM classification and clinical staging system: review of the changes and clinical implications". Quantitative Imaging in Medicine and Surgery. 8 (7): 709–718. doi:10.21037/qims.2018.08.02. PMC 6127520. PMID 30211037.
- Mc Laughlin J (November 2012). "An historical overview of radon and its progeny: applications and health effects". Radiat Prot Dosimetry. 152 (1–3): 2–8. doi:10.1093/rpd/ncs189. PMID 22914338.
- Nasim F, Sabath BF, Eapen GA (May 2019). "Lung Cancer". Med Clin North Am. 103 (3): 463–473. doi:10.1016/j.mcna.2018.12.006. PMID 30955514. S2CID 102349766.
- Obeng C, Folch E, Fernando Santacruz J (December 2018). "Management of malignant airway obstruction". AME Medical Journal. 3: 115. doi:10.21037/amj.2018.11.06. S2CID 80791599.
- Parascandola M (March 2020). "The other Surgeon General's report: history of the U.S. public health response to air pollution, cigarette smoking, and lung cancer". Annals of Cancer Epidemiology. 4: 3. doi:10.21037/ace.2020.03.01. S2CID 216205576.
- Peruga A, López MJ, Martinez C, Fernández E (March 2021). "Tobacco control policies in the 21st century: achievements and open challenges". Mol Oncol. 15 (3): 744–752. doi:10.1002/1878-0261.12918. PMC 7931122. PMID 33533185.
- Proctor RN (March 2012). "The history of the discovery of the cigarette-lung cancer link: evidentiary traditions, corporate denial, global toll". Tob Control. 21 (2): 87–91. doi:10.1136/tobaccocontrol-2011-050338. PMID 22345227. S2CID 2734836.
- Rudin CM, Brambilla E, Faivre-Finn C, Sage J (January 2021). "Small-cell lung cancer". Nat Rev Dis Primers. 7 (1): 3. doi:10.1038/s41572-020-00235-0. PMC 8177722. PMID 33446664.
- Schabath MB, Cote ML (October 2019). "Cancer Progress and Priorities: Lung Cancer". Cancer Epidemiol Biomarkers Prev. 28 (10): 1563–1579. doi:10.1158/1055-9965.EPI-19-0221. PMC 6777859. PMID 31575553.
- Spencer K, Parrish R, Barton R, Henry A (March 2018). "Palliative radiotherapy". BMJ. 360: k821. doi:10.1136/bmj.k821. PMC 5865075. PMID 29572337.
- Spiro SG, Silvestri GA (September 2005). "One hundred years of lung cancer". American Journal of Respiratory and Critical Care Medicine. 172 (5): 523–529. doi:10.1164/rccm.200504-531OE. PMID 15961694.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (May 2021). "Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries". CA: A Cancer Journal for Clinicians. 71 (3): 209–249. doi:10.3322/caac.21660. PMID 33538338.
- Temel JS, Petrillo LA, Greer JA (February 2022). "Patient-Centered Palliative Care for Patients With Advanced Lung Cancer". J Clin Oncol. 40 (6): 626–634. doi:10.1200/JCO.21.01710. PMID 34985932. S2CID 245772225.
- Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS (August 2021). "Lung cancer". Lancet. 398 (10299): 535–554. doi:10.1016/S0140-6736(21)00312-3. PMID 34273294. S2CID 236034814.
- Walcott-Sapp S, Sukumar M (8 December 2016). "The history of pulmonary lobectomy: Two phases of innovation". CTSNet. Retrieved 28 April 2023.
- Witschi H (November 2001). "A short history of lung cancer". Toxicological Sciences. 64 (1): 4–6. doi:10.1093/toxsci/64.1.4. PMID 11606795.
External links
| Classification | D |
|---|---|
| External resources |
| Cancer involving the respiratory tract | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper RT | |||||||||||||
| Lower RT |
| ||||||||||||
| Pleura | |||||||||||||
| Mediastinum | |||||||||||||