| Revision as of 21:57, 29 November 2005 editWouterstomp (talk | contribs)Administrators19,999 edits →Treatment: moved follow-up to treatment intro← Previous edit | Latest revision as of 21:26, 12 December 2024 edit undoAjpolino (talk | contribs)Autopatrolled, Administrators17,027 edits Undid revision 1262723583 by 129.112.109.42 (talk) This reads as a WP:SELFCITE issue to radathand.com. I assume it's a good resource for clinicians, but it's not a great encyclopedia reference, and this is an encyclopedia not a practice manual.Tag: Undo | ||
| Line 1: | Line 1: | ||
| {{Short description|Male reproductive organ cancer}} | |||
| ] | |||
| {{Featured article}} | |||
| '''Prostate cancer''' is a ] in which ] develops in the ], a gland important in ] ]. Cancer occurs when ]s that form the basic building blocks of the prostate multiply out of control. These cells may spread (]) from the prostate to other parts of the body, especially the ] and ]s. Prostate cancer can cause severe ], weight loss, ], difficulty ] and can result in ]. | |||
| {{Use dmy dates|date=April 2024}} | |||
| {{cs1 config |name-list-style=vanc |display-authors=6}} | |||
| Prostate cancer only occurs in men and develops most frequently in individuals over fifty years old. It is the second most common type of cancer in men and is responsible for more deaths than any cancer except for ]. However, many men who develop prostate cancer never have ]s, undergo no therapy, and die of unrelated illness. Many things, including ], have been implicated in the development of prostate cancer but, ], it is not a preventable disease. | |||
| {{Infobox medical condition | |||
| | name = Prostate cancer | |||
| Prostate cancer is most often discovered by screening ] tests or by ] of the prostate gland by a health care provider. Confirmation of prostate cancer is typically accomplished by removing a piece of the prostate (]) and examining it under a ]. Further tests, such as ] and ]s, may be performed to determine whether prostate cancer has spread. | |||
| | synonyms = Prostate carcinoma | |||
| | image = Diagram showing prostate cancer pressing on the urethra CRUK 182.svg | |||
| Prostate cancer can be treated with ], ] therapy, ], ], or a combination thereof. The age and underlying health of the individual, as well as the extent of spread, appearance under the microscope, and response of the cancer to initial treatment are important in determining the outcome of the disease. {{an|ACS}} Since prostate cancer is a disease of older men, many men will die of other causes before the prostate cancer can spread or cause symptoms; this makes selecting treatment options difficult. | |||
| | caption = Diagram of prostate tumor pressing on ] | |||
| {{DiseaseDisorder infobox | | |||
| | field = ], ] | |||
| Name = Prostate cancer | | |||
| | symptoms = Typically none. Sometimes trouble urinating, erectile dysfunction, or pain in the back/pelvis. | |||
| ICD10 = C61 | | |||
| | complications = | |||
| ICD9 = 185 | | |||
| | onset = Age over 40 | |||
| | duration = | |||
| | types = | |||
| | causes = | |||
| | risks = Older age, family history, race | |||
| | diagnosis = ] followed by ] | |||
| | differential = ] | |||
| | prevention = | |||
| | treatment = ], ], ], ], ] | |||
| | medication = | |||
| | prognosis = ]s range from 30–99%, depending on stage.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Figure 3: Prostate cancer stages and progression."}} | |||
| }} | }} | ||
| == The prostate == | |||
| {{main|prostate}} | |||
| '''Prostate cancer''' is the ] in the ], a ] in the ] below the ]. Abnormal growth of prostate tissue is usually detected through screening tests, typically blood tests that check for ] (PSA) levels. Those with high levels of PSA in their blood are at increased risk for developing prostate cancer. Diagnosis requires a ] of the prostate. If cancer is present, the pathologist assigns a ], and a higher score represents a more dangerous tumor. Medical imaging is performed to look for cancer that has spread outside the prostate. Based on the Gleason score, PSA levels, and imaging results, a cancer case is assigned a ] 1 to 4. A higher stage signifies a more advanced, more dangerous disease. | |||
| The ] is an ] in the male ] which helps make and store ]. The normal adult prostate is about the size of a walnut, is three centimers long, and weighs twenty grams.{{an|Aumüller}} It is located in the ], beneath the ] and in front of the ]. It surrounds the upper part of the ], the tube that carries ] from the bladder during ] and semen during ].{{an|Moore}} Because of its location, diseases of the prostate may cause symptoms which affect urination, ejaculation, or ]. | |||
| Most prostate tumors remain small and cause no health problems. These are managed with ], monitoring the tumor with regular tests to ensure it has not grown. Tumors more likely to be dangerous can be destroyed with ] or surgically removed by ]. Those whose cancer spreads beyond the prostate are treated with ] therapy which reduces levels of the ]s (male sex hormones) that prostate cells need to survive. Eventually cancer cells can grow resistant to this treatment. This most-advanced stage of the disease, called castration-resistant prostate cancer, is treated with continued hormone therapy alongside the ] drug ]. Some tumors ] (spread) to other areas of the body, particularly the ]s and ]s. There, tumors cause severe ], leg weakness or paralysis, and eventually death. Prostate cancer ] depends on how far the cancer has spread at diagnosis. Most men diagnosed have tumors confined to the prostate; 99% of them survive more than 10 years from their diagnoses. Tumors that have metastasized to distant body sites are most dangerous, with ]s of 30–40%. | |||
| The prostate is composed of 30-50 small ]s which create twenty percent of the fluid comprising semen.{{an|Stieve}} These glands are formed by the ]s which are ] in prostate cancer. These cells require male ]s, known as ]s, to work properly. Androgens include ], which is made in the ], and ], made in the ]s. These hormones are also responsible for male ]s such as facial hair and testicular growth. | |||
| The risk of developing prostate cancer increases with age; the average age of diagnosis is 67. Those with a ] of any cancer are more likely to have prostate cancer, particularly those who inherit ] of the '']'' gene. Each year 1.2 million cases of prostate cancer are diagnosed, and 350,000 die of the disease,{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Epidemiology"}} making it the second-leading cause of cancer and cancer death in men. One in eight men is diagnosed with prostate cancer in his lifetime and one in forty dies of the disease.{{sfn|Scher|Eastham|2022|loc="Prostate cancer"}} Prostate tumors were first described in the mid-19th century, during surgeries on men with urinary obstructions. Initially, prostatectomy was the primary treatment for prostate cancer. By the mid-20th century, radiation treatments and hormone therapies were developed to improve prostate cancer treatment. The invention of hormone therapies for prostate cancer was recognized with the 1966 Nobel Prize to ] and the 1977 Prize to ]. | |||
| == Symptoms == | |||
| Early stage prostate cancer does not usually cause ]s. The majority of the time, it is diagnosed during the workup for an elevated ] detected on a routine check-up. | |||
| Sometimes, however, prostate cancer causes symptoms which are more common in diseases such as ]. These include ], ], difficulty initiating and maintaining a stream of urine, weak flow of urine, ], and painful urination. Prostate cancer may also interfere with sexual function, causing difficulty achieving ] or painful ].{{an|Miller}} | |||
| More advanced prostate cancer may cause symptoms which result from spread of the disease to parts of the body outside of the pelvis. The most common symptom is ] from involvement of the bone with prostate cancer. This is often in the lower back, from spread of the tumor to the spine. Prostate cancer in the ] may also compress the ], resulting in weakness of the ] and ] and ].{{an|Van}} | |||
| == Pathophysiology == | |||
| ]; cancer cells, however, avoid apoptosis.]] | |||
| ] is a group of many related diseases. These diseases begin in ], the body's basic unit of life. Cells have many important functions throughout the body. Normally, cells grow and divide to form new cells in an orderly way. They perform their functions for a while, and then they die (]). This process helps keep the body healthy. Sometimes, however, cells do not die. Instead, they keep dividing and creating new cells that the body does not need. They form a mass of tissue, called a growth or tumor. | |||
| Tumors can be benign or malignant: | |||
| * Benign tumors are not cancer. They can usually be removed, and in most cases, they do not come back. Cells from benign tumors do not spread to other parts of the body. Most important, benign tumors of the prostate are not a threat to life. ] (BPH) is the abnormal growth of benign prostate cells. In BPH, the prostate grows larger and presses against the urethra and bladder, interfering with the normal flow of urine. More than half of the men in the United States between the ages of 60 and 70 and as many as 90 percent between the ages of 70 and 90 have symptoms of BPH. For some men, the symptoms may be severe enough to require treatment. | |||
| * Malignant tumors are cancer. Cells in these tumors are abnormal. They divide without control or order, and they do not die. They can invade and damage nearby tissues and organs. Also, cancer cells can break away from a malignant tumor and enter the bloodstream and lymphatic system. This is how cancer spreads from the original (primary) cancer site to form new (secondary) tumors in other organs. The spread of cancer is called ]. | |||
| When prostate cancer spreads (metastasizes) outside the prostate, cancer cells are often found in nearby lymph nodes. If the cancer has reached these nodes, it means that cancer cells may have spread to other parts of the body -- other lymph nodes and other organs, such as the bones, bladder, or rectum. When cancer spreads from its original location to another part of the body, the new tumor has the same kind of abnormal cells and the same name as the primary tumor. For example, if prostate cancer spreads to the bones, the cancer cells in the new tumor are prostate cancer cells. The disease is metastatic prostate cancer; it is not bone cancer. | |||
| == Epidemiology == | |||
| The risk of developing prostate cancer is related to ], ], ], ], ], and ]s. Prostate cancer only occurs in men, and is more common with advancing age. Prostate cancer is uncommon in men less than 45. The average age of patients at the time of diagnosis is 70.{{an|Hankey}} | |||
| {{TOC limit}} | |||
| A genetic predisposition to prostate cancer is suggested by an increased ] among certain racial groups, in identical ]s of men with prostate cancer, and in men with certain ]s. In the United States, prostate cancer affects black men more commonly than white or Hispanic men. Prostate cancer is more common and more deadly in black men.{{an|Hoffman}} Men who have a brother or father with prostate cancer are twice as likely to themselves develop prostate cancer.{{an|Steinberg}} ]n ] suggest that forty percent of prostate cancer risk can be explained by ].{{an|Lichtenstein}} There is no one single gene which is responsible for prostate cancer, however. Many different genes have been implicated. Two genes (] and ]) that are important risk factors for ] and ] in women have also been implicated in prostate cancer.{{an|Struewing}} | |||
| == Signs and symptoms == | |||
| Several different ]s, ]s, and ] contribute to prostate cancer risk. The strongest link has been found between increased consumption of ] and increased risk for prostate cancer. Decreased intake of ]s and ]s is also related to an increase in prostate cancer risk. Indeed this seems to be related to increased intake of ] (found in animal fat) at the expense of ] (found in ]s). Less firm connections have been found between an increased risk for prostate cancer and a decreased intake of ] (found in green leafy vegetables), ] (found in tomatoes), ]s (found in fatty fishes like ]), and the mineral ]. Lower ] levels of ] also may increase risk. Less ] (UV) exposure also increases risk; because UV radiation is partly responsible for increasing levels of vitamin D in the blood, this further supports vitamin D's role in the development of prostate cancer.{{an|Schulman}} | |||
| Early prostate cancer usually causes no symptoms. As the cancer advances, it may cause ], blood in the ] or ], or trouble urinating – commonly including frequent urination and slow or weak urine stream.<ref name=ACS-Signs/> More than half of men over age 50 experience some form of urination problem,{{sfn|Merriel|Funston|Hamilton|2018|loc="Symptoms and signs"}} typically due to issues other than prostate cancer such as ] (non-cancerous enlargement of the prostate).<ref name=ACS-Signs/> | |||
| Advanced prostate tumors can ] to nearby lymph nodes and bones, particularly in the pelvis, hips, spine, ribs, head, and neck.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Figure 3: Prostate cancer stages and progression"}} There they can cause ], unexplained weight loss, and back or bone pain that does not improve with rest.{{sfn|Coleman|Croucher|Padhani|Clézardin|2020|loc="Prevalence of SREs"}}<ref>{{cite web|url=https://www.cancerresearchuk.org/about-cancer/prostate-cancer/symptoms |access-date=21 May 2023 |title=Symptoms of Prostate Cancer |publisher=Cancer Research UK |date=15 March 2022}}</ref> Metastases can damage the bones around them, and around a quarter of those with metastatic prostate cancer develop a ].{{sfn|Coleman|Croucher|Padhani|Clézardin|2020|loc="Prostate cancer"}} Growing metastases can also ] causing weakness in the legs and feet, or limb paralysis.{{sfn|Clinical Overview|2022|loc="Clinical presentation"}}{{sfn|Scher|Eastham|2022|loc="Metastatic disease: noncastrate"}} | |||
| There are links between prostate cancer and medications, medical procedures, and medical conditions. Daily use of ] (NSAIDs) such as ], ], and ] potentially decrease prostate cancer risk.{{an|Jacobs}} The group of ] lowering drugs collectively known as the ] (eg ]) may lower prostate cancer risk.{{an|Shannon}} ] by ] may increase the risk of prostate cancer, though there are conflicting data.{{an|Giovannucci}} Men who are ] may have decreased rates of prostate cancer.{{an|Ross}} Increased ejaculation frequency also may decrease the chance for prostate cancer; one study showed that men who had five episodes of ejaculation a week in their 20s had a decreased rate of prostate cancer.{{an|Giles}} ] or ] of the prostate, ], may increase the chance for prostate cancer. In particular, infection with the ]s ], ], and ] seem to increase risk.{{an|Dennis}} Finally, ]{{an|Calle}} and elevated blood levels of ]{{an|Gann}} may also increase the risk for prostate cancer. | |||
| == Screening == | == Screening == | ||
| {{Main|Prostate cancer screening}} | |||
| Screening means looking for signs of disease in people who have no symptoms. So screening for prostate cancer is looking for early-stage disease when treatment may be more effective. The main screening tools for prostate cancer are the digital rectal examination (DRE) and the prostate–specific antigen (PSA) test. The DRE and PSA test cannot tell if you have cancer; they can only suggest the need for further tests. Some medical experts believe all men should be offered regular screening tests for prostate cancer. Other medical experts do not recommend screening. | |||
| Most cases of prostate cancer are diagnosed through screening tests, when tumors are too small to cause any symptoms.<ref name=ACS-Signs>{{cite web |title=Prostate Cancer Signs and Symptoms |url=https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/signs-symptoms.html |access-date=21 May 2023 |publisher=American Cancer Society |date=1 August 2019 |language=en}}</ref> This is done through blood tests to measure levels of the protein ] (PSA), which are elevated in those with enlarged prostates, whether due to prostate cancer or ].{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Screening and early detection"}}<ref>{{cite web|url=https://www.cdc.gov/cancer/prostate/basic_info/screening.htm |access-date=17 May 2023 |title=What Is Screening for Prostate Cancer? |publisher=U.S. Centers for Disease Control and Prevention |date=25 August 2022}}</ref> The typical man's blood has around 1 ] (ng) of PSA per ] (mL) of blood tested.{{sfn|Carlsson|Vickers|2020|loc="3. Tailor screening frequency based on PSA-level"}} Those with PSA levels below average are very unlikely to develop dangerous prostate cancer over the next 8 to 10 years.{{sfn|Carlsson|Vickers|2020|loc="3. Tailor screening frequency based on PSA-level"}} Men with PSA levels above 4 ng/mL are at increased risk – around 1 in 4 will develop prostate cancer – and are often referred for a prostate biopsy.<ref name=ACS-Screen>{{cite web|url=https://www.cancer.org/cancer/types/prostate-cancer/detection-diagnosis-staging/tests.html |accessdate=11 December 2023 |title=Screening Tests for Prostate Cancer |publisher=American Cancer Society |date=4 January 2021}}</ref>{{sfn|Scher|Eastham|2022|loc="Prostate-specific antigen"}} PSA levels over 10 ng/mL indicate an even higher risk: over half of men in this group develop prostate cancer.<ref name=ACS-Screen/> <!--Looking for sources to add more here about PSA levels that rise over time-->Men with high PSA levels are often recommended to repeat the blood test four to six weeks later, as PSA levels can fluctuate unrelated to prostate cancer.{{sfn|Carlsson|Vickers|2020|loc="4. For men with elevated PSA (≥3 ng/mL), repeat PSA."}} ], ], recent ], and some urological procedures can increase PSA levels; taking ] can decrease PSA levels.<ref name=ACS-Screen/> | |||
| Those with elevated PSA may undergo secondary screening blood tests that measure subtypes of PSA and other molecules to better predict the likelihood that a person will develop aggressive prostate cancer. Many measure "free PSA" – the fraction of PSA unbound to other blood proteins, usually around 10% to 30%. Men who have a lower percentage of free PSA are more likely to have prostate cancer.{{sfn|Duffy|2020|loc="Percent free PSA"}} Several common tests more accurately detect prostate cancer cases by also measuring subtypes of free PSA, including the Prostate Health Index (measures a fragment called −2proPSA) and 4K score (measures intact free PSA).{{sfn|Duffy|2020|loc="Prostate Health Index (PHI)"}}{{sfn|Duffy|2020|loc="4K score"}} Other tests measure blood levels of additional prostate-related proteins such as ] (also measured by 4K score), or urine levels of ] molecules common to prostate tumors like ] and ] fused to ].{{sfn|Duffy|2020|loc=Table 2}} | |||
| * ] (DRE) -- During this procedure, the physician inserts a gloved, lubricated finger into the rectum in order to feel the size and shape of the prostate to find areas that are for hard or lumpy, which may indicate cancer. DRE can only detect abnormalities in one area of the prostate (the zone that can be felt through the rectum). Fortunately, this is where most prostate cancers arise. | |||
| *Blood test for ] (PSA) -- a lab measures the levels of PSA in a blood sample. Under normal circumstances, PSA is not found in the blood, but levels lower than 4 ng/mL (nanograms per milliliter) are considered normal. Levels greater than 10 ng/mL are considered abnormal. PSA levels between 4 and 10 ng/mL are considered to be borderline. PSA tests for prostate cancer are a subject of some controversy among clinicians and researchers. This is because some men who have prostate cancer do not have elevated PSA (>4 ng/mL), while some men with elevated levels do not have prostate cancer. This is far from being a perfect test, but it is the best method currently and used commonly. | |||
| Several large studies have found that men screened for prostate cancer have a reduced risk of dying from the disease;{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Box 1: Screening for prostate cancer in different regions"}} however, detection of cancer cases that would not have otherwise impacted health can cause anxiety, and lead to unneeded biopsies and treatments, both of which can cause unwanted complications.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Screening and early detection"}} Major national health organizations offer differing recommendations, attempting to balance the benefits of early diagnosis with the potential harms of treating people whose tumors are unlikely to impact health.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Screening and early detection"}} Most ] recommend that men at high risk of prostate cancer (due to age, family history, ethnicity, or prior evidence of high blood PSA levels) be counseled on the risks and benefits of PSA testing, and be offered access to screening tests.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Screening and early detection"}} Medical guidelines generally recommend against screening for men over age 70, or with a life expectancy of less than 10 years, as a newly diagnosed prostate cancer is unlikely to impact their natural lifespan.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Screening and early detection"}}{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Box 1: Screening for prostate cancer in different regions"}} Uptake of screening varies by geography – more than 80% of men are screened in the US and Western Europe, 20% of men in Japan, and screening is rare in regions with a low ].{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Box 1: Screening for prostate cancer in different regions"}} | |||
| Elevated PSA levels can occur for many reasons. They may rise in men who have prostate cancer, benign prostate hyperplasia (BPH), or an infection in the prostate (prostatitis). To maximize the accuracy of a PSA test: (1) don't ejaculate for 2 days prior to having a PSA test as this can raise PSA levels, and (2)inform your physician if you are taking finasteride (marketed as Proscar or Propecia) or dutasteride (marketed as Avodart). These drugs, used to treat BPH and baldness affect the metabolism of testosterone throughout your body and will likely lower your PSA levels. Also, (3) the DRE needs to be performed after drawing blood for the PSA test, as palpation of the prostate can stimulate it to produce PSA and lead to elevated PSA levels in the serum. Some herbal supplements can also affect PSA levels. Discuss any supplements you are taking with your physician prior to having a PSA test. | |||
| The most recent trend is to consider the rate of change of the PSA level as an indication of the risk of cancer. This requires at least two PSA tests be done over a period of time. Abnormal DRE or high serum PSA levels are reasons for a medical follow up. The results of these tests will help to determine whether further tests are necessary to check for cancer. | |||
| When the total PSA blood test is in the grey zone (between 4 and 10 ng/mL) and the DRE is normal, the percentage of free PSA (unbound to other proteins) in the blood is used to distinguish between BPH and prostate cancer. A low value for percent-free PSA indicates a higher probability of prostate cancer. | |||
| Currently, a biopsy is the only procedure that can definitively diagnose prostate cancer. It is performed when digital rectal examination shows abnormalities or a patient has high total PSA in the serum. A biopsy gun inserts and removes hollow core needles (usually three to six for each side of the prostate) in less than a second. The needles are very fine and remove only small cores of tissue. In this way, small 'samples' of the prostate are removed. The tissue samples are then examined under a microscope to determine if cancer cells are present and to evaluate the extent of the cancer. Some men have reported that this is the most physically uncomfortable part of their experience of being diagnosed with prostate cancer. (Patients can request that an appropriate anesthetic be used.) | |||
| == Diagnosis == | == Diagnosis == | ||
| ] | |||
| Men suspected of having prostate cancer may undergo several tests to assess the prostate. One common procedure is the ], in which a doctor inserts a lubricated finger into the ] to feel the nearby prostate.<ref>{{cite web|url=https://www.cancer.org/cancer/types/prostate-cancer/detection-diagnosis-staging/how-diagnosed.html |access-date=18 May 2023 |title=Tests to Diagnose and Stage Prostate Cancer |publisher=American Cancer Society |date=21 February 2023}}</ref>{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Diagnosis"}} Tumors feel like stiff, irregularly shaped lumps against the rest of the prostate. Hardening of the prostate can also be due to ]; around 20–25% of those with abnormal findings on their rectal exams have prostate cancer.{{sfn|Scher|Eastham|2022|loc="Physical examination"}} Several urological societies' guidelines recommend ] (MRI) to evaluate the prostate for potential tumors in men with high PSA levels. MRI results can help distinguish those who have potentially dangerous tumors from those who do not.{{sfn|Maffei|Giganti|Moore|2023|loc="MRI as a test for clinically referred men with a raised PSA or abnormal digital rectal exam}} | |||
| If a man has symptoms or test results that suggest prostate cancer, his doctor asks about his personal and family medical history, performs a physical exam, and may order laboratory tests. The exams and tests may include a digital rectal exam, a urine test to check for blood or infection, and a blood test to measure PSA. In some cases, the doctor also may check the level of ] (PAP) in the blood, especially if the results of the PSA indicate there might be a problem. | |||
| A definitive diagnosis of prostate cancer requires a ] of the prostate. Prostate biopsies are typically taken by a needle passing through the rectum or ], guided by ], MRI, or a combination of the two.{{sfn|Scher|Eastham|2022|loc="Prostate biopsy"}}{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Diagnosis"}} Ten to twelve samples are taken from several regions of the prostate to improve the chances of finding any tumors.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Diagnosis"}} Biopsies are sent for a ], wherein they are examined under a microscope by a ], who determines the type and extent of cancerous cells present. Cancers are first classified based on their appearance under a microscope. Over 95% of prostate cancers are classified as ]s (resembling ] tissue), with the rest largely ] (resembling ]s, a type of ]) and ] (resembling ]s).{{sfn|Scher|Eastham|2022|loc="Pathology"}} | |||
| The doctor may order exams to learn more about the cause of the symptoms. These may include: | |||
| ] (left) and ] (right) showing prostate cancer metastases in the bone (red arrows). The dye used for PSMA scans is also absorbed by the kidneys, liver, and spleen (large dark objects at right). |alt=Medical images of a man's torso. Arrows indicate tumor metastases, visible as dots in the man's spine and pelvis, in both scans.]] | |||
| *] -- sound waves that cannot be heard by humans (ultrasound) are sent out by a probe inserted into the rectum. The waves bounce off the prostate, and a computer uses the echoes to create a picture called a sonogram. | |||
| *] -- a series of x-rays of the organs of the urinary tract. | |||
| *] -- a procedure in which a doctor looks into the urethra and bladder through a thin, lighted tube. | |||
| Next, tumor samples are ] based on how much the tumor tissue differs from normal prostate tissue; the more different the tumor appears, the faster the tumor is likely to grow. The ] is commonly used, where the pathologist assigns numbers ranging from 3 (most similar to healthy prostate tissue) to 5 (least similar) to different regions of the biopsied tissue. They then calculate a "Gleason score" by adding the two numbers that represent the largest areas of the biopsy sample.{{sfn|Scher|Eastham|2022|loc="Pathology"}} The lowest possible Gleason score of 6 represents a biopsy most similar to healthy prostate; the highest Gleason score of 10 represents the most severely cancerous.{{efn|group=note|The original 1966 Gleason grading system allowed pathologist scores of 1 to 5, resulting in Gleason scores of 2 to 10; however, a 2005 update by the ] largely eliminated the pathologist scores 1 and 2. In common practice, tumors are now scored 3 to 5, resulting in Gleason scores of 6 to 10.{{sfn|Epstein|2018|loc="Historical background", "2005 and 2014 ISUP grading conferences", and "Gleason patterns"}}}} Gleason scores are commonly grouped into "Gleason grade groups", which predict disease prognosis: a Gleason score of 6 is Gleason grade group 1 (best prognosis). A score of 7 (with Gleason scores 4 + 3, or Gleason scores 3 + 4, with the most prominent listed first) can be grade group 2 or 3; it is grade group 2 if the less severe Gleason score (3) covered more area; grade group 3 if the more severe Gleason score (4) covered more area. A score of 8 is grade group 4. A score of 9 or 10 is grade group 5 (worst prognosis).{{sfn|Scher|Eastham|2022|loc="Pathology"}} | |||
| If test results suggest that cancer may be present, the man will need to have a ]. During a biopsy, the doctor removes tissue samples from the prostate, usually with a needle. A pathologist looks at the tissue under a microscope to check for cancer cells. If cancer is present, the pathologist usually reports the grade of the tumor. The grade tells how much the tumor tissue differs from normal prostate tissue and suggests how fast the tumor is likely to grow. The most common method of grading prostate cancer, called the Gleason system, uses scores of 2 to 10, with 10 indicating the most aberrant growing and 'cancerous' samples. The pathologist assigns a number between 1 and 5 to the most common pattern observed under the microscope. The second most common pattern is also assigned a number. The sum of these numbers makes up the Gleason score. Another system uses G1 through G4. It is important that the pathologist grading the tumor have a lot of experience looking at prostate tumors, as the grade of the tumor is one of the major factors in determining the treatment recommendation. This is because tumors with higher scores or grades are more likely to grow and spread than tumors with lower scores. | |||
| The extent of cancer spread is assessed by MRI or ] – a ] (PET) imaging technique where a ] that binds the prostate protein ] is used to detect metastases distant from the prostate.{{sfn|Scher|Eastham|2022|loc="Prostate cancer staging"}}{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Diagnosis"}} ]s may also be used, but are less able to detect spread outside the prostate than MRI. ] is used to test for spread of cancer to bones.{{sfn|Scher|Eastham|2022|loc="Prostate cancer staging"}} | |||
| If the physical exam and test results do not suggest cancer, the doctor may recommend treatment for ]. | |||
| == |
=== Staging === | ||
| {{ |
{{Main|Prostate cancer staging}} | ||
| ] | |||
| An important part of the evaluation of prostate cancer is determination of the ], or how far the cancer has spread. The most common system is the ] system, which evaluates the size of the tumor, the number of involved ]s, and any other ]. As with many other cancers, these are often grouped into four stages (I–IV). The ] is another method sometimes used. The purpose of staging is to help determine ] and to assist in selecting therapies; a good staging system will have similar characteristics for most cancers at a given stage. | |||
| After diagnosis, the tumor is ] to determine the extent of its growth and spread. Prostate cancer is typically staged using the ]'s (AJCC) three-component ], with scores assigned for the extent of the tumor (T), spread to any lymph nodes (N), and the presence of ] (M).<ref name=ACSStage>{{cite web |url=https://www.cancer.org/cancer/types/prostate-cancer/detection-diagnosis-staging/staging.html |title=Prostate Cancer Staging |date=8 October 2021 |publisher=American Cancer Society |access-date=14 May 2023}}</ref> Scores of T1 and T2 represent tumors that remain within the prostate: T1 is for tumors not detectable by imaging or digital rectal exam; T2 is for tumors detectable by imaging or rectal exam, but still confined within the prostate.{{sfn|Scher|Eastham|2022|loc="Table 87-1 TNM classification"}} T3 is for tumors that grow beyond the prostate – T3a for tumors with any extension outside the prostate; T3b for tumors that invade the adjacent ]s. T4 is for tumors that have grown into organs beyond the seminal vesicles.{{sfn|Scher|Eastham|2022|loc="Table 87-1 TNM classification"}} The N and M scores are binary (yes or no). N1 represents any spread to the nearby lymph nodes. M1 represents any metastases to other body sites.{{sfn|Scher|Eastham|2022|loc="Table 87-1 TNM classification"}} | |||
| == Treatment == | |||
| The AJCC then combines the TNM scores, Gleason grade group, and results of the PSA blood test to categorize cancer cases into one of four stages, and their subdivisions. Cancer cases with localized tumors (T1 or T2), no spread (N0 and M0), Gleason grade group 1, and PSA less than 10 ng/mL are designated stage I. Those with localized tumors and PSA between 10 and 20 ng/mL are designated stage II – subdivided into IIA for Gleason grade group 1, IIB for grade group 2, and IIC for grade group 3 or 4. Stage III is the designation for any of three higher risk factors: IIIA is for a PSA level about 20 ng/mL; IIIB is for T3 or T4 tumors; IIIC is for a Gleason grade group of 5. Stage IV is for cancers that have spread to lymph nodes (N1, stage IVA) or other organs (M1, stage IVB).<ref name=ACSStage/> | |||
| Treatment for prostate cancer depends on the stage of the disease and the grade of the tumor (which indicates how abnormal the cells look, and how likely they are to grow or spread). Other important factors in planning treatment are the man's age and general health and his feelings about the treatments and their possible side effects. | |||
| {| class="wikitable floatright" style="text-align:center;font-size:90%;margin-left:1em;" | |||
| |+AJCC stage for prostate cancer | |||
| !AJCC Stage | |||
| !TNM scores | |||
| !Gleason grade group | |||
| !PSA | |||
| |- | |||
| |Stage I | |||
| |T1 or T2, N0, M0 | |||
| |1 | |||
| |<10 ng/mL | |||
| |- | |||
| |Stage IIA | |||
| | rowspan="3" |T1 or T2, N0, M0 | |||
| |1 | |||
| | rowspan="3" |10-20 ng/mL | |||
| |- | |||
| |Stage IIB | |||
| |2 | |||
| |- | |||
| |Stage IIC | |||
| |3 or 4 | |||
| |- | |||
| |Stage IIIA | |||
| |T1 or T2, N0, M0 | |||
| | rowspan="2" |3 or 4 | |||
| |> 20 ng/mL | |||
| |- | |||
| |Stage IIIB | |||
| |T3 or T3, N0, M0 | |||
| | rowspan="2" |10–20 ng/mL | |||
| |- | |||
| |Stage IIIC | |||
| |T1 or T2, N0, M0 | |||
| |5 | |||
| |- | |||
| |Stage IVA | |||
| |Any T, N1 | |||
| | rowspan="2" |Any group | |||
| | rowspan="2" |Any PSA | |||
| |- | |||
| |Stage IVB | |||
| |Any T, M1 | |||
| |} | |||
| The United Kingdom ] recommends a five-stage system based on disease prognosis called the Cambridge Prognostic Group, with prognostic groups CPG 1 to CPG 5.<ref name=":2">{{cite web |title=Prostate Cancer: Diagnosis and Management. NICE Guideline |url=https://www.nice.org.uk/guidance/ng131/chapter/Recommendations#assessment-and-diagnosis |access-date=3 October 2022 |website=National Institute for Health and Care Excellence (NICE)|date=9 May 2019 }}</ref> CPG 1 is the same as AJCC stage I. Cases with localized tumors (T1 or T2) and either Gleason grade group 2 or higher PSA levels (10 to 20 ng/mL) are designated CPG 2. CPG 3 represents either Gleason grade group 3, or the combination of the CPG 2 criteria. CPG 4 is similar to AJCC stage 3 – any of Gleason grade group 4, PSA levels above 20 ng/mL, or a tumor that has grown beyond the prostate (T3). CPG 5 is for the highest risk cases: either a T4 tumor, Gleason grade group 5, or any two of the CPG 4 criteria.<ref>{{cite web|url=https://www.cancerresearchuk.org/about-cancer/prostate-cancer/stages/cambridge-prognostic-group-cpg |access-date=25 June 2023 |title=Prostate Cancer Risk Groups and the Cambridge Prognostic Group (CPG) |publisher=Cancer Research UK |date=24 May 2022}}</ref> | |||
| Treatment for prostate cancer may involve watchful waiting, surgery, radiation therapy, or hormonal therapy. Some patients receive a combination of therapies. The patient and his doctor may want to consider both the benefits and possible side effects of each option, especially the effects on sexual activity and urinary control, and other concerns about quality of life. | |||
| == Prevention == | |||
| It is hard to limit the effects of treatment so that only cancer cells are removed or destroyed. Because healthy cells and tissues may be damaged, treatment often causes unwanted side effects. The side effects of cancer treatment depend mainly on the type and extent of the treatment. Also, each patient reacts differently. | |||
| No drug or vaccine is approved by regulatory agencies for the prevention of prostate cancer. Several studies have shown ]s to reduce the total incidence of prostate cancer; however, it is unclear as of 2022 whether they reduce any cases of dangerous disease.{{sfn|Scher|Eastham|2022|loc="No cancer diagnosis"}} | |||
| == Management == | |||
| During and after treatment, the doctor will continue to follow the patient. The doctor will examine the man regularly to be sure that the disease has not returned or progressed, and will decide what other medical care may be needed. Follow-up exams may include x-rays, scans, and lab tests, such as the PSA blood test. | |||
| {{Main|Management of prostate cancer}} | |||
| Treatment of prostate cancer varies based on how advanced the cancer is, the risk it may spread, and the affected person's health and personal preferences.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Management"}} Those with localized disease at low risk for spread are often more likely to be harmed by the side effects of treatment than the disease itself, and so are regularly tested for a worsening of their disease.{{sfn|Scher|Eastham|2022|loc="Active surveillance"}} Those at higher risk may receive treatment to eliminate the tumor – typically ] (surgery to remove the prostate) or ], sometimes alongside ].<ref name=ACSTreat>{{cite web|url=https://www.cancer.org/cancer/types/prostate-cancer/treating/by-stage.html |access-date=28 May 2023 |title=Initial Treatment of Prostate Cancer, by Stage and Risk Group |publisher=American Cancer Society |date=9 August 2022}}</ref> Those with metastatic disease are treated with ], as well as radiation or other agents to alleviate the symptoms of metastatic tumors.<ref name=ACSTreat/> Blood PSA levels are monitored every few months to assess the effectiveness of treatments, and whether the disease is recurring or advancing.<ref>{{cite web|url=https://www.cancer.org/cancer/types/prostate-cancer/treating/psa-levels-after-treatment.html |title=Following PSA Levels During and After Prostate Cancer Treatment |access-date=28 May 2023 |publisher=American Cancer Society |date=1 August 2019}}</ref> | |||
| === |
=== Localized disease === | ||
| ] | |||
| Men diagnosed with low-risk cases of prostate cancer often defer treatment and are monitored regularly for cancer progression by ], which involves testing for tumor growth at fixed intervals by PSA tests (around every six months), digital rectal exam (annually), and MRI or repeat biopsies (every one to three years).<ref>{{cite web|url=https://www.cancer.org/cancer/types/prostate-cancer/treating/watchful-waiting.html |accessdate=16 April 2024 |title=Observation or Active Surveillance for Prostate Cancer |publisher=American Cancer Society |date=22 November 2023}}</ref> This program continues until increases in PSA levels, Gleason grade, or tumor size indicate a higher-risk tumor that may require intervention.{{sfn|Liu|Patel|Haney|Epstein|2021|loc="Reclassification and progression"}} At least half of men remain on active surveillance, never requiring more direct treatment for their prostate tumors.{{sfn|Liu|Patel|Haney|Epstein|2021|loc="Abstract"}} | |||
| Those who elect to have therapy receive ] or a ]; these have similar rates of cancer control, but different side effects.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Localized Disease"}}{{sfn|Scher|Eastham|2022|loc="Clinically localized prostate cancer"}} Radiation can be delivered by ] (IMRT), which allows for high doses (greater than 80 ]) to be delivered to the prostate with relatively little radiation to other organs, or by ], where a radioactive source is surgically inserted into the prostate.{{sfn|Scher|Eastham|2022|loc="External beam radiation therapy"}}{{sfn|Scher|Eastham|2022|loc="Brachytherapy"}} IMRT is given over several sessions, with treatments repeated five days per week for several weeks. Brachytherapy is typically performed in a single session, with the radioactive source permanently implanted into the prostate, where it expends its radioactivity within the next few months.<ref>{{cite web|url=https://www.cancer.org/cancer/types/prostate-cancer/treating/radiation-therapy.html |accessdate=5 December 2023 |title=Radiation Therapy for Prostate Cancer |publisher=American Cancer Society |date=13 February 2023}}</ref> With either technique, radiation damage to nearby organs can increase the risk of subsequent ] and cause ], ], ]<ref>{{cite journal |last1=Brejt |first1=Nick |last2=Berry |first2=Jonathan |last3=Nisbet |first3=Angus |last4=Bloomfield |first4=David |last5=Burkill |first5=Guy |title=Pelvic radiculopathies, lumbosacral plexopathies, and neuropathies in oncologic disease: a multidisciplinary approach to a diagnostic challenge |journal=Cancer Imaging |date=December 30, 2013 |volume=13 |issue=4 |pages=591–601 |doi=10.1102/1470-7330.2013.0052 |pmid=24433993 |pmc=3893894}}</ref> and ] – damage to the rectum that can cause ], ], ], and pain.{{sfn|Brawley|Mohan|Nein|2018|loc="Radiation therapy"}} | |||
| If the doctor recommends watchful waiting, the man's health will be monitored closely, including frequent PSA monitoring, and he will be treated only if symptoms occur or worsen or if the rate of PSA rise is concerning. Watchful waiting may be suggested for some men who have prostate cancer that is found at an early stage and appears to be slow growing. Also, watchful waiting may be advised for older men or men with other serious medical problems. For these men, the risks and possible side effects of surgery, radiation therapy, or hormonal therapy may outweigh the possible benefits. Men with early stage prostate cancer are taking part in a study to determine when or whether treatment may be necessary and effective. | |||
| ] | |||
| Although men who choose watchful waiting avoid the side effects of surgery and radiation, there can be some negative aspects to this choice. Watchful waiting may reduce the chance of controlling the disease before it spreads. Also, older men should keep in mind that it may be harder to manage surgery and radiation therapy as they age. | |||
| Radical prostatectomy aims to surgically remove the cancerous part of the prostate, along with the seminal vesicles, and the end of the ] (the duct that delivers sperm from the testes).{{sfn|Dall'Era|2023|loc="Radical prostatectomy"}} In wealthier countries, this is typically done by ], where robotic tools inserted through small holes in the abdomen allow a surgeon to make small and exact movements during surgery.{{sfn|Costello|2020|loc="The rise of robotic surgery"}} This method results in shorter hospital stays, less blood loss, and fewer complications than traditional open surgery.{{sfn|Costello|2020|loc="The rise of robotic surgery"}} In places where robot-assisted surgery is unavailable, prostatectomy can be performed ] (using a camera and hand tools through small holes in the abdomen), or through traditional open surgery with an incision above the penis (retropubic approach) or below the scrotum (perineal approach).{{sfn|Scher|Eastham|2022|loc="Radical prostatectomy"}}{{sfn|Costello|2020|loc="The rise of robotic surgery"}} The four approaches result in similar rates of cancer control.{{sfn|Scher|Eastham|2022|loc="Radical prostatectomy"}} Damage to nearby tissue during surgery can result in erectile dysfunction and ]. Erectile dysfunction is more likely in those who are older or had previous erectile issues.{{sfn|Scher|Eastham|2022|loc="Radical prostatectomy"}} Incontinence is more common in those who are older and have shorter ]s.{{sfn|Scher|Eastham|2022|loc="Radical prostatectomy"}} Both for cancer progression outcomes and surgical side effects, the skill and experience of the individual surgeon doing the procedure are among the greatest determinants of success.{{sfn|Scher|Eastham|2022|loc="Radical prostatectomy"}} | |||
| After prostatectomy, PSA levels drop rapidly, reaching very low or undetectable levels within two months. Radiotherapy also substantially reduces PSA levels, but more slowly and less completely, with PSA levels reaching their nadir two years after radiotherapy.<ref>{{cite web|url=https://www.cancer.org/cancer/types/prostate-cancer/treating/psa-levels-after-treatment.html |accessdate=5 December 2023 |title=Following PSA Levels During and After Prostate Cancer Treatment |publisher=American Cancer Society |date=1 August 2019}}</ref> After either treatment, PSA levels are monitored regularly. Up to half of those treated will eventually have a rise in PSA levels, suggesting the tumor or small metastases are growing again.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Biochemical recurrence and residual disease"}} People with high or rising PSA levels are often offered another round of radiation therapy directed at the former tumor site. This reduces risk for further progression by 75%.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Biocehmical recurrence and residual disease"}} Those suspected of metastases can undergo PET scanning with sensitive ] C-11 choline, F-18 fluciclovine, and F-18 or Ga-68 attached to a PSMA-targeting drug, each of which is able to detect small metastases more sensitively than alternative imaging methods.{{sfn|Scher|Eastham|2022|loc="Rising PSA after definitive local therapy"}}{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Biocehmical recurrence and residual disease"}} | |||
| Some men may decide against watchful waiting because they feel they would be uncomfortable living with an untreated cancer, even one that appears to be growing slowly or not at all. A man who chooses watchful waiting but later becomes concerned or anxious should discuss his feelings with his doctor. A different treatment approach is nearly always available. | |||
| === |
=== Metastatic disease === | ||
| ] | |||
| For those with metastatic disease, the standard of care is ] (also called "chemical castration"), drugs that reduce levels of ]s (male sex hormones) that prostate cells require to grow.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Metastatic hormone-sensitive prostate cancer"}} Various drugs are used to lower androgen levels by blocking the synthesis or action of ], the primary androgen. The first line of treatment typically involves ]s like ], ], or ] by injection monthly or less frequently as needed.<ref>{{cite web|url=https://www.cancer.org/cancer/types/prostate-cancer/treating/hormone-therapy.html |access-date=15 May 2023 |title=Hormone Therapy for Prostate Cancer |date=9 August 2022 |publisher=American Cancer Society}}</ref>{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Metastatic hormone-sensitive prostate cancer"}} GnRH agonists cause a brief rise in testosterone levels at treatment initiation, which can worsen disease in people with significant symptoms of metastases.{{sfn|Scher|Eastham|2022|loc="Testosterone-lowering agents"}} In these people, ] like ] or ] are given instead, and can also rapidly reduce testosterone levels.{{sfn|Scher|Eastham|2022|loc="Testosterone-lowering agents"}} Reducing testosterone can cause various side effects, including ]es, reduction in muscle mass and bone density, reduced sex drive, fatigue, personality changes, and an increased risk of diabetes, cardiovascular disease, and depression.{{sfn|Scher|Eastham|2022|loc="Testosterone-lowering agents"}} Hormone therapy halts tumor growth in more than 95% of those treated,{{sfn|Achard|Putora|Omlin|Zilli|2022|loc="Introduction"}} and PSA levels return to normal in up to 70%.{{sfn|Scher|Eastham|2022|loc="Outcomes of androgen deprivation"}} | |||
| Despite reduced testosterone levels, metastatic prostate tumors eventually continue to grow – manifested by rising blood PSA levels, and metastases to nearby bones.{{sfn|Scher|Eastham|2022|loc="Metastatic disease: castrate"}}{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Metastatic castration-resistant prostate cancer"}} This is the most advanced stage of the disease, called castration-resistant prostate cancer (CRPC). CRPC tumors continuously evolve resistance to treatments, necessitating several lines of therapy, each used in sequence to extend survival. The standard of care is the chemotherapy ] along with ] drugs, namely the androgen receptor antagonists ], ], and ], as well as the testosterone production inhibitor ].{{sfn|Teo|Rathkopf|Kantoff|2019|loc="Management of metastatic castration-resistant prostate cancer"}}{{sfn|Scher|Eastham|2022|loc="Metastatic disease: castrate"}}{{sfn|Teo|Rathkopf|Kantoff|2019|loc="Abiraterone acetate"}} An alternative is the ] procedure ], where the affected person's immune cells are removed, treated to more effectively target prostate cancer cells, and re-injected.{{sfn|Scher|Eastham|2022|loc="Metastatic disease: castrate"}} Tumors that evolve resistance to docetaxel may receive the second-generation ] drug ].{{sfn|Scher|Eastham|2022|loc="Metastatic disease: castrate"}} | |||
| Surgery is a common treatment for early stage prostate cancer. The doctor may remove all of the prostate (a type of surgery called radical prostatectomy) or only part of it. In some cases, the doctor can use a new technique known as nerve-sparing surgery. This type of surgery may save the nerves that control erection. However, men with large tumors or tumors that are very close to the nerves may not be able to have this surgery. | |||
| Some CRPC treatments are used only in men whose tumors have certain characteristics that make the therapy more likely to be effective. Men whose tumors express the protein ] may receive the radiopharmaceutical ], which binds to and destroys PSMA-positive cells.<ref>{{cite web|url=https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer |accessdate=9 January 2024 |title=FDA Approves Pluvicto for Metastatic Castration-Resistant Prostate Cancer |publisher=U.S. Food & Drug Administration |date=23 March 2022}}</ref>{{sfn|Scher|Eastham|2022|loc="Metastatic disease: castrate"}} Those whose tumors have defective ] benefit from treatment with the ] drug ] and ] inhibitors, namely ], ], or ].{{sfn|Scher|Eastham|2022|loc="Metastatic disease: castrate"}} | |||
| The doctor can describe the types of surgery and can discuss and compare their benefits and risks. | |||
| *In radical retropubic prostatectomy, the doctor removes the entire prostate and nearby lymph nodes through an incision in the abdomen. | |||
| *In radical perineal prostatectomy, the doctor removes the entire prostate through an incision between the ] and the ]. Nearby lymph nodes are sometimes removed through a separate incision in the abdomen. | |||
| === Supportive care === | |||
| Surgical removal of the prostate and surrounding tissue (radical prostatectomy) has long been recognized as one of the most effective forms of therapy for prostate cancer. A conventional radical prostatectomy involves an open incision across the abdomen. Robotic laparoscopic prostatectomy represents a major advance in the treatment of prostate cancer by offering men a less-invasive alternative to traditional surgery. The surgeon performs the same nerve-sparing procedure done in conventional laparoscopic prostatectomy, but with greater precision. The prostate, nearby lymph nodes, seminal vesicles and adjacent tissue are removed through the small incisions, which are closed with a few stitches. | |||
| Bone metastases – present in around 85% of those with metastatic prostate cancer – are the primary cause of symptoms and death from metastatic prostate cancer.{{sfn|Coleman|Croucher|Padhani|Clézardin|2020|loc="Prevalence of bone metastases"}}{{sfn|Coleman|Croucher|Padhani|Clézardin|2020|loc="Prevalence of SREs"}} Those with constant pain are prescribed ]s.{{sfn|Coleman|Croucher|Padhani|Clézardin|2020|loc="Analgesics used in CIBP"}} However, people with bone metastases can experience "breakthrough pain", sudden bursts of severe pain that resolve within around 15 minutes, before ] can take effect.{{sfn|Coleman|Croucher|Padhani|Clézardin|2020|loc="Analgesics used in CIBP"}} Single sites of pain can be treated with ] to shrink nearby tumors.{{sfn|Scher|Eastham|2022|loc="Pain management"}} More dispersed bone pain can be treated with radioactive compounds that disproportionately accumulate in bone, like ] and ], which help reduce the size of bone tumors. Similarly, the systemic chemotherapeutics used for metastatic prostate cancer can reduce pain as they shrink tumors.{{sfn|Scher|Eastham|2022|loc="Pain management"}} Other bone modifying agents like ] and ] can reduce prostate cancer bone pain, even though they have little effect on tumor size.{{sfn|Scher|Eastham|2022|loc="Pain management"}} Metastases compress the spinal cord in up to 12% of those with metastatic prostate cancer causing pain, weakness, numbness, and paralysis.{{sfn|Thompson|Wood|Feuer|2007|loc="Cord compression"}}<ref name=MSCC>{{cite web|url=https://prostatecanceruk.org/prostate-information-and-support/advanced-prostate-cancer/metastatic-spinal-cord-compression-mscc?scrollTo=symptoms-of-MSCC |access-date=25 June 2023 |title=What is Metastatic Spinal Cord Compression (MSCC)? |publisher=Prostate Cancer UK |date=June 2022}}</ref> Inflammation in the spine can be treated with high-dose steroids, as well as surgery and radiotherapy to shrink spinal tumors and relieve pressure on the spinal cord.{{sfn|Thompson|Wood|Feuer|2007|loc="Cord compression"}}<ref name=MSCC/> | |||
| *In transurethral resection of the prostate (TURP), the doctor removes part of the prostate with an instrument that is inserted through the urethra. The cancer is cut from the prostate by electricity passing through a small wire loop on the end of the instrument. This method is used mainly to remove tissue that blocks urine flow. | |||
| Those with advanced prostate cancer suffer fatigue, lethargy, and a generalized weakness. This is caused in part by gastrointestinal problems, with ], weight loss, nausea, and ] all common. These are typically treated with appetite-increasing drugs – ] or corticosteroids – ]s, or treatments that focus on underlying gastrointestinal issues.{{sfn|Thompson|Wood|Feuer|2007|loc="Gastrointestinal symptoms"}} General weakness can also be caused by ], itself caused by a combination of the disease itself, poor nutrition, and damage to the bone marrow from cancer treatments or bone metastases.{{sfn|Thompson|Wood|Feuer|2007|loc="General debility"}} Anemia can be treated in various ways depending on the cause, or can be addressed directly with ]s.{{sfn|Thompson|Wood|Feuer|2007|loc="General debility"}} Organ damage and metastases in the lymph nodes can lead to uncomfortable accumulation of fluid (called ]) in the genitals or lower limbs. These swellings can be extremely painful, curtailing an affected person's ability to urinate, have sex, or walk normally. Lymphedema can be treated by applying pressure to aid drainage, surgically draining pooled fluid, and cleaning and treating nearby damaged skin.{{sfn|Thompson|Wood|Feuer|2007|loc="Lymphoedema"}} | |||
| If the pathologist finds cancer cells in the lymph nodes, it is likely that the disease has spread to other parts of the body. Sometimes, lymph nodes are removed before doing a prostatectomy. If the prostate cancer has not spread to the lymph nodes, the doctor then removes the prostate. But if cancer has spread to the nodes, the doctor usually does not remove the prostate, but may suggest other treatment. | |||
| People with prostate cancer are around twice as likely to experience ] or ] compared to those without cancer.{{sfn|Mundle|Afenya|Agarwal|2021|loc="Estimates of anxiety, depression, and distress"}} When added to normal prostate cancer treatments, psychological interventions such as ] and ] can help reduce anxiety, depression, and general distress.{{sfn|Mundle|Afenya|Agarwal|2021|loc="Abstract"}} | |||
| While the above was the standard of care through the 1980s and early 1990s recent Journal publications indicate that "Radical prostatectomy combined with early adjunctive hormonal therapy for patients with nodal metastasis is superior to all other forms of therapy and should be considered the standard of care. This approach provides survival rates comparable with patients with clinically organ-confined prostate cancer." Radical prostatectomy for the patient with locally advanced prostate cancer.{{an|Ward}} | |||
| As those severely ill with metastatic prostate cancer approach the end of their lives, most experience confusion and may ] or have trouble recognizing loved ones.{{sfn|Thompson|Wood|Feuer|2007|loc="Delirium"}}<ref name=PCUK>{{cite web|url=https://prostatecanceruk.org/prostate-information-and-support/advanced-prostate-cancer/dying-from-prostate-cancer/what-to-expect |access-date= 25 June 2023 |title=Dying from Prostate Cancer – What to Expect |publisher=Prostate Cancer UK |date=July 2018}}</ref> Confusion is caused by various conditions, including ], ], ], and as a side effect of various drugs, especially ]s.{{sfn|Thompson|Wood|Feuer|2007|loc="Delirium"}} Most people sleep for long periods, and some feel drowsy when awake.<ref name=PCUK/> Restlessness is also common, sometimes caused by physical discomfort from constipation or ], sometimes caused by anxiety.<ref name=PCUK/> In their last few days, affected men's breathing may become shallow and slow, with long pauses between breaths. Breathing may be accompanied by a ] as fluid lingers in the throat, but this is not uncomfortable for the affected person.<ref name=PCUK/><ref name=ASCO>{{cite web|url=https://www.cancer.net/navigating-cancer-care/advanced-cancer/care-through-final-days |access-date=25 June 2023 |title=Care Through the Final Days |date=November 2022 |publisher=American Society of Clinical Oncology}}</ref> Their hands and feet may cool to the touch, and skin become blotchy or blue due to weaker blood circulation. Many stop eating and drinking, resulting in dry-feeling mouth, which can be aided by moistening the mouth and lips.<ref name=PCUK/> The person becomes less and less responsive, and eventually the heart and breathing stop.<ref name=ASCO/> | |||
| Patients are often uncomfortable for the first few days after surgery. Pain usually can be controlled with medicine, and patients should discuss pain relief with the doctor or nurse. The patient will wear a ] (a tube inserted into the urethra) to drain urine for 10 days to 3 weeks. The nurse or doctor will show the man how to care for the catheter. It is also common for patients to feel extremely tired or weak for a while. The length of time it takes to recover from an operation varies. | |||
| ==Prognosis== | |||
| Surgery to remove the prostate can cause long-term problems, including ] and/or fecal or urinary ]. Nerve-sparing surgery is an attempt to avoid the problem of impotence. When the doctor can use nerve-sparing surgery and the operation is fully successful, impotence may be only temporary or partial. Still, some men who have this procedure may be permanently impotent. Different men experience these side effects to be a greater or lesser problem. Men who have a prostatectomy no longer produce semen, so they have dry orgasms. Men who wish to father children may consider sperm banking or a sperm retrieval procedure. | |||
| The prognosis of diagnosed prostate cancer varies widely based on the cancer's grade and stage at the time of diagnosis; those with lower stage disease have vastly improved prognoses. Around 80% of prostate cancer diagnoses are in men whose cancer is still confined to the prostate. These men can survive long after diagnosis, with as many as 99% still alive 10 years from diagnosis.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Prognosis and survival"}} Men whose cancer has metastasized to a nearby part of the body (around 15% of diagnoses) have poorer prognoses, with ] rates of 60–80%.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Figure 3: Prostate cancer stages and progression."}} Those with metastases in distant body sites (around 5% of diagnoses) have relatively poor prognoses, with five-year survival rates of 30–40%.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Figure 3: Prostate cancer stages and progression."}} | |||
| Those who have low blood PSA levels at diagnosis, and whose tumors have a low Gleason grade and less-advanced clinical stage tend to have better prognoses.{{sfn|Pilié|Viscuse|Logothetis|Corn|2022|loc=Table 45-2}} After prostatectomy or radiotherapy, those who have a short time between treatment and a subsequent rise in PSA levels, or quickly rising PSA levels are more likely to die from their cancers.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Biochemical recurrence and residual disease"}} Castration-resistant metastatic prostate cancer is incurable,{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Abstract"}} and kills a majority of those whose disease reaches this stage.{{sfn|Scher|Eastham|2022|loc="Metastatic disease: castrate"}} | |||
| === Radiation therapy === | |||
| == Cause == | |||
| ] (also called ]) uses high-energy x-rays to kill cancer cells. Like surgery, radiation therapy is local therapy; it can affect cancer cells only in the treated area. In early stage prostate cancer, radiation can be used instead of surgery, or it may be used after surgery to destroy any cancer cells that may remain in the area. In advanced stages, it may be given to relieve pain or other problems. | |||
| Prostate cancer is caused by the accumulation of ]s to the ] of cells in the prostate. These mutations affect genes involved in cell growth, replication, ], and ].{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Genetics"}} With these processes dysregulated, some cells replicate abnormally, forming a clump of cells called a tumor.<ref>{{cite web|url=https://www.cancer.org/cancer/types/prostate-cancer/causes-risks-prevention/what-causes.html |access-date=17 May 2023 |title=What Causes Prostate Cancer? |publisher=American Cancer Society |date=1 August 2019}}</ref> As the tumor grows, its cells accumulate more mutations, allowing it to stimulate the growth of new blood vessels to support further growth.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Disease progression"}} Eventually, a tumor can grow large enough to invade nearby organs such as the ] or ].<ref>{{cite web|url=https://www.cancerresearchuk.org/about-cancer/prostate-cancer/stages/locally-advanced-prostate-cancer |access-date=21 May 2023 |title=Locally Advanced Prostate Cancer |publisher=Cancer Research UK |date=31 May 2022}}</ref> In advanced tumors, cells can develop the ability to detach from their original tissue site, and evade the ].{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Disease progression"}} These cells can spread through the ] to nearby ]s, or through the bloodstream to the bone marrow and (more rarely) other body sites.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Disease progression"}} At these new sites, the cancer cells disrupt normal body function and continue to grow. Metastases cause most of the discomfort associated with prostate cancer, and can eventually kill the affected person.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Disease progression"}} | |||
| == Pathophysiology == | |||
| Radiation may be directed at the body by a ], or it may come from tiny radioactive seeds placed inside or near the tumor (internal or implant radiation, or brachytherapy). Men who receive radioactive seeds alone usually have small tumors. Some men with prostate cancer receive both kinds of radiation therapy. | |||
| Most prostate tumors begin in the peripheral zone – the outermost part of the prostate.{{sfn|Sandhu|Moore|Chiong|Beltran|2021|loc="The biology of prostate cancer"}} As cells begin to grow out of control, they form a small clump of disregulated cells called a ] (PIN).<ref name=ACS-Pins>{{cite web|url=https://www.cancer.org/cancer/diagnosis-staging/tests/understanding-your-pathology-report/prostate-pathology/high-grade-prostatic-intraepithelial-neoplasia.html |access-date=25 May 2023 |title=Understanding Your Pathology Report: Prostatic Intraepithelial Neoplasia (PIN) and Intraductal Carcinoma |publisher=American Cancer Society}}</ref> Some PINs continue to grow, forming layers of tissue that stop expressing genes common to their original tissue location – ], ], and ] – and instead begin expressing genes typical of cells in the innermost lining of the pancreatic duct – ] and ].{{sfn|Sandhu|Moore|Chiong|Beltran|2021|loc="The biology of prostate cancer"}} These multilayered PINs also often overexpress the gene ], which is associated with prostate cancer progression.{{sfn|Sandhu|Moore|Chiong|Beltran|2021|loc="The biology of prostate cancer"}} | |||
| For external radiation therapy, patients go to the hospital or clinic, usually 5 days a week for several weeks. Patients may stay in the hospital for a short time for implant radiation. | |||
| Some PINs can eventually grow into tumors.<ref name=ACS-Pins/> This is commonly accompanied by large-scale changes to the ], with ] sequences being rearranged or copied repeatedly. Some genomic alterations are particularly common in early prostate cancer, namely ] between ] and the oncogene ] (up to 60% of prostate tumors), mutations that disable ] (up to 15% of tumors), and mutations that hyperactivate ] (up to 5% of tumors).{{sfn|Sandhu|Moore|Chiong|Beltran|2021|loc="The biology of prostate cancer"}} | |||
| ] may cause patients to become extremely tired, especially in the later weeks of treatment. Resting is important, but doctors usually encourage men to try to stay as active as they can. Some men may have diarrhea or frequent and uncomfortable urination. | |||
| Metastatic prostate cancer tends to have more genetic mutations than localized disease.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Metastatic disease"}} Many of these mutations are in genes that protect from DNA damage, such as ] (mutated in 8% of localized tumors, more than 27% of metastatic ones) and ] (1% of localized tumors, more than 5% of metastatic ones).{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Metastatic disease"}} Similarly mutations in the DNA repair-related genes ] and ] are rare in localized disease but found in at least 7% and 5% of metastatic disease cases respectively.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Metastatic disease"}} | |||
| When men with prostate cancer receive external radiation therapy, it is uncommon for the skin in the treated area to become red, dry, or tender, however there may be hair loss in the treated area. The loss is usually temporary. | |||
| The transition from castrate-sensitive to castrate-resistant prostate cancer is also accompanied by the acquisition of various gene mutations. In castrate-resistant disease, more than 70% of tumors have mutations in the ] signaling pathway – amplifications and gain-of-function mutations in the receptor gene itself, amplification of its activators (for example, FOXA1), or inactivating mutations in its negative regulators (for example, ] and ]).{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Metastatic disease"}} These androgen receptor disruptions are only found in up to 6% of biopsies of castrate-sensitive metastatic disease.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Metastatic disease"}} Similarly, deletions of the tumor suppressor ] are harbored by 12–17% of castrate-sensitive tumors, but over 40% of castrate-resistant tumors.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Metastatic disease"}} Less commonly, tumors have aberrant activation of the ] via disruption of members ] (9% of tumors) or ] (4% of tumors); or dysregulation of the ] pathway via ]/] mutations (6% of tumors) or ] (2% of tumors).{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Metastatic disease"}} | |||
| Both types of radiation therapy may cause impotence in some men. While internal radiation therapy may cause temporary urinary incontinence, external radiation therapy causes temporary bowel inflammation. Long-term side effects from internal radiation therapy are uncommon. | |||
| == Epidemiology == | |||
| External beam radiotherapy with curative intent for localised prostate cancer is frequently given with concurrent hormone ablation therapy. The indications for adding hormone therapy are currently (September 2005) in a state of flux, as is the recommended interval for such treatment. Generally, patients who are thought to have a significant (>15%) risk of lymph node involvement or spread beyond the prostate are given concurrent hormone ablation drugs. Oncologists give such treatment anywhere from 2 months to 3 years in overall duration. | |||
| ] | |||
| Prostate cancer is the second-most frequently diagnosed cancer in men, and the second-most frequent cause of cancer death in men (after ]).{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Epidemiology"}}{{sfn|Scher|Eastham|2022|loc="Prostate cancer"}} Around 1.2 million new cases of prostate cancer are diagnosed each year, and over 350,000 people die of the disease, annually.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Epidemiology"}} One in eight men are diagnosed with prostate cancer in their lifetime, and around one in forty die of the disease.{{sfn|Scher|Eastham|2022|loc="Prostate cancer"}} Rates of prostate cancer rise with age. Due to this, prostate cancer rates are generally higher in parts of the world with higher life expectancy, which also tend to be areas with higher ] and higher ].{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Epidemiology"}} Australia, Europe, North America, New Zealand, and parts of South America have the highest incidence. South Asia, Central Asia, and sub-Saharan Africa have the lowest incidence of prostate cancer; though incidence is increasing quickly in these regions.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Epidemiology"}} Prostate cancer is the most diagnosed cancer in men in over half of the world's countries, and the leading cause of cancer death in men in around a quarter of countries.{{sfn|Bergengren|Pekala|Matsoukas|Fainberg|2023|loc="3.1 Epidemiology"}} | |||
| External beam radiotherapy, prostate implant brachytherapy, and radical surgery all appear equally efficacious in curing localised prostate cancer. | |||
| Prostate cancer is rare in those under 40 years old,{{sfn|Pernar|Ebot|Wilson|Mucci|2018|loc="Risk factors for total prostate cancer"}} and most cases occur in those over 60 years,{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Epidemiology"}} with the average person diagnosed at 67.{{sfn|Stephenson|Abouassaly|Klein|2021|loc="Age at diagnosis"}} The average age of those who die from prostate cancer is 77.{{sfn|Stephenson|Abouassaly|Klein|2021|loc="Age at diagnosis"}} Only a minority of prostate cancer cases are diagnosed. ] of men who died at various ages have shown cancer in the prostates of over 40% of men over age 50. Incidence rises with age, and nearly 70% of men autopsied at age 80–89 had cancer in their prostates.{{sfn|Dall'Era|2023|loc="General considerations"}} | |||
| === Cryotherapy === | |||
| === Genetics === | |||
| ] is another method of treating prostate cancer. Cryotherapy is the insertion of metal rods into the prostate and circulating liquid nitrogen through these rods. This process lowers the temperature to about minus 374° F. As the tissue freezes, the formation and expansion of ice crystals within the cancerous cells cause them to rupture and die. A catheter is placed inside the urethra and a warming solution is circulated to prevent damage to the urethra. Short term results have been good however long term results appear to suggest that it is not as effective as surgery or radiation. Additionally, impotence results from cryotherapy 90 percent of the time. | |||
| Prostate cancer is more common in families with a history of any cancer.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Genetic predisposition"}} Men with an affected first-degree relative (father or brother) have more than twice the risk of developing prostate cancer, and those with two first-degree relatives have a five-fold greater risk compared with men with no family history.{{sfn|Scher|Eastham|2022|loc="Epidemiology"}} Increased risk also runs in some ethnic groups, with men of African and ] ancestry at particularly high risk – having prostate cancer at higher rates, and having more-aggressive prostate cancers that develop at earlier ages.{{sfn|McHugh|Saunders|Dadaev|McGrowder|2022|loc="Introduction"}} Large ] have identified over 100 gene variants associated with increased prostate cancer risk.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Genetic predisposition"}} The greatest risk increase is associated with variations in ] (up to an eight-fold increased risk) and ] (three-fold increased risk), both of which are involved in repairing ].{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Genetic predisposition"}} Variants in other genes involved in DNA damage repair have also been associated with an increased risk of developing prostate cancer – particularly early-onset prostate cancer – including ], ], ], ], ], ], ], ], and ].{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Genetic predisposition"}} Additionally, variants in the genome near the ] ] are associated with increased risk.{{sfn|Rebello|Oing|Knudsen|Loeb|2021|loc="Genetic predisposition"}} As are ]s in the ] common in African-Americans, and in the ], ], and ] involved in ] synthesis and signaling.{{sfn|Scher|Eastham|2022|loc="Epidemiology"}} Together, known gene variants are estimated to cause around 25% of prostate cancer cases, including 40% of early-onset prostate cancers.{{sfn|Scher|Eastham|2022|loc="Epidemiology"}} | |||
| === |
=== Body and lifestyle === | ||
| Men who are taller are at a slightly increased risk for developing prostate cancer, as are men who are ].{{sfn|Pernar|Ebot|Wilson|Mucci|2018|loc="Risk factors for advanced and fatal prostate cancer"}} High levels of blood cholesterol are also associated with increased prostate cancer risk; consequently, those who take the cholesterol-lowering drugs, ]s, have a reduced risk of advanced prostate cancer.{{sfn|Pernar|Ebot|Wilson|Mucci|2018|loc="Statins"}} Chronic inflammation can cause various cancers. Potential links between infection (or other sources of inflammation) and prostate cancer have been studied but none definitively found, and one large study found no link between prostate cancer and a history of ], ], ], or infection with various ]es.{{sfn|Stephenson|Abouassaly|Klein|2021|loc="Inflammation and infection"}} | |||
| Regular vigorous exercise may reduce one's chance of developing advanced prostate cancer, as can several dietary interventions.{{sfn|Pernar|Ebot|Wilson|Mucci|2018|loc="Exercise"}} Those with a diet rich in ]s (certain leafy greens, broccoli, and cauliflower), ], ] (found in ]), or ] (found in tomatoes) are at a reduced risk of symptomatic prostate cancer.{{sfn|Scher|Eastham|2022|loc="Epidemiology"}}{{sfn|Pernar|Ebot|Wilson|Mucci|2018|loc="Fish"}} Conversely, those who consume high levels of dietary fats, ] (from cooking red meats), or calcium may be at an increased risk of developing advanced prostate cancer.{{sfn|Scher|Eastham|2022|loc="Epidemiology"}}{{sfn|Pernar|Ebot|Wilson|Mucci|2018|loc="Calcium, dairy products, and vitamin D"}} Several dietary supplements have been studied and found not to impact prostate cancer risk, including ], ], ], and ].{{sfn|Scher|Eastham|2022|loc="No cancer diagnosis"}}{{sfn|Pernar|Ebot|Wilson|Mucci|2018|loc="Calcium, dairy products, and vitamin D"}} | |||
| ] keeps cancer cells from getting the male hormones they need to grow. It is called systemic therapy because it can affect cancer cells throughout the body. Systemic therapy is used to treat cancer that has spread. Sometimes this type of therapy is used to try to prevent the cancer from coming back after surgery or radiation treatment. | |||
| == Special populations == | |||
| There are several forms of hormonal therapy: | |||
| ] and ] people who have prostates can develop prostate cancer. Those who have undergone ] or ] have reduced risk of developing prostate cancer, relative to ] men of similar age.{{sfn|Bertoncelli Tanaka|Sahota|Burn|Falconer|2022|loc="Conclusions"}} Screening tests in this group are complicated, as transgender women may have lower PSA levels than cisgender men due to their reduced testosterone levels.{{sfn|Bertoncelli Tanaka|Sahota|Burn|Falconer|2022|loc="Interpretation of PSA in transgender women on GAHT"}} PSA levels greater than 1 ng/mL are generally considered above normal by gender care specialists.{{sfn|Bertoncelli Tanaka|Sahota|Burn|Falconer|2022|loc="Screening for prostate cancer in transgender women"}} Digital rectal exams of the prostate are often impossible in women who have undergone ], as the length and rigidity of the new vagina can obstruct access to the prostate from the rectum.{{sfn|Bertoncelli Tanaka|Sahota|Burn|Falconer|2022|loc="Screening for prostate cancer in transgender women"}} | |||
| *] is surgery to remove the testicles, which are the main source of male hormones. | |||
| *Drugs known as luteinizing hormone-releasing hormone (LHRH) agonists can prevent the testicles from producing testosterone. Examples are ], ], and ]. | |||
| *Drugs known as ]s can block the action of androgens. Two examples are ] and ]. | |||
| *Drugs that can prevent the adrenal glands from making androgens include ] and ]. These drugs are rarely used for this purpose. | |||
| == History == | |||
| After orchiectomy or treatment with an LHRH agonist, the body no longer gets testosterone from the testicles. However, the adrenal glands still produce small amounts of male hormones. Sometimes, the patient is also given an antiandrogen, which blocks the effect of any remaining male hormones. This combination of treatments is known as total androgen blockade (TAB), combined hormonal therapy (CHT), combined androgen blockade (CAB), or maximal androgen deprivation (MAD). Doctors do not know for sure whether total androgen blockade is more effective than orchiectomy or LH-RH agonist alone. | |||
| ] | |||
| A prostate mass was first described in 1817 by the English surgeon ], following the autopsy of a man who had died at age 68 with lower-body pain and urinary issues.{{sfn|Valier|2016|pp=15–18}}<ref>{{cite journal |vauthors=Lawrence W |title=Cases of Fungus Hæmatodes, with Observations, by George Langstaff, Esq. and an Appendix, Containing two Cases of Analogous Affections |journal=Med Chir Trans |volume=8 |issue= |pages=272–314 |date=1817 |pmid=20895322 |pmc=2129005 |doi=10.1177/095952871700800114 }}</ref> In 1853, ] surgeon John Adams described another prostate tumor from a man who had died with urinary issues; Adams had a pathologist examine the tumor, providing the first confirmed case of a cancerous tumor in the prostate.{{sfn|Valier|2016|pp=15–18}}<ref>{{cite journal | vauthors = Adams J | title = The Case of Scirrhous of the Prostate Gland with Corresponding Affliction of the Lymphatic Glands in the Lumbar Region and in the Pelvis | journal = Lancet | volume = 1 | issue = 1547 | pages = 393–94 | year = 1853 | doi = 10.1016/S0140-6736(02)68759-8 }}</ref> The disease was initially rarely described; an 1893 report found only 50 cases described in the medical literature.{{sfn|Lytton|2001|p=1859}} Around the turn of the 19th century, prostate surgery to relieve urinary obstruction became more common, allowing surgeons and pathologists to examine the removed prostate tissue. Two studies around the time found cancer in as many as 10% of surgical specimens, suggesting prostate cancer was a fairly common cause of prostate enlargement.{{sfn|Lytton|2001|p=1859}} | |||
| Prostate cancer that has spread to other parts of the body usually can be controlled with hormonal therapy for a period of time, often several years. Eventually, however, most prostate cancers are able to grow with very little or no male hormones. When this happens, hormonal therapy is no longer effective, and the doctor may suggest other forms of treatment that are under study. | |||
| For much of the 20th century, the primary therapy for prostate cancer was surgery to remove the prostate. Perineal prostatectomy was first performed in 1904 by ] at ].{{sfn|Denmeade|Isaacs|2002|loc="Prostatectomy"}}<ref>{{cite journal | vauthors = Young HH | title = Four Cases of Radical Prostatectomy | journal = Johns Hopkins Bull. | volume = 16 | year = 1905 }}</ref> Young's method became the widespread standard, initially done primarily to relieve symptoms of urinary blockage.{{sfn|Denmeade|Isaacs|2002|loc="Prostatectomy"}} In 1931 a new surgical method, ], became available, replacing perineal prostatectomy for symptomatic relief of obstruction.{{sfn|Lytton|2001|p=1859}} In 1945, ] described a retropubic prostatectomy approach, which provided easier access to pelvic lymph nodes to assist in staging the extent of disease, and was easier for surgeons to learn.{{sfn|Denmeade|Isaacs|2002|loc="Prostatectomy"}} This was improved upon by ]'s 1983 description of a retropubic prostatectomy approach that avoided damage to the nerves near the prostate, preserving erectile function.{{sfn|Denmeade|Isaacs|2002|loc="Prostatectomy"}}<ref>{{cite journal | vauthors = Walsh PC, Lepor H, Eggleston JC | title = Radical Prostatectomy with Preservation of Sexual Function: Anatomical and Pathological Considerations | journal = The Prostate | volume = 4 | issue = 5 | pages = 473–485 | year = 1983 | pmid = 6889192 | doi = 10.1002/pros.2990040506 | s2cid = 30740301 }}</ref> | |||
| The side effects of hormonal therapy depend largely on the type of treatment. Orchiectomy and LH-RH agonists often cause side effects such as impotence, hot flashes and loss of sexual desire. When first taken, an LH-RH agonist may make a patient's symptoms worse for a short time. This temporary problem is called "flare." Gradually, however, the treatment causes a man's testosterone level to fall. Without testosterone, tumor growth slows down and the patient's condition improves. (To prevent flare, the doctor may give the man an antiandrogen for a while along with the LH-RH agonist.) In some cases, men may be prescribed intermittent courses of hormone therapy, with careful monitoring by their doctor to determine when to begin the next course of treatment. | |||
| Radiation therapy for prostate cancer was used occasionally in the early 20th century, with ] implanted into the ] or rectum to reduce the tumor size and associated symptoms.{{sfn|Denmeade|Isaacs|2002|loc="Radiation therapy"}} In the 1950s the advent of more powerful radiation machines allowed for external beam radiotherapy to reach the prostate. By the 1960s, this was often combined with hormone therapy to improve the potency of therapy.{{sfn|Denmeade|Isaacs|2002|loc="Radiation therapy"}} In the 1970s, ] pioneered an open surgery technique where needles of ] were placed directly into the prostate. This was improved upon by Henrik H. Holm in 1983 by using transrectal ultrasound to guide the implantation of radioactive material.{{sfn|Denmeade|Isaacs|2002|loc="Radiation therapy"}} | |||
| Antiandrogens can cause nausea, vomiting, diarrhea, or breast growth or tenderness. If used a long time, ketoconazole may cause liver problems, and aminoglutethimide can cause skin rashes. Men who receive total androgen blockade may experience more side effects than men who receive a single method of hormonal therapy. Any method of hormonal therapy that lowers androgen levels can contribute to weakening of the bones in older men. | |||
| The observation that the testicles (and the hormones they secrete) influence prostate size was made as early as the late 18th century via castration experiments in animals. However, occasional experimentation over the next century bore mixed results, likely due to the inability to separate prostate tumors from prostates enlarged due to benign prostatic hyperplasia. In 1941, ] and ] published two studies using surgical castration or oral ] to reduce androgen levels and improve prostate cancer symptoms. Huggins was awarded the 1966 Nobel Prize in Physiology or Medicine for this discovery, the first systemic therapy for prostate cancer.{{sfn|Denmeade|Isaacs|2002|loc="Androgen-ablation therapy"}}<ref>{{cite journal | vauthors = Huggins CB, Hodges CV | title = Studies on Prostate Cancer: 1. The Effects of Castration, of Estrogen and Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate | journal = Cancer Res | volume = 1 | issue = 4 | pages = 293 | year = 1941 | url = http://cancerres.aacrjournals.org/content/1/4/293 | archive-url = https://web.archive.org/web/20170630121943/http://cancerres.aacrjournals.org/content/1/4/293 | archive-date = 30 June 2017 | access-date = 2 September 2015 }}</ref> In the 1960s, large studies showed estrogen therapy to be as effective as surgical castration at treating prostate cancer, but that those on estrogen therapy were at increased risk of suffering ]s.{{sfn|Denmeade|Isaacs|2002|loc="Androgen-ablation therapy"}} Through the 1980s, ]'s studies of GnRH led to the development of GnRH agonists, which were found to be as effective as estrogen without the increased risk of clotting.{{sfn|Denmeade|Isaacs|2002|loc="Androgen-ablation therapy"}}<ref>{{cite journal | vauthors = Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, Comaru-Schally AM, Schally AV | title = Tumor Growth Inhibition in Patients with Prostatic Carcinoma Treated with Luteinizing Hormone-Releasing Hormone Agonists | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 79 | issue = 5 | pages = 1658–1662 | date = March 1982 | pmid = 6461861 | pmc = 346035 | doi = 10.1073/pnas.79.5.1658 | doi-access = free | title-link = doi | bibcode = 1982PNAS...79.1658T }}</ref> Schally was awarded the 1977 Nobel Prize in Physiology or Medicine for his work on GnRH and prostate cancer.{{sfn|Denmeade|Isaacs|2002|loc="Androgen-ablation therapy"}} | |||
| === Support for men with prostate cancer === | |||
| Systemic chemotherapy for prostate cancer has been studied since the 1950s but clinical trials failed to show benefits in most people who receive the drugs.{{sfn|Denmeade|Isaacs|2002|loc="Cytotoxic chemotherapy"}} In 1996, the US ] approved the systemic chemotherapy ] for those with castration-resistant prostate cancer based on trials showing that it improved symptoms even though it failed to enhance survival.{{sfn|Desai|McManus|Sharifi|2021|loc="Evolution of treatment"}} In 2004, docetaxel was approved as the first chemotherapy to increase survival in those with castration-resistant prostate cancer.{{sfn|Desai|McManus|Sharifi|2021|loc="Evolution of treatment"}} After additional trials in 2015, docetaxel use was extended to those with castration-sensitive prostate cancer.{{sfn|Teo|Rathkopf|Kantoff|2019|loc="Figure 1"}} | |||
| Living with a serious disease such as cancer is not easy. Some people find they need help coping with the emotional as well as the practical aspects of their disease. Patients often get together in support groups, where they can share what they have learned about coping with their disease and the effects of treatment. Patients may want to talk with a member of their health care team about finding a support group. | |||
| == Society and culture == | |||
| People living with cancer may worry about caring for their families, keeping their jobs, or continuing daily activities. Concerns about treatments and managing side effects, hospital stays, and medical bills are also common. Doctors, nurses, dietitians and other members of the health care team can answer questions about treatment, working, or other activities. Meeting with a social worker, counselor, or member of the clergy can be helpful to those who want to talk about their feelings or discuss their concerns. Often, a social worker can suggest resources for help with rehabilitation, emotional support, financial aid, transportation, or home care. | |||
| Prostate cancer screening and awareness have been widely promoted since the early 2000s by ] in September and ] in November.{{sfn|Patel|Halpern|Desai|Keeter|2020|p=64}} However, an analysis of internet searches suggests neither event changes the level of prostate cancer interest or discussion much, in contrast to the more established ].{{sfn|Patel|Halpern|Desai|Keeter|2020|p=64}} | |||
| == Research == | |||
| It is natural for a man and his partner to be concerned about the effects of prostate cancer and its treatment on their sexual relationship. They may want to talk with the doctor about possible side effects and whether these are likely to be temporary or permanent. Whatever the outlook, it is usually helpful for patients and their partners to talk about their concerns and help one another find ways to be intimate during and after treatment. | |||
| Prostate cancer is a major topic of ongoing research. From 2016–2020, over $1.26 billion was invested in prostate cancer research, representing around 5% of global cancer research funds.{{sfn|McIntosh|Alam|Adams|Boon|2023|loc=Table 1}} This places prostate cancer 10th among 18 common cancer types in funding per cancer death, and 9th in funding per ] lost.{{sfn|McIntosh|Alam|Adams|Boon|2023|loc=Table 2}} | |||
| == Current research == | |||
| Research into prostate cancer relies on a number of laboratory models to test aspects of the disease. Several prostate ]s are widely used, namely the classic lines ], ], and ], as well as more recent cell lines 22Rv1, ], ], and MDA-PCa-2a and −2b.{{sfn|Mai|Chin|Foong|Pang|2022|loc="2.1 Human prostate cancer cell lines"}} Research requiring more complex models of the prostate uses ]s – clusters of prostate cells that can be grown from human prostate tumors or stem cells.{{sfn|Mai|Chin|Foong|Pang|2022|loc="2.4. Organoids"}} Modeling tumor growth and metastasis requires a ], typically a mouse. Researchers can either surgically implant human prostate tumors into immunocompromised mice (a technique called a ]),{{sfn|Mai|Chin|Foong|Pang|2022|loc="2.2 PDX lines"}} or induce prostate tumors in mice with ].{{sfn|Mai|Chin|Foong|Pang|2022|loc="2.3 Genetically engineered mouse model (GEMM)"}} These genetically engineered mouse models typically use a ] system to disrupt ]s or activate ]s specifically in prostate cells.{{sfn|Ittmann|Huang|Radaelli|Martin|2013|loc="Introduction"}} | |||
| Many types of ]s on prostate cancer are being conducted. These include studies of ways to prevent, detect, diagnose, and treat prostate cancer; studies of the psychological effects of the disease; and studies of ways to improve comfort and ]. | |||
| == |
== Notes == | ||
| {{reflist|group=note}} | |||
| {{notelist}} | |||
| == References == | |||
| Although several risk factors for prostate cancer are known, it is still not sure why one man develops the disease and another doesn't. Some aspects of a man's lifestyle may affect his chances of developing prostate cancer. For example, some evidence suggests a link between diet and this disease. These studies show that prostate cancer is more common in populations that consume a high-fat diet (particularly animal fat), and in populations that have diets lacking certain nutrients. Although it is not known whether a diet low in fat will prevent prostate cancer, a low-fat diet may have many other health benefits. | |||
| {{Reflist}} | |||
| === Works cited === | |||
| There are large differences in prostate cancer risk between racial groups, but it is not yet clear why this is so. African Americans have the highest incidence of prostate cancer in the world. | |||
| {{refbegin|32em}} | |||
| * {{cite journal |vauthors=Achard V, Putora PM, Omlin A, Zilli T, Fischer S |title=Metastatic Prostate Cancer: Treatment Options |journal=Oncology |volume=100 |issue=1 |pages=48–59 |date=2022 |pmid=34781285 |doi=10.1159/000519861 |s2cid=244132770 }} | |||
| Researchers are interested in genes that may influence the risk of developing prostate cancer. They are studying the genes of men who were diagnosed with prostate cancer at a relatively young age (less than 55 years old) and the genes of families who have several members with the disease. However, there are, as yet, few identified genes that are known to affect prostate cancer risk. | |||
| * {{cite journal |vauthors=Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, Bray F, Brawley O, Luckenbaugh AN, Mucci L, Morgan TM, Carlsson SV |title=2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review |journal=Eur Urol |volume= 84|issue= 2|pages= 191–206|date=May 2023 |pmid=37202314 |doi=10.1016/j.eururo.2023.04.021 |s2cid=258780321 |doi-access = free | title-link = doi |pmc=10851915 }} | |||
| * {{cite journal |vauthors=Bertoncelli Tanaka M, Sahota K, Burn J, Falconer A, Winkler M, Ahmed HU, Rashid TG |title=Prostate Cancer in Transgender Women: What Does a Urologist Need to Know? |journal=BJU Int |volume=129 |issue=1 |pages=113–122 |date=January 2022 |pmid=34157213 |doi=10.1111/bju.15521 |url=}} | |||
| === Prevention === | |||
| * {{cite journal |vauthors=Brawley S, Mohan R, Nein CD |title=Localized Prostate Cancer: Treatment Options |journal=American Family Physician |volume=97 |issue=12 |pages=798–805 |date=June 2018 |pmid=30216009 | url=https://www.aafp.org/pubs/afp/issues/2018/0615/p798.html }} | |||
| * {{cite journal |vauthors=Carlsson SV, Vickers AJ |title=Screening for Prostate Cancer |journal=Med Clin North Am |volume=104 |issue=6 |pages=1051–1062 |date=November 2020 |pmid=33099450 |pmc=8287565 |doi=10.1016/j.mcna.2020.08.007 }} | |||
| Several studies are under way to explore how prostate cancer might be prevented. These include the use of dietary supplements, such as ] and ]. In addition, recent studies suggest that a diet that regularly includes tomato-based foods may help protect men from prostate cancer. | |||
| * {{cite journal |vauthors=Coleman RE, Croucher PI, Padhani AR, Clézardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R, Costa L |title=Bone Metastases |journal=Nat Rev Dis Primers |volume=6 |issue=1 |pages=83 |date=October 2020 |pmid=33060614 |doi=10.1038/s41572-020-00216-3 |hdl=20.500.11820/6bac9e59-0afa-4b4a-bebf-4e747b889917 |s2cid=222350837 |hdl-access=free }} | |||
| * {{cite journal |vauthors=Costello AJ |title=Considering the Role of Radical Prostatectomy in 21st Century Prostate Cancer Care |journal=Nat Rev Urol |volume=17 |issue=3 |pages=177–188 |date=March 2020 |pmid=32086498 |doi=10.1038/s41585-020-0287-y |s2cid=211234353 |url=}} | |||
| The drug ] was studied in the Prostate Cancer Prevention Trial. This drug is used to treat hair loss (marketed as Propecia). It functions by blocking the conversion of ] to ]. Thousands of men from the United States participated in the study for a period of 7 years. The results of the study are a key finding in prostate cancer research, especially because of its findings about the prevalence of prostate cancer among men with supposedly 'normal' PSA results. | |||
| * {{cite book|vauthors=Dall'Era MA |chapter=39-17: Prostate Cancer |title=Current Medical Diagnosis & Treatment 2023 |date=2023 |veditors=Papadakis MA, McPhee SJ, Rabow MW, McQuaid KR |publisher=McGraw Hill |edition=62 |isbn=978-1-264-68734-3}} | |||
| * {{cite journal | vauthors = Denmeade SR, Isaacs JT | title = A history of prostate cancer treatment | journal = Nature Reviews. Cancer | volume = 2 | issue = 5 | pages = 389–396 | date = May 2002 | pmid = 12044015 | pmc = 4124639 | doi = 10.1038/nrc801 }} | |||
| Scientists are also looking at ways to prevent recurrence among men who have been treated for prostate cancer. These approaches involve the use of drugs such as finasteride, ], and ]. Studies have shown that hormonal therapy after radiation therapy or after radical prostatectomy can benefit certain men whose cancer has spread to nearby tissues. | |||
| * {{cite journal |vauthors=Desai K, McManus JM, Sharifi N |title=Hormonal Therapy for Prostate Cancer |journal=Endocr Rev |volume=42 |issue=3 |pages=354–373 |date=May 2021 |pmid=33480983 |pmc=8152444 |doi=10.1210/endrev/bnab002 }} | |||
| * {{cite journal |vauthors=Duffy MJ |title=Biomarkers for Prostate Cancer: Prostate-Specific Antigen and Beyond |journal=Clin Chem Lab Med |volume=58 |issue=3 |pages=326–339 |date=February 2020 |pmid=31714881 |doi=10.1515/cclm-2019-0693 |url=|doi-access=free }} | |||
| Researchers also are investigating whether diets that are low in ] and high in ], ]s, s and other food products might prevent a recurrence. For example, the Adventist Health Study found that meat-eaters had a 54% increased risk for prostate cancer when compared with vegetarians, even after adjusting for age, sex, and smoking.{{an|Other}} | |||
| * {{cite journal |vauthors=Epstein JI |title=Prostate Cancer Grading: a Decade After the 2005 Modified System |journal=Mod Pathol |volume=31 |issue=S1 |pages=S47–63 |date=January 2018 |pmid=29297487 |doi=10.1038/modpathol.2017.133 |url=|doi-access=free }} | |||
| * {{cite encyclopedia |publisher=Elsevier |series=Point of Care |encyclopedia=Clinical Overviews |title=Prostate Cancer |editor=Hessen MT |date=August 2022 |ref={{harvid|Clinical Overview|2022}} }} | |||
| In July, ], an ]n research team lead Graham Giles of The Cancer Council released a report of a medical study that concluded that frequent ] by males may be an effective preventative measure. It was speculated by the researchers that the resulting ]s helps remove ]s from the gland area. A subsequent study from the Health Professionals Study found no link between the two.{{an|Leitzmann}} | |||
| * {{cite journal |vauthors=Ittmann M, Huang J, Radaelli E, Martin P, Signoretti S, Sullivan R, Simons BW, Ward JM, Robinson BD, Chu GC, Loda M, Thomas G, Borowsky A, Cardiff RD |title=Animal Models of Human Prostate Cancer: the Consensus Report of the New York Meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee |journal=Cancer Res |volume=73 |issue=9 |pages=2718–36 |date=May 2013 |pmid=23610450 |pmc=3644021 |doi=10.1158/0008-5472.CAN-12-4213 }} | |||
| * {{cite journal |vauthors=Liu JL, Patel HD, Haney NM, Epstein JI, Partin AW |title=Advances in the Selection of Patients with Prostate Cancer for Active Surveillance |journal=Nat Rev Urol |volume=18 |issue=4 |pages=197–208 |date=April 2021 |pmid=33623103 |doi=10.1038/s41585-021-00432-w |s2cid=232024016 |url=}} | |||
| Due to old and limited studies, it was thought that increased levels of sexual activity led to an increased risk of prostate cancer. The results of a much larger study published in '']'' seem to suggest the opposite, however. Culminating in ], the study found that men who ejaculated (through sexual activity or ]) 21 times or more a month had decreased levels of occurrence. This was true across all age groups. | |||
| * {{cite journal | vauthors = Lytton B | title = Prostate cancer: a brief history and the discovery of hormonal ablation treatment | journal = The Journal of Urology | volume = 165 | issue = 6 Pt 1 | pages = 1859–1862 | date = June 2001 | pmid = 11371867 | doi = 10.1016/S0022-5347(05)66228-3 }} | |||
| * {{cite journal |vauthors=Maffei D, Giganti F, Moore CM |title=Seminar: Revisiting the Value of PSA-Based Prostate Cancer Screening Essay No 5: Should Men Undergo MRI Before Prostate Biopsy? (Pro) |journal=Urol Oncol |volume=41 |issue=2 |pages=88–91 |date=February 2023 |pmid=35871993 |doi=10.1016/j.urolonc.2022.04.016 |url=https://discovery.ucl.ac.uk/id/eprint/10157007/}} | |||
| === Screening/Early detection === | |||
| * {{cite journal |vauthors=Mai CW, Chin KY, Foong LC, Pang KL, Yu B, Shu Y, Chen S, Cheong SK, Chua CW |title=Modeling Prostate Cancer: What Does it Take to Build an Ideal Tumor Model? |journal=Cancer Lett |volume=543 |issue= |pages=215794 |date=September 2022 |pmid=35718268 |doi=10.1016/j.canlet.2022.215794 |s2cid=249831438 }} | |||
| * {{cite journal |vauthors=McHugh J, Saunders EJ, Dadaev T, McGrowder E, Bancroft E, Kote-Jarai Z, Eeles R |title=Prostate Cancer Risk in Men of Differing Genetic Ancestry and Approaches to Disease Screening and Management in these Groups |journal=Br J Cancer |volume=126 |issue=10 |pages=1366–1373 |date=June 2022 |pmid=34923574 |pmc=9090767 |doi=10.1038/s41416-021-01669-3 }} | |||
| Researchers are studying ways to screen men for prostate cancer. At this time, it is not known whether screening for prostate cancer actually saves lives, even if the disease is found at an earlier stage. | |||
| * {{cite journal |vauthors=McIntosh SA, Alam F, Adams L, Boon IS, Callaghan J, Conti I, Copson E, Carson V, Davidson M, Fitzgerald H, Gautam A, Jones CM, Kargbo S, Lakshmipathy G, Maguire H, McFerran K, Mirandari A, Moore N, Moore R, Murray A, Newman L, Robinson SD, Segaran A, Soong CN, Walker A, Wijayaweera K, Atun R, Cutress RI, Head MG |title=Global Funding for Cancer Research Between 2016 and 2020: a Content Analysis of Public and Philanthropic Investments |journal=Lancet Oncol |volume=24 |issue=6 |pages=636–645 |date=June 2023 |pmid=37269844 |doi=10.1016/S1470-2045(23)00182-1 |url=https://eprints.ncl.ac.uk/fulltext.aspx?url=291767/C1967B92-EEE6-42F7-97E9-B4641C8A5D2D.pdf&pub_id=291767}} | |||
| * {{cite journal |vauthors=Merriel SW, Funston G, Hamilton W |title=Prostate Cancer in Primary Care |journal=Adv Ther |volume=35 |issue=9 |pages=1285–1294 |date=September 2018 |pmid=30097885 |pmc=6133140 |doi=10.1007/s12325-018-0766-1 }} | |||
| === Treatment === | |||
| * {{cite journal |vauthors=Mundle R, Afenya E, Agarwal N |title=The Effectiveness of Psychological Intervention for Depression, Anxiety, and Distress in Prostate Cancer: a Systematic Review of Literature |journal=Prostate Cancer Prostatic Dis |volume=24 |issue=3 |pages=674–687 |date=September 2021 |pmid=33750905 |doi=10.1038/s41391-021-00342-3 |s2cid=232325496 }} | |||
| * {{cite journal |vauthors=Patel MS, Halpern JA, Desai AS, Keeter MK, Bennett NE, Brannigan RE |title=Success of Prostate and Testicular Cancer Awareness Campaigns Compared to Breast Cancer Awareness Month According to Internet Search Volumes: A Google Trends Analysis |journal=Urology |volume=139 |issue= |pages=64–70 |date=May 2020 |pmid=32001306 |doi=10.1016/j.urology.2019.11.062 |s2cid=210982209 }} | |||
| Many studies of new approaches for men with prostate cancer take the form of ]. | |||
| * {{cite journal |vauthors=Pernar CH, Ebot EM, Wilson KM, Mucci LA |title=The Epidemiology of Prostate Cancer |journal=Cold Spring Harb Perspect Med |volume=8 |issue=12 |pages= a030361|date=December 2018 |pmid=29311132 |pmc=6280714 |doi=10.1101/cshperspect.a030361 }} | |||
| * {{cite book|vauthors=Pilié P, Viscuse P, Logothetis CJ, Corn PG |chapter=45: Prostate Cancer |title=The MD Anderson Manual of Medical Oncology |edition=4 |veditors=Kantarjian HM, Wolff RA, Rieber AG |date=2022 |publisher=McGraw Hill |isbn=978-1-260-46764-2}} | |||
| Cryosurgery is under study as an alternative to surgery and radiation therapy. The doctor tries to avoid damaging healthy tissue by placing an instrument known as a cryoprobe in direct contact with the tumor to freeze it. The extreme cold destroys the cancer cells. | |||
| * {{cite journal |vauthors=Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, Gillessen S, Van der Kwast T, Bristow RG |title=Prostate cancer |journal=Nat Rev Dis Primers |volume=7 |issue=1 |pages=9 |date=February 2021 |pmid=33542230 |doi=10.1038/s41572-020-00243-0 |s2cid=231794303 }} | |||
| * {{cite book|vauthors=Scher HI, Eastham JA |chapter=87: Benign and Malignant Diseases of the Prostate |title=] |edition=21 |publisher=McGraw Hill |date=2022|veditors= Loscalzo J, Fauci A, Kasper D, ''et al'' |isbn= 978-1-264-26850-4}} | |||
| Doctors are studying new ways of using radiation therapy and hormonal therapy. They also are testing the effectiveness of chemotherapy and biological therapy for men whose cancer does not respond or stops responding to hormonal therapy. In addition, scientists are exploring new treatment schedules and new ways of combining various types of treatment. For example, they are studying the usefulness of hormonal therapy before primary therapy (surgery or radiation) to shrink the tumor. | |||
| * {{cite book|vauthors=Stephenson AJ, Abouassaly R, Klein EA |chapter=Epidemiology, Etiology, and Prevention of Prostate Cancer |date=2021 |title=Cambell-Walsh-Wein Urology |edition=12 |publisher=Elsevier |veditors=Partin AW, Dmochowski RR, Kavoussi LR, Peters CA |pages=3457–3477 |isbn=978-0-323-54642-3}} | |||
| * {{cite journal |vauthors=Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG |title=Prostate cancer |journal=Lancet |volume=398 |issue=10305 |pages=1075–1090 |date=September 2021 |pmid=34370973 |doi=10.1016/S0140-6736(21)00950-8 |s2cid=236941733 |doi-access = free | title-link = doi }} | |||
| For men with early stage prostate cancer, researchers also are comparing treatment with watchful waiting. The results of this work will help doctors know whether to treat early stage prostate cancer immediately or only later on, if symptoms occur or worsen. | |||
| * {{cite journal |vauthors=Teo MY, Rathkopf DE, Kantoff P |title=Treatment of Advanced Prostate Cancer |journal=Annu Rev Med |volume=70 |issue= |pages=479–499 |date=January 2019 |pmid=30691365 |pmc=6441973 |doi=10.1146/annurev-med-051517-011947 }} | |||
| * {{cite journal |vauthors=Thompson JC, Wood J, Feuer D |title=Prostate Cancer: Palliative Care and Pain Relief |journal=Br Med Bull |volume=83 |issue= |pages=341–354 |date=2007 |pmid=17628024 |doi=10.1093/bmb/ldm018 |doi-access = free | title-link = doi }} | |||
| == References == | |||
| * {{cite book|title=A History of Prostate Cancer |vauthors=Valier H |isbn=978-1-4039-8803-4 |date=2016 |publisher=Springer Nature |chapter=The Problematic Prehistory of Prostate Cancer |pages=15–42}} | |||
| # {{anb|ACS}} American Cancer Society webpage. ''Detailed Guide: prostate cancer.'' Found at | |||
| {{refend}} | |||
| # {{anb|Aumüller}} Aumüller, G. ''Prostate Gland and Seminal Vesicles.'' Springer-Verlag Berlin-Heidelberg. 1979. | |||
| # {{anb|Moore}} Moore, K., Dalley, A. ''Clinically Oriented Anatomy.'' Lippincott Williams & Wilkins, Baltimore, Maryland. 1999. | |||
| # {{anb|Stieve}} Steive, H. ''Männliche Genitalorgane.'' In: Handbuch der mikroskopischen Anatomie des Menschen. Vol. VII Part 2, pp. 1-399. Berlin: Springer 1930. | |||
| # {{anb|Miller}} Miller, DC, Hafez, KS, Stewart, A, et al. ''Prostate carcinoma presentation, diagnosis, and staging: an update form the National Cancer Data Base.'' Cancer 2003; 98:1169. PMID 12973840 | |||
| # {{anb|Van}} van der Cruijsen-Koeter IW, Vis AN, Roobol MJ, Wildhagen MF, de Koning HJ, van der Kwast TH, Schroder FH. ''Comparison of screen detected and clinically diagnosed prostate cancer in the European randomized study of screening for prostate cancer, section rotterdam.'' Urol. 2005 Jul;174(1):121-5. PMID 15947595 | |||
| # {{anb|Hankey}} Hankey BF, Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, Ries LA, Merrill RM, Kaplan RS. ''Cancer surveillance series: interpreting trends in prostate cancer--part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates.'' J Natl Cancer Inst. 1999 Jun 16;91(12):1017-24. PMID 10379964 | |||
| # {{anb|Hoffman}} Hoffman RM; Gilliland FD; Eley JW; Harlan LC; Stephenson RA; Stanford JL; Albertson PC; Hamilton AS; Hunt WC; Potosky AL. ''Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study.'' J Natl Cancer Inst. 2001 Mar 7;93(5):388-95. PMID 11238701 | |||
| # {{anb|Steinberg}} Steinberg GD; Carter BS; Beaty TH; Childs B; Walsh PC. ''Family history and the risk of prostate cancer.'' Prostate. 1990;17(4):337-47. PMID 2251225 | |||
| # {{anb|Lichtenstein}} Lichtenstein P; Holm NV; Verkasalo PK; Iliadou A; Kaprio J; Koskenvuo M; Pukkala E; Skytthe A; Hemminki K. ''Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland.'' N Engl J Med. 2000 Jul 13;343(2):78-85. PMID 10891514 | |||
| # {{anb|Struewing}} Struewing JP; Hartge P; Wacholder S; Baker SM; Berlin M; McAdams M; Timmerman MM; Brody LC; Tucker MA. ''The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews.'' N Engl J Med. 1997 May 15;336(20):1401-8. PMID 9145676 | |||
| # {{anb|Schulman}} Schulman CC; Ekane S; Zlotta AR. ''Nutrition and prostate cancer: evidence or suspicion?'' Urology. 2001 Sep;58(3):318-34. PMID 11549473 | |||
| # {{anb|Jacobs}} Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, Thun MJ. ''A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence.'' J Natl Cancer Inst. 2005 Jul 6;97(13):975-80. PMID 15998950 | |||
| # {{anb|Shannon}} Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, Farris PE. ''Statins and prostate cancer risk: a case-control study.'' Am J Epidemiol. 2005 Aug 15;162(4):318-25. Epub 2005 Jul 13. PMID 16014776 | |||
| # {{anb|Giovannucci}} Giovannucci E, Tosteson TD, Speizer FE, Ascherio A, Vessey MP, Colditz GA. ''A retrospective cohort study of vasectomy and prostate cancer in US men.'' JAMA. 1993 Feb 17;269(7):878-82. PMID 8123059 | |||
| # {{anb|Ross}}{{Journal reference issue | Author=Ross RK, Shimizu H, Paganini-Hill A, Honda G, Henderson BE | Title=Case-control studies of prostate cancer in blacks and whites in southern California | Journal=J Natl Cancer Inst | Year=1987 | Pages=869-74 | Volume=78 | Issue=5 }} PMID 3471995 | |||
| # {{anb|Giles}} Giles GG; Severi G; English DR; McCredie MR; Borland R; Boyle P; Hopper JL. ''Sexual factors and prostate cancer.'' BJU Int. 2003 Aug;92(3):211-6. PMID 12887469 | |||
| # {{anb|Dennis}} Dennis LK; Lynch CF; Torner JC. ''Epidemiologic association between prostatitis and prostate cancer.'' Urology. 2002 Jul;60(1):78-83. PMID 12100928 | |||
| # {{anb|Calle}} Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. ''Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults.'' N Engl J Med. 2003 Apr 24;348(17):1625-38. PMID 12711737 | |||
| # {{anb|Gann}} Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. ''Prospective study of sex hormone levels and risk of prostate cancer.'' J Natl Cancer Inst. 1996 Aug 21;88(16):1118-26. PMID 8757191 | |||
| # {{anb|Ward}}{{Journal reference issue | Author=Ward JF, Zincke H | Title=Radical prostatectomy for the patient with locally advanced prostate cancer | Journal=Curr Urol Rep | Year=2003 | Pages=196-204 | Volume=4 | Issue=3 }} PMID 12756082 | |||
| # {{anb|Other}}{{Journal reference issue | Author= | Title=Position of the American Dietetic Association and Dietitians of Canada: Vegetarian diets | Journal=J Am Diet Assoc | Year=2003 | Pages=748-65 | Volume=103 | Issue=6 }} PMID 12778049 | |||
| # {{anb|Leitzmann}}{{Journal reference issue | Author=Leitzmann MF, Platz EA, Stampfer MJ, Willett WC, Giovannucci E | Title=Ejaculation frequency and subsequent risk of prostate cancer | Journal=JAMA | Year=2004 | Pages=1578-86 | Volume=291 | Issue=13 }} PMID 15069045 | |||
| == External links == | == External links == | ||
| <!-- | |||
| * | |||
| *********************** ({{No More Links}}) *************************** | |||
| * | |||
| * Please be cautious in adding more links to this article. Misplaced Pages * | |||
| * | |||
| * is not a collection of links nor should it be used for advertising. * | |||
| * | |||
| * * | |||
| * | |||
| * Excessive or inappropriate links Will-Be-Deleted. * | |||
| * One survivor's story | |||
| * See ] & ] for details. * | |||
| * Many NCI publications may be viewed or ordered on the Internet at http://cancer.gov/publications | |||
| * * | |||
| * If there are already plentiful links, please propose additions or * | |||
| * replacements on this article's discussion page, or submit your link * | |||
| * to the relevant category at the Open Directory Project (dmoz.org) * | |||
| * and link back to that category using the {{dmoz}} template. * | |||
| ********************** ({{No More Links}}) **************************** | |||
| --> | |||
| {{Sister project links|display=Prostate cancer}} | |||
| {{Medical resources | |||
| | ICD11 = {{ICD11|2C82}} | |||
| | ICD10 = {{ICD10|C61}} | |||
| | ICD9 = {{ICD9|185}} | |||
| | ICDO = | |||
| | OMIM = 176807 | |||
| | DiseasesDB = 10780 | |||
| | MedlinePlus = 000380 | |||
| | eMedicineSubj = radio | |||
| | eMedicineTopic = 574 | |||
| | MeshID = D011471 | |||
| }} | |||
| {{Male genital neoplasia}} | |||
| Patient support sources: | |||
| {{Authority control}} | |||
| * news://alt.support.cancer.prostate is a newsgroup quite active with men currently dealing with the disease. | |||
| * | |||
| * | |||
| {{DEFAULTSORT:Prostate Cancer}} | |||
| <i>The original text of this article was taken from the ] document | |||
| ] | |||
| NIH Publication No. 00-1576, which can be found at http://www.cancer.gov/cancerinfo/wyntk/prostate </i> | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 21:26, 12 December 2024
Male reproductive organ cancerMedical condition
| Prostate cancer | |
|---|---|
| Other names | Prostate carcinoma |
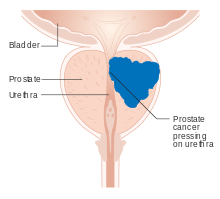 | |
| Diagram of prostate tumor pressing on urethra | |
| Specialty | Oncology, urology |
| Symptoms | Typically none. Sometimes trouble urinating, erectile dysfunction, or pain in the back/pelvis. |
| Usual onset | Age over 40 |
| Risk factors | Older age, family history, race |
| Diagnostic method | PSA test followed by tissue biopsy |
| Differential diagnosis | Benign prostatic hyperplasia |
| Treatment | Active surveillance, prostatectomy, radiation therapy, hormone therapy, chemotherapy |
| Prognosis | Five-year survival rates range from 30–99%, depending on stage. |
Prostate cancer is the uncontrolled growth of cells in the prostate, a gland in the male reproductive system below the bladder. Abnormal growth of prostate tissue is usually detected through screening tests, typically blood tests that check for prostate-specific antigen (PSA) levels. Those with high levels of PSA in their blood are at increased risk for developing prostate cancer. Diagnosis requires a biopsy of the prostate. If cancer is present, the pathologist assigns a Gleason score, and a higher score represents a more dangerous tumor. Medical imaging is performed to look for cancer that has spread outside the prostate. Based on the Gleason score, PSA levels, and imaging results, a cancer case is assigned a stage 1 to 4. A higher stage signifies a more advanced, more dangerous disease.
Most prostate tumors remain small and cause no health problems. These are managed with active surveillance, monitoring the tumor with regular tests to ensure it has not grown. Tumors more likely to be dangerous can be destroyed with radiation therapy or surgically removed by radical prostatectomy. Those whose cancer spreads beyond the prostate are treated with hormone therapy which reduces levels of the androgens (male sex hormones) that prostate cells need to survive. Eventually cancer cells can grow resistant to this treatment. This most-advanced stage of the disease, called castration-resistant prostate cancer, is treated with continued hormone therapy alongside the chemotherapy drug docetaxel. Some tumors metastasize (spread) to other areas of the body, particularly the bones and lymph nodes. There, tumors cause severe bone pain, leg weakness or paralysis, and eventually death. Prostate cancer prognosis depends on how far the cancer has spread at diagnosis. Most men diagnosed have tumors confined to the prostate; 99% of them survive more than 10 years from their diagnoses. Tumors that have metastasized to distant body sites are most dangerous, with five-year survival rates of 30–40%.
The risk of developing prostate cancer increases with age; the average age of diagnosis is 67. Those with a family history of any cancer are more likely to have prostate cancer, particularly those who inherit cancer-associated variants of the BRCA2 gene. Each year 1.2 million cases of prostate cancer are diagnosed, and 350,000 die of the disease, making it the second-leading cause of cancer and cancer death in men. One in eight men is diagnosed with prostate cancer in his lifetime and one in forty dies of the disease. Prostate tumors were first described in the mid-19th century, during surgeries on men with urinary obstructions. Initially, prostatectomy was the primary treatment for prostate cancer. By the mid-20th century, radiation treatments and hormone therapies were developed to improve prostate cancer treatment. The invention of hormone therapies for prostate cancer was recognized with the 1966 Nobel Prize to Charles B. Huggins and the 1977 Prize to Andrzej W. Schally.
Signs and symptoms
Early prostate cancer usually causes no symptoms. As the cancer advances, it may cause erectile dysfunction, blood in the urine or semen, or trouble urinating – commonly including frequent urination and slow or weak urine stream. More than half of men over age 50 experience some form of urination problem, typically due to issues other than prostate cancer such as benign prostatic hyperplasia (non-cancerous enlargement of the prostate).
Advanced prostate tumors can metastasize to nearby lymph nodes and bones, particularly in the pelvis, hips, spine, ribs, head, and neck. There they can cause fatigue, unexplained weight loss, and back or bone pain that does not improve with rest. Metastases can damage the bones around them, and around a quarter of those with metastatic prostate cancer develop a bone fracture. Growing metastases can also compress the spinal cord causing weakness in the legs and feet, or limb paralysis.
Screening
Main article: Prostate cancer screeningMost cases of prostate cancer are diagnosed through screening tests, when tumors are too small to cause any symptoms. This is done through blood tests to measure levels of the protein prostate-specific antigen (PSA), which are elevated in those with enlarged prostates, whether due to prostate cancer or benign prostatic hyperplasia. The typical man's blood has around 1 nanogram (ng) of PSA per milliliter (mL) of blood tested. Those with PSA levels below average are very unlikely to develop dangerous prostate cancer over the next 8 to 10 years. Men with PSA levels above 4 ng/mL are at increased risk – around 1 in 4 will develop prostate cancer – and are often referred for a prostate biopsy. PSA levels over 10 ng/mL indicate an even higher risk: over half of men in this group develop prostate cancer. Men with high PSA levels are often recommended to repeat the blood test four to six weeks later, as PSA levels can fluctuate unrelated to prostate cancer. Benign prostatic hyperplasia, prostate infection, recent ejaculation, and some urological procedures can increase PSA levels; taking 5α-reductase inhibitors can decrease PSA levels.
Those with elevated PSA may undergo secondary screening blood tests that measure subtypes of PSA and other molecules to better predict the likelihood that a person will develop aggressive prostate cancer. Many measure "free PSA" – the fraction of PSA unbound to other blood proteins, usually around 10% to 30%. Men who have a lower percentage of free PSA are more likely to have prostate cancer. Several common tests more accurately detect prostate cancer cases by also measuring subtypes of free PSA, including the Prostate Health Index (measures a fragment called −2proPSA) and 4K score (measures intact free PSA). Other tests measure blood levels of additional prostate-related proteins such as kallikrein-2 (also measured by 4K score), or urine levels of mRNA molecules common to prostate tumors like PCA3 and TMPRSS2 fused to ERG.
Several large studies have found that men screened for prostate cancer have a reduced risk of dying from the disease; however, detection of cancer cases that would not have otherwise impacted health can cause anxiety, and lead to unneeded biopsies and treatments, both of which can cause unwanted complications. Major national health organizations offer differing recommendations, attempting to balance the benefits of early diagnosis with the potential harms of treating people whose tumors are unlikely to impact health. Most medical guidelines recommend that men at high risk of prostate cancer (due to age, family history, ethnicity, or prior evidence of high blood PSA levels) be counseled on the risks and benefits of PSA testing, and be offered access to screening tests. Medical guidelines generally recommend against screening for men over age 70, or with a life expectancy of less than 10 years, as a newly diagnosed prostate cancer is unlikely to impact their natural lifespan. Uptake of screening varies by geography – more than 80% of men are screened in the US and Western Europe, 20% of men in Japan, and screening is rare in regions with a low Human Development Index.
Diagnosis

Men suspected of having prostate cancer may undergo several tests to assess the prostate. One common procedure is the digital rectal examination, in which a doctor inserts a lubricated finger into the rectum to feel the nearby prostate. Tumors feel like stiff, irregularly shaped lumps against the rest of the prostate. Hardening of the prostate can also be due to benign prostatic hyperplasia; around 20–25% of those with abnormal findings on their rectal exams have prostate cancer. Several urological societies' guidelines recommend magnetic resonance imaging (MRI) to evaluate the prostate for potential tumors in men with high PSA levels. MRI results can help distinguish those who have potentially dangerous tumors from those who do not.
A definitive diagnosis of prostate cancer requires a biopsy of the prostate. Prostate biopsies are typically taken by a needle passing through the rectum or perineum, guided by transrectal ultrasonography, MRI, or a combination of the two. Ten to twelve samples are taken from several regions of the prostate to improve the chances of finding any tumors. Biopsies are sent for a histopathologic diagnosis of prostate cancer, wherein they are examined under a microscope by a pathologist, who determines the type and extent of cancerous cells present. Cancers are first classified based on their appearance under a microscope. Over 95% of prostate cancers are classified as adenocarcinomas (resembling gland tissue), with the rest largely squamous-cell carcinoma (resembling squamous cells, a type of epithelial cell) and transitional cell carcinoma (resembling transitional cells).

Next, tumor samples are graded based on how much the tumor tissue differs from normal prostate tissue; the more different the tumor appears, the faster the tumor is likely to grow. The Gleason grading system is commonly used, where the pathologist assigns numbers ranging from 3 (most similar to healthy prostate tissue) to 5 (least similar) to different regions of the biopsied tissue. They then calculate a "Gleason score" by adding the two numbers that represent the largest areas of the biopsy sample. The lowest possible Gleason score of 6 represents a biopsy most similar to healthy prostate; the highest Gleason score of 10 represents the most severely cancerous. Gleason scores are commonly grouped into "Gleason grade groups", which predict disease prognosis: a Gleason score of 6 is Gleason grade group 1 (best prognosis). A score of 7 (with Gleason scores 4 + 3, or Gleason scores 3 + 4, with the most prominent listed first) can be grade group 2 or 3; it is grade group 2 if the less severe Gleason score (3) covered more area; grade group 3 if the more severe Gleason score (4) covered more area. A score of 8 is grade group 4. A score of 9 or 10 is grade group 5 (worst prognosis).
The extent of cancer spread is assessed by MRI or PSMA scan – a positron emission tomography (PET) imaging technique where a radioactive label that binds the prostate protein prostate-specific membrane antigen is used to detect metastases distant from the prostate. CT scans may also be used, but are less able to detect spread outside the prostate than MRI. Bone scintigraphy is used to test for spread of cancer to bones.
Staging
Main article: Prostate cancer staging
After diagnosis, the tumor is staged to determine the extent of its growth and spread. Prostate cancer is typically staged using the American Joint Committee on Cancer's (AJCC) three-component TNM system, with scores assigned for the extent of the tumor (T), spread to any lymph nodes (N), and the presence of metastases (M). Scores of T1 and T2 represent tumors that remain within the prostate: T1 is for tumors not detectable by imaging or digital rectal exam; T2 is for tumors detectable by imaging or rectal exam, but still confined within the prostate. T3 is for tumors that grow beyond the prostate – T3a for tumors with any extension outside the prostate; T3b for tumors that invade the adjacent seminal vesicles. T4 is for tumors that have grown into organs beyond the seminal vesicles. The N and M scores are binary (yes or no). N1 represents any spread to the nearby lymph nodes. M1 represents any metastases to other body sites.
The AJCC then combines the TNM scores, Gleason grade group, and results of the PSA blood test to categorize cancer cases into one of four stages, and their subdivisions. Cancer cases with localized tumors (T1 or T2), no spread (N0 and M0), Gleason grade group 1, and PSA less than 10 ng/mL are designated stage I. Those with localized tumors and PSA between 10 and 20 ng/mL are designated stage II – subdivided into IIA for Gleason grade group 1, IIB for grade group 2, and IIC for grade group 3 or 4. Stage III is the designation for any of three higher risk factors: IIIA is for a PSA level about 20 ng/mL; IIIB is for T3 or T4 tumors; IIIC is for a Gleason grade group of 5. Stage IV is for cancers that have spread to lymph nodes (N1, stage IVA) or other organs (M1, stage IVB).
| AJCC Stage | TNM scores | Gleason grade group | PSA |
|---|---|---|---|
| Stage I | T1 or T2, N0, M0 | 1 | <10 ng/mL |
| Stage IIA | T1 or T2, N0, M0 | 1 | 10-20 ng/mL |
| Stage IIB | 2 | ||
| Stage IIC | 3 or 4 | ||
| Stage IIIA | T1 or T2, N0, M0 | 3 or 4 | > 20 ng/mL |
| Stage IIIB | T3 or T3, N0, M0 | 10–20 ng/mL | |
| Stage IIIC | T1 or T2, N0, M0 | 5 | |
| Stage IVA | Any T, N1 | Any group | Any PSA |
| Stage IVB | Any T, M1 |
The United Kingdom National Institute for Health and Care Excellence recommends a five-stage system based on disease prognosis called the Cambridge Prognostic Group, with prognostic groups CPG 1 to CPG 5. CPG 1 is the same as AJCC stage I. Cases with localized tumors (T1 or T2) and either Gleason grade group 2 or higher PSA levels (10 to 20 ng/mL) are designated CPG 2. CPG 3 represents either Gleason grade group 3, or the combination of the CPG 2 criteria. CPG 4 is similar to AJCC stage 3 – any of Gleason grade group 4, PSA levels above 20 ng/mL, or a tumor that has grown beyond the prostate (T3). CPG 5 is for the highest risk cases: either a T4 tumor, Gleason grade group 5, or any two of the CPG 4 criteria.
Prevention
No drug or vaccine is approved by regulatory agencies for the prevention of prostate cancer. Several studies have shown 5α-reductase inhibitors to reduce the total incidence of prostate cancer; however, it is unclear as of 2022 whether they reduce any cases of dangerous disease.
Management
Main article: Management of prostate cancerTreatment of prostate cancer varies based on how advanced the cancer is, the risk it may spread, and the affected person's health and personal preferences. Those with localized disease at low risk for spread are often more likely to be harmed by the side effects of treatment than the disease itself, and so are regularly tested for a worsening of their disease. Those at higher risk may receive treatment to eliminate the tumor – typically prostatectomy (surgery to remove the prostate) or radiation therapy, sometimes alongside hormone therapy. Those with metastatic disease are treated with chemotherapy, as well as radiation or other agents to alleviate the symptoms of metastatic tumors. Blood PSA levels are monitored every few months to assess the effectiveness of treatments, and whether the disease is recurring or advancing.
Localized disease

Men diagnosed with low-risk cases of prostate cancer often defer treatment and are monitored regularly for cancer progression by active surveillance, which involves testing for tumor growth at fixed intervals by PSA tests (around every six months), digital rectal exam (annually), and MRI or repeat biopsies (every one to three years). This program continues until increases in PSA levels, Gleason grade, or tumor size indicate a higher-risk tumor that may require intervention. At least half of men remain on active surveillance, never requiring more direct treatment for their prostate tumors.
Those who elect to have therapy receive radiation therapy or a prostatectomy; these have similar rates of cancer control, but different side effects. Radiation can be delivered by intensity-modulated radiation therapy (IMRT), which allows for high doses (greater than 80 Gy) to be delivered to the prostate with relatively little radiation to other organs, or by brachytherapy, where a radioactive source is surgically inserted into the prostate. IMRT is given over several sessions, with treatments repeated five days per week for several weeks. Brachytherapy is typically performed in a single session, with the radioactive source permanently implanted into the prostate, where it expends its radioactivity within the next few months. With either technique, radiation damage to nearby organs can increase the risk of subsequent bladder cancer and cause erectile dysfunction, infertility, irreversible lumbar plexopathy and radiation proctitis – damage to the rectum that can cause diarrhea, bloody stools, fecal incontinence, and pain.
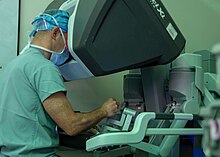
Radical prostatectomy aims to surgically remove the cancerous part of the prostate, along with the seminal vesicles, and the end of the vas deferens (the duct that delivers sperm from the testes). In wealthier countries, this is typically done by robot-assisted surgery, where robotic tools inserted through small holes in the abdomen allow a surgeon to make small and exact movements during surgery. This method results in shorter hospital stays, less blood loss, and fewer complications than traditional open surgery. In places where robot-assisted surgery is unavailable, prostatectomy can be performed laparoscopically (using a camera and hand tools through small holes in the abdomen), or through traditional open surgery with an incision above the penis (retropubic approach) or below the scrotum (perineal approach). The four approaches result in similar rates of cancer control. Damage to nearby tissue during surgery can result in erectile dysfunction and urinary incontinence. Erectile dysfunction is more likely in those who are older or had previous erectile issues. Incontinence is more common in those who are older and have shorter urethras. Both for cancer progression outcomes and surgical side effects, the skill and experience of the individual surgeon doing the procedure are among the greatest determinants of success.
After prostatectomy, PSA levels drop rapidly, reaching very low or undetectable levels within two months. Radiotherapy also substantially reduces PSA levels, but more slowly and less completely, with PSA levels reaching their nadir two years after radiotherapy. After either treatment, PSA levels are monitored regularly. Up to half of those treated will eventually have a rise in PSA levels, suggesting the tumor or small metastases are growing again. People with high or rising PSA levels are often offered another round of radiation therapy directed at the former tumor site. This reduces risk for further progression by 75%. Those suspected of metastases can undergo PET scanning with sensitive radiotracers C-11 choline, F-18 fluciclovine, and F-18 or Ga-68 attached to a PSMA-targeting drug, each of which is able to detect small metastases more sensitively than alternative imaging methods.
Metastatic disease
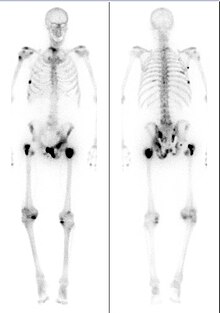
For those with metastatic disease, the standard of care is androgen deprivation therapy (also called "chemical castration"), drugs that reduce levels of androgens (male sex hormones) that prostate cells require to grow. Various drugs are used to lower androgen levels by blocking the synthesis or action of testosterone, the primary androgen. The first line of treatment typically involves GnRH agonists like leuprolide, goserelin, or triptorelin by injection monthly or less frequently as needed. GnRH agonists cause a brief rise in testosterone levels at treatment initiation, which can worsen disease in people with significant symptoms of metastases. In these people, GnRH antagonists like degarelix or relugolix are given instead, and can also rapidly reduce testosterone levels. Reducing testosterone can cause various side effects, including hot flashes, reduction in muscle mass and bone density, reduced sex drive, fatigue, personality changes, and an increased risk of diabetes, cardiovascular disease, and depression. Hormone therapy halts tumor growth in more than 95% of those treated, and PSA levels return to normal in up to 70%.
Despite reduced testosterone levels, metastatic prostate tumors eventually continue to grow – manifested by rising blood PSA levels, and metastases to nearby bones. This is the most advanced stage of the disease, called castration-resistant prostate cancer (CRPC). CRPC tumors continuously evolve resistance to treatments, necessitating several lines of therapy, each used in sequence to extend survival. The standard of care is the chemotherapy docetaxel along with antiandrogen drugs, namely the androgen receptor antagonists enzalutamide, apalutamide, and darolutamide, as well as the testosterone production inhibitor abiraterone acetate. An alternative is the cell therapy procedure Sipuleucel-T, where the affected person's immune cells are removed, treated to more effectively target prostate cancer cells, and re-injected. Tumors that evolve resistance to docetaxel may receive the second-generation taxane drug cabazitaxel.
Some CRPC treatments are used only in men whose tumors have certain characteristics that make the therapy more likely to be effective. Men whose tumors express the protein PSMA may receive the radiopharmaceutical Lu-177 PSMA, which binds to and destroys PSMA-positive cells. Those whose tumors have defective DNA damage repair benefit from treatment with the immune checkpoint inhibitor drug pembrolizumab and PARP inhibitors, namely olaparib, rucaparib, or niraparib.
Supportive care
Bone metastases – present in around 85% of those with metastatic prostate cancer – are the primary cause of symptoms and death from metastatic prostate cancer. Those with constant pain are prescribed nonsteroidal anti-inflammatory drugs. However, people with bone metastases can experience "breakthrough pain", sudden bursts of severe pain that resolve within around 15 minutes, before pain medications can take effect. Single sites of pain can be treated with external beam radiation therapy to shrink nearby tumors. More dispersed bone pain can be treated with radioactive compounds that disproportionately accumulate in bone, like radium-223 and samarium-153-EDTMP, which help reduce the size of bone tumors. Similarly, the systemic chemotherapeutics used for metastatic prostate cancer can reduce pain as they shrink tumors. Other bone modifying agents like zoledronic acid and denosumab can reduce prostate cancer bone pain, even though they have little effect on tumor size. Metastases compress the spinal cord in up to 12% of those with metastatic prostate cancer causing pain, weakness, numbness, and paralysis. Inflammation in the spine can be treated with high-dose steroids, as well as surgery and radiotherapy to shrink spinal tumors and relieve pressure on the spinal cord.
Those with advanced prostate cancer suffer fatigue, lethargy, and a generalized weakness. This is caused in part by gastrointestinal problems, with loss of appetite, weight loss, nausea, and constipation all common. These are typically treated with appetite-increasing drugs – megestrol acetate or corticosteroids – antiemetics, or treatments that focus on underlying gastrointestinal issues. General weakness can also be caused by anemia, itself caused by a combination of the disease itself, poor nutrition, and damage to the bone marrow from cancer treatments or bone metastases. Anemia can be treated in various ways depending on the cause, or can be addressed directly with blood transfusions. Organ damage and metastases in the lymph nodes can lead to uncomfortable accumulation of fluid (called lymphedema) in the genitals or lower limbs. These swellings can be extremely painful, curtailing an affected person's ability to urinate, have sex, or walk normally. Lymphedema can be treated by applying pressure to aid drainage, surgically draining pooled fluid, and cleaning and treating nearby damaged skin.
People with prostate cancer are around twice as likely to experience anxiety or depression compared to those without cancer. When added to normal prostate cancer treatments, psychological interventions such as psychoeducation and cognitive behavioral therapy can help reduce anxiety, depression, and general distress.
As those severely ill with metastatic prostate cancer approach the end of their lives, most experience confusion and may hallucinate or have trouble recognizing loved ones. Confusion is caused by various conditions, including kidney failure, sepsis, dehydration, and as a side effect of various drugs, especially opioids. Most people sleep for long periods, and some feel drowsy when awake. Restlessness is also common, sometimes caused by physical discomfort from constipation or urinary retention, sometimes caused by anxiety. In their last few days, affected men's breathing may become shallow and slow, with long pauses between breaths. Breathing may be accompanied by a rattling noise as fluid lingers in the throat, but this is not uncomfortable for the affected person. Their hands and feet may cool to the touch, and skin become blotchy or blue due to weaker blood circulation. Many stop eating and drinking, resulting in dry-feeling mouth, which can be aided by moistening the mouth and lips. The person becomes less and less responsive, and eventually the heart and breathing stop.
Prognosis
The prognosis of diagnosed prostate cancer varies widely based on the cancer's grade and stage at the time of diagnosis; those with lower stage disease have vastly improved prognoses. Around 80% of prostate cancer diagnoses are in men whose cancer is still confined to the prostate. These men can survive long after diagnosis, with as many as 99% still alive 10 years from diagnosis. Men whose cancer has metastasized to a nearby part of the body (around 15% of diagnoses) have poorer prognoses, with five-year survival rates of 60–80%. Those with metastases in distant body sites (around 5% of diagnoses) have relatively poor prognoses, with five-year survival rates of 30–40%.
Those who have low blood PSA levels at diagnosis, and whose tumors have a low Gleason grade and less-advanced clinical stage tend to have better prognoses. After prostatectomy or radiotherapy, those who have a short time between treatment and a subsequent rise in PSA levels, or quickly rising PSA levels are more likely to die from their cancers. Castration-resistant metastatic prostate cancer is incurable, and kills a majority of those whose disease reaches this stage.
Cause
Prostate cancer is caused by the accumulation of genetic mutations to the DNA of cells in the prostate. These mutations affect genes involved in cell growth, replication, cell death, and DNA damage repair. With these processes dysregulated, some cells replicate abnormally, forming a clump of cells called a tumor. As the tumor grows, its cells accumulate more mutations, allowing it to stimulate the growth of new blood vessels to support further growth. Eventually, a tumor can grow large enough to invade nearby organs such as the seminal vesicles or bladder. In advanced tumors, cells can develop the ability to detach from their original tissue site, and evade the immune system. These cells can spread through the lymphatic system to nearby lymph nodes, or through the bloodstream to the bone marrow and (more rarely) other body sites. At these new sites, the cancer cells disrupt normal body function and continue to grow. Metastases cause most of the discomfort associated with prostate cancer, and can eventually kill the affected person.
Pathophysiology
Most prostate tumors begin in the peripheral zone – the outermost part of the prostate. As cells begin to grow out of control, they form a small clump of disregulated cells called a prostatic intraepithelial neoplasia (PIN). Some PINs continue to grow, forming layers of tissue that stop expressing genes common to their original tissue location – p63, cytokeratin 5, and cytokeratin 14 – and instead begin expressing genes typical of cells in the innermost lining of the pancreatic duct – cytokeratin 8 and cytokeratin 18. These multilayered PINs also often overexpress the gene AMACR, which is associated with prostate cancer progression.
Some PINs can eventually grow into tumors. This is commonly accompanied by large-scale changes to the genome, with chromosome sequences being rearranged or copied repeatedly. Some genomic alterations are particularly common in early prostate cancer, namely gene fusion between TMPRSS2 and the oncogene ERG (up to 60% of prostate tumors), mutations that disable SPOP (up to 15% of tumors), and mutations that hyperactivate FOXA1 (up to 5% of tumors).
Metastatic prostate cancer tends to have more genetic mutations than localized disease. Many of these mutations are in genes that protect from DNA damage, such as p53 (mutated in 8% of localized tumors, more than 27% of metastatic ones) and RB1 (1% of localized tumors, more than 5% of metastatic ones). Similarly mutations in the DNA repair-related genes BRCA2 and ATM are rare in localized disease but found in at least 7% and 5% of metastatic disease cases respectively.
The transition from castrate-sensitive to castrate-resistant prostate cancer is also accompanied by the acquisition of various gene mutations. In castrate-resistant disease, more than 70% of tumors have mutations in the androgen receptor signaling pathway – amplifications and gain-of-function mutations in the receptor gene itself, amplification of its activators (for example, FOXA1), or inactivating mutations in its negative regulators (for example, ZBTB16 and NCOR1). These androgen receptor disruptions are only found in up to 6% of biopsies of castrate-sensitive metastatic disease. Similarly, deletions of the tumor suppressor PTEN are harbored by 12–17% of castrate-sensitive tumors, but over 40% of castrate-resistant tumors. Less commonly, tumors have aberrant activation of the Wnt signaling pathway via disruption of members APC (9% of tumors) or CTNNB1 (4% of tumors); or dysregulation of the PI3K pathway via PI3KCA/PI3KCB mutations (6% of tumors) or AKT1 (2% of tumors).
Epidemiology

Prostate cancer is the second-most frequently diagnosed cancer in men, and the second-most frequent cause of cancer death in men (after lung cancer). Around 1.2 million new cases of prostate cancer are diagnosed each year, and over 350,000 people die of the disease, annually. One in eight men are diagnosed with prostate cancer in their lifetime, and around one in forty die of the disease. Rates of prostate cancer rise with age. Due to this, prostate cancer rates are generally higher in parts of the world with higher life expectancy, which also tend to be areas with higher gross domestic product and higher human development index. Australia, Europe, North America, New Zealand, and parts of South America have the highest incidence. South Asia, Central Asia, and sub-Saharan Africa have the lowest incidence of prostate cancer; though incidence is increasing quickly in these regions. Prostate cancer is the most diagnosed cancer in men in over half of the world's countries, and the leading cause of cancer death in men in around a quarter of countries.
Prostate cancer is rare in those under 40 years old, and most cases occur in those over 60 years, with the average person diagnosed at 67. The average age of those who die from prostate cancer is 77. Only a minority of prostate cancer cases are diagnosed. Autopsies of men who died at various ages have shown cancer in the prostates of over 40% of men over age 50. Incidence rises with age, and nearly 70% of men autopsied at age 80–89 had cancer in their prostates.
Genetics
Prostate cancer is more common in families with a history of any cancer. Men with an affected first-degree relative (father or brother) have more than twice the risk of developing prostate cancer, and those with two first-degree relatives have a five-fold greater risk compared with men with no family history. Increased risk also runs in some ethnic groups, with men of African and African-Caribbean ancestry at particularly high risk – having prostate cancer at higher rates, and having more-aggressive prostate cancers that develop at earlier ages. Large genome-wide association studies have identified over 100 gene variants associated with increased prostate cancer risk. The greatest risk increase is associated with variations in BRCA2 (up to an eight-fold increased risk) and HOXB13 (three-fold increased risk), both of which are involved in repairing DNA damage. Variants in other genes involved in DNA damage repair have also been associated with an increased risk of developing prostate cancer – particularly early-onset prostate cancer – including BRCA1, ATM, NBS1, MSH2, MSH6, PMS2, CHEK2, RAD51D, and PALB2. Additionally, variants in the genome near the oncogene MYC are associated with increased risk. As are single-nucleotide polymorphisms in the vitamin D receptor common in African-Americans, and in the androgen receptor, CYP3A4, and CYP17 involved in testosterone synthesis and signaling. Together, known gene variants are estimated to cause around 25% of prostate cancer cases, including 40% of early-onset prostate cancers.
Body and lifestyle
Men who are taller are at a slightly increased risk for developing prostate cancer, as are men who are obese. High levels of blood cholesterol are also associated with increased prostate cancer risk; consequently, those who take the cholesterol-lowering drugs, statins, have a reduced risk of advanced prostate cancer. Chronic inflammation can cause various cancers. Potential links between infection (or other sources of inflammation) and prostate cancer have been studied but none definitively found, and one large study found no link between prostate cancer and a history of gonorrhea, syphilis, chlamydia, or infection with various human papillomaviruses.
Regular vigorous exercise may reduce one's chance of developing advanced prostate cancer, as can several dietary interventions. Those with a diet rich in cruciferous vegetables (certain leafy greens, broccoli, and cauliflower), fish, genistein (found in soy), or lycopene (found in tomatoes) are at a reduced risk of symptomatic prostate cancer. Conversely, those who consume high levels of dietary fats, polycyclic aromatic hydrocarbons (from cooking red meats), or calcium may be at an increased risk of developing advanced prostate cancer. Several dietary supplements have been studied and found not to impact prostate cancer risk, including selenium, vitamin C, vitamin D, and vitamin E.
Special populations
Transgender women and gender non-conforming people who have prostates can develop prostate cancer. Those who have undergone gender-affirming hormone therapy or gender-affirming surgery have reduced risk of developing prostate cancer, relative to cisgender men of similar age. Screening tests in this group are complicated, as transgender women may have lower PSA levels than cisgender men due to their reduced testosterone levels. PSA levels greater than 1 ng/mL are generally considered above normal by gender care specialists. Digital rectal exams of the prostate are often impossible in women who have undergone vaginoplasty, as the length and rigidity of the new vagina can obstruct access to the prostate from the rectum.
History

A prostate mass was first described in 1817 by the English surgeon George Langstaff, following the autopsy of a man who had died at age 68 with lower-body pain and urinary issues. In 1853, London Hospital surgeon John Adams described another prostate tumor from a man who had died with urinary issues; Adams had a pathologist examine the tumor, providing the first confirmed case of a cancerous tumor in the prostate. The disease was initially rarely described; an 1893 report found only 50 cases described in the medical literature. Around the turn of the 19th century, prostate surgery to relieve urinary obstruction became more common, allowing surgeons and pathologists to examine the removed prostate tissue. Two studies around the time found cancer in as many as 10% of surgical specimens, suggesting prostate cancer was a fairly common cause of prostate enlargement.
For much of the 20th century, the primary therapy for prostate cancer was surgery to remove the prostate. Perineal prostatectomy was first performed in 1904 by Hugh H. Young at Johns Hopkins Hospital. Young's method became the widespread standard, initially done primarily to relieve symptoms of urinary blockage. In 1931 a new surgical method, transurethral resection of the prostate, became available, replacing perineal prostatectomy for symptomatic relief of obstruction. In 1945, Terence Millin described a retropubic prostatectomy approach, which provided easier access to pelvic lymph nodes to assist in staging the extent of disease, and was easier for surgeons to learn. This was improved upon by Patrick C. Walsh's 1983 description of a retropubic prostatectomy approach that avoided damage to the nerves near the prostate, preserving erectile function.
Radiation therapy for prostate cancer was used occasionally in the early 20th century, with radium implanted into the urethra or rectum to reduce the tumor size and associated symptoms. In the 1950s the advent of more powerful radiation machines allowed for external beam radiotherapy to reach the prostate. By the 1960s, this was often combined with hormone therapy to improve the potency of therapy. In the 1970s, Willet Whitmore pioneered an open surgery technique where needles of Iodine-125 were placed directly into the prostate. This was improved upon by Henrik H. Holm in 1983 by using transrectal ultrasound to guide the implantation of radioactive material.
The observation that the testicles (and the hormones they secrete) influence prostate size was made as early as the late 18th century via castration experiments in animals. However, occasional experimentation over the next century bore mixed results, likely due to the inability to separate prostate tumors from prostates enlarged due to benign prostatic hyperplasia. In 1941, Charles B. Huggins and Clarence V. Hodges published two studies using surgical castration or oral estrogen to reduce androgen levels and improve prostate cancer symptoms. Huggins was awarded the 1966 Nobel Prize in Physiology or Medicine for this discovery, the first systemic therapy for prostate cancer. In the 1960s, large studies showed estrogen therapy to be as effective as surgical castration at treating prostate cancer, but that those on estrogen therapy were at increased risk of suffering blood clots. Through the 1980s, Andrzej W. Schally's studies of GnRH led to the development of GnRH agonists, which were found to be as effective as estrogen without the increased risk of clotting. Schally was awarded the 1977 Nobel Prize in Physiology or Medicine for his work on GnRH and prostate cancer.
Systemic chemotherapy for prostate cancer has been studied since the 1950s but clinical trials failed to show benefits in most people who receive the drugs. In 1996, the US Food and Drug Administration approved the systemic chemotherapy mitoxantrone for those with castration-resistant prostate cancer based on trials showing that it improved symptoms even though it failed to enhance survival. In 2004, docetaxel was approved as the first chemotherapy to increase survival in those with castration-resistant prostate cancer. After additional trials in 2015, docetaxel use was extended to those with castration-sensitive prostate cancer.
Society and culture
Prostate cancer screening and awareness have been widely promoted since the early 2000s by Prostate Cancer Awareness Month in September and Movember in November. However, an analysis of internet searches suggests neither event changes the level of prostate cancer interest or discussion much, in contrast to the more established Breast Cancer Awareness Month.
Research
Prostate cancer is a major topic of ongoing research. From 2016–2020, over $1.26 billion was invested in prostate cancer research, representing around 5% of global cancer research funds. This places prostate cancer 10th among 18 common cancer types in funding per cancer death, and 9th in funding per disability-adjusted life year lost.
Research into prostate cancer relies on a number of laboratory models to test aspects of the disease. Several prostate immortalized cell lines are widely used, namely the classic lines DU145, PC-3, and LNCaP, as well as more recent cell lines 22Rv1, LAPC-4, VCaP, and MDA-PCa-2a and −2b. Research requiring more complex models of the prostate uses organoids – clusters of prostate cells that can be grown from human prostate tumors or stem cells. Modeling tumor growth and metastasis requires a model organism, typically a mouse. Researchers can either surgically implant human prostate tumors into immunocompromised mice (a technique called a patient derived xenograft), or induce prostate tumors in mice with genetic engineering. These genetically engineered mouse models typically use a Cre recombinase system to disrupt tumor suppressors or activate oncogenes specifically in prostate cells.
Notes
- The original 1966 Gleason grading system allowed pathologist scores of 1 to 5, resulting in Gleason scores of 2 to 10; however, a 2005 update by the International Society of Urological Pathology largely eliminated the pathologist scores 1 and 2. In common practice, tumors are now scored 3 to 5, resulting in Gleason scores of 6 to 10.
- Cancer incidence often plateaus or falls in the oldest age groups due to reduced diagnosis in people who are already in poor health.
References
- ^ Rebello et al. 2021, "Figure 3: Prostate cancer stages and progression.".
- ^ Rebello et al. 2021, "Epidemiology".
- ^ Scher & Eastham 2022, "Prostate cancer".
- ^ "Prostate Cancer Signs and Symptoms". American Cancer Society. 1 August 2019. Retrieved 21 May 2023.
- Merriel, Funston & Hamilton 2018, "Symptoms and signs".
- Rebello et al. 2021, "Figure 3: Prostate cancer stages and progression".
- ^ Coleman et al. 2020, "Prevalence of SREs".
- "Symptoms of Prostate Cancer". Cancer Research UK. 15 March 2022. Retrieved 21 May 2023.
- Coleman et al. 2020, "Prostate cancer".
- Clinical Overview 2022, "Clinical presentation".
- Scher & Eastham 2022, "Metastatic disease: noncastrate".
- ^ Rebello et al. 2021, "Screening and early detection".
- "What Is Screening for Prostate Cancer?". U.S. Centers for Disease Control and Prevention. 25 August 2022. Retrieved 17 May 2023.
- ^ Carlsson & Vickers 2020, "3. Tailor screening frequency based on PSA-level".
- ^ "Screening Tests for Prostate Cancer". American Cancer Society. 4 January 2021. Retrieved 11 December 2023.
- Scher & Eastham 2022, "Prostate-specific antigen".
- Carlsson & Vickers 2020, "4. For men with elevated PSA (≥3 ng/mL), repeat PSA.".
- Duffy 2020, "Percent free PSA".
- Duffy 2020, "Prostate Health Index (PHI)".
- Duffy 2020, "4K score".
- Duffy 2020, Table 2.
- ^ Rebello et al. 2021, "Box 1: Screening for prostate cancer in different regions".
- "Tests to Diagnose and Stage Prostate Cancer". American Cancer Society. 21 February 2023. Retrieved 18 May 2023.
- ^ Rebello et al. 2021, "Diagnosis".
- Scher & Eastham 2022, "Physical examination".
- Maffei, Giganti & Moore 2023, "MRI as a test for clinically referred men with a raised PSA or abnormal digital rectal exam.
- Scher & Eastham 2022, "Prostate biopsy".
- ^ Scher & Eastham 2022, "Pathology".
- Epstein 2018, "Historical background", "2005 and 2014 ISUP grading conferences", and "Gleason patterns".
- ^ Scher & Eastham 2022, "Prostate cancer staging".
- ^ "Prostate Cancer Staging". American Cancer Society. 8 October 2021. Retrieved 14 May 2023.
- ^ Scher & Eastham 2022, "Table 87-1 TNM classification".
- "Prostate Cancer: Diagnosis and Management. NICE Guideline [NG131]". National Institute for Health and Care Excellence (NICE). 9 May 2019. Retrieved 3 October 2022.
- "Prostate Cancer Risk Groups and the Cambridge Prognostic Group (CPG)". Cancer Research UK. 24 May 2022. Retrieved 25 June 2023.
- ^ Scher & Eastham 2022, "No cancer diagnosis".
- Rebello et al. 2021, "Management".
- Scher & Eastham 2022, "Active surveillance".
- ^ "Initial Treatment of Prostate Cancer, by Stage and Risk Group". American Cancer Society. 9 August 2022. Retrieved 28 May 2023.
- "Following PSA Levels During and After Prostate Cancer Treatment". American Cancer Society. 1 August 2019. Retrieved 28 May 2023.
- "Observation or Active Surveillance for Prostate Cancer". American Cancer Society. 22 November 2023. Retrieved 16 April 2024.
- Liu et al. 2021, "Reclassification and progression".
- Liu et al. 2021, "Abstract".
- Rebello et al. 2021, "Localized Disease".
- Scher & Eastham 2022, "Clinically localized prostate cancer".
- Scher & Eastham 2022, "External beam radiation therapy".
- Scher & Eastham 2022, "Brachytherapy".
- "Radiation Therapy for Prostate Cancer". American Cancer Society. 13 February 2023. Retrieved 5 December 2023.
- Brejt N, Berry J, Nisbet A, Bloomfield D, Burkill G (30 December 2013). "Pelvic radiculopathies, lumbosacral plexopathies, and neuropathies in oncologic disease: a multidisciplinary approach to a diagnostic challenge". Cancer Imaging. 13 (4): 591–601. doi:10.1102/1470-7330.2013.0052. PMC 3893894. PMID 24433993.
- Brawley, Mohan & Nein 2018, "Radiation therapy".
- Dall'Era 2023, "Radical prostatectomy".
- ^ Costello 2020, "The rise of robotic surgery".
- ^ Scher & Eastham 2022, "Radical prostatectomy".
- "Following PSA Levels During and After Prostate Cancer Treatment". American Cancer Society. 1 August 2019. Retrieved 5 December 2023.
- ^ Rebello et al. 2021, "Biochemical recurrence and residual disease".
- ^ Rebello et al. 2021, "Biocehmical recurrence and residual disease".
- Scher & Eastham 2022, "Rising PSA after definitive local therapy".
- ^ Rebello et al. 2021, "Metastatic hormone-sensitive prostate cancer".
- "Hormone Therapy for Prostate Cancer". American Cancer Society. 9 August 2022. Retrieved 15 May 2023.
- ^ Scher & Eastham 2022, "Testosterone-lowering agents".
- Achard et al. 2022, "Introduction".
- Scher & Eastham 2022, "Outcomes of androgen deprivation".
- ^ Scher & Eastham 2022, "Metastatic disease: castrate".
- Rebello et al. 2021, "Metastatic castration-resistant prostate cancer".
- Teo, Rathkopf & Kantoff 2019, "Management of metastatic castration-resistant prostate cancer".
- Teo, Rathkopf & Kantoff 2019, "Abiraterone acetate".
- "FDA Approves Pluvicto for Metastatic Castration-Resistant Prostate Cancer". U.S. Food & Drug Administration. 23 March 2022. Retrieved 9 January 2024.
- Coleman et al. 2020, "Prevalence of bone metastases".
- ^ Coleman et al. 2020, "Analgesics used in CIBP".
- ^ Scher & Eastham 2022, "Pain management".
- ^ Thompson, Wood & Feuer 2007, "Cord compression".
- ^ "What is Metastatic Spinal Cord Compression (MSCC)?". Prostate Cancer UK. June 2022. Retrieved 25 June 2023.
- Thompson, Wood & Feuer 2007, "Gastrointestinal symptoms".
- ^ Thompson, Wood & Feuer 2007, "General debility".
- Thompson, Wood & Feuer 2007, "Lymphoedema".
- Mundle, Afenya & Agarwal 2021, "Estimates of anxiety, depression, and distress".
- Mundle, Afenya & Agarwal 2021, "Abstract".
- ^ Thompson, Wood & Feuer 2007, "Delirium".
- ^ "Dying from Prostate Cancer – What to Expect". Prostate Cancer UK. July 2018. Retrieved 25 June 2023.
- ^ "Care Through the Final Days". American Society of Clinical Oncology. November 2022. Retrieved 25 June 2023.
- Rebello et al. 2021, "Prognosis and survival".
- Pilié et al. 2022, Table 45-2.
- Rebello et al. 2021, "Abstract".
- Rebello et al. 2021, "Genetics".
- "What Causes Prostate Cancer?". American Cancer Society. 1 August 2019. Retrieved 17 May 2023.
- ^ Rebello et al. 2021, "Disease progression".
- "Locally Advanced Prostate Cancer". Cancer Research UK. 31 May 2022. Retrieved 21 May 2023.
- ^ Sandhu et al. 2021, "The biology of prostate cancer".
- ^ "Understanding Your Pathology Report: Prostatic Intraepithelial Neoplasia (PIN) and Intraductal Carcinoma". American Cancer Society. Retrieved 25 May 2023.
- ^ Rebello et al. 2021, "Metastatic disease".
- "Prostate Cancer Incidence Statistics". Cancer Research UK. 15 May 2015. Retrieved 2 April 2024.
- Bergengren et al. 2023, "3.1 Epidemiology".
- Pernar et al. 2018, "Risk factors for total prostate cancer".
- ^ Stephenson, Abouassaly & Klein 2021, "Age at diagnosis".
- Dall'Era 2023, "General considerations".
- ^ Rebello et al. 2021, "Genetic predisposition".
- ^ Scher & Eastham 2022, "Epidemiology".
- McHugh et al. 2022, "Introduction".
- Pernar et al. 2018, "Risk factors for advanced and fatal prostate cancer".
- Pernar et al. 2018, "Statins".
- Stephenson, Abouassaly & Klein 2021, "Inflammation and infection".
- Pernar et al. 2018, "Exercise".
- Pernar et al. 2018, "Fish".
- ^ Pernar et al. 2018, "Calcium, dairy products, and vitamin D".
- Bertoncelli Tanaka et al. 2022, "Conclusions".
- Bertoncelli Tanaka et al. 2022, "Interpretation of PSA in transgender women on GAHT".
- ^ Bertoncelli Tanaka et al. 2022, "Screening for prostate cancer in transgender women".
- ^ Valier 2016, pp. 15–18.
- Lawrence W (1817). "Cases of Fungus Hæmatodes, with Observations, by George Langstaff, Esq. and an Appendix, Containing two Cases of Analogous Affections". Med Chir Trans. 8: 272–314. doi:10.1177/095952871700800114. PMC 2129005. PMID 20895322.
- Adams J (1853). "The Case of Scirrhous of the Prostate Gland with Corresponding Affliction of the Lymphatic Glands in the Lumbar Region and in the Pelvis". Lancet. 1 (1547): 393–94. doi:10.1016/S0140-6736(02)68759-8.
- ^ Lytton 2001, p. 1859.
- ^ Denmeade & Isaacs 2002, "Prostatectomy".
- Young HH (1905). "Four Cases of Radical Prostatectomy". Johns Hopkins Bull. 16.
- Walsh PC, Lepor H, Eggleston JC (1983). "Radical Prostatectomy with Preservation of Sexual Function: Anatomical and Pathological Considerations". The Prostate. 4 (5): 473–485. doi:10.1002/pros.2990040506. PMID 6889192. S2CID 30740301.
- ^ Denmeade & Isaacs 2002, "Radiation therapy".
- ^ Denmeade & Isaacs 2002, "Androgen-ablation therapy".
- Huggins CB, Hodges CV (1941). "Studies on Prostate Cancer: 1. The Effects of Castration, of Estrogen and Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate". Cancer Res. 1 (4): 293. Archived from the original on 30 June 2017. Retrieved 2 September 2015.
- Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, et al. (March 1982). "Tumor Growth Inhibition in Patients with Prostatic Carcinoma Treated with Luteinizing Hormone-Releasing Hormone Agonists". Proceedings of the National Academy of Sciences of the United States of America. 79 (5): 1658–1662. Bibcode:1982PNAS...79.1658T. doi:10.1073/pnas.79.5.1658. PMC 346035. PMID 6461861.
- Denmeade & Isaacs 2002, "Cytotoxic chemotherapy".
- ^ Desai, McManus & Sharifi 2021, "Evolution of treatment".
- Teo, Rathkopf & Kantoff 2019, "Figure 1".
- ^ Patel et al. 2020, p. 64.
- McIntosh et al. 2023, Table 1.
- McIntosh et al. 2023, Table 2.
- Mai et al. 2022, "2.1 Human prostate cancer cell lines".
- Mai et al. 2022, "2.4. Organoids".
- Mai et al. 2022, "2.2 PDX lines".
- Mai et al. 2022, "2.3 Genetically engineered mouse model (GEMM)".
- Ittmann et al. 2013, "Introduction".
Works cited
- Achard V, Putora PM, Omlin A, Zilli T, Fischer S (2022). "Metastatic Prostate Cancer: Treatment Options". Oncology. 100 (1): 48–59. doi:10.1159/000519861. PMID 34781285. S2CID 244132770.
- Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, et al. (May 2023). "2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review". Eur Urol. 84 (2): 191–206. doi:10.1016/j.eururo.2023.04.021. PMC 10851915. PMID 37202314. S2CID 258780321.
- Bertoncelli Tanaka M, Sahota K, Burn J, Falconer A, Winkler M, Ahmed HU, et al. (January 2022). "Prostate Cancer in Transgender Women: What Does a Urologist Need to Know?". BJU Int. 129 (1): 113–122. doi:10.1111/bju.15521. PMID 34157213.
- Brawley S, Mohan R, Nein CD (June 2018). "Localized Prostate Cancer: Treatment Options". American Family Physician. 97 (12): 798–805. PMID 30216009.
- Carlsson SV, Vickers AJ (November 2020). "Screening for Prostate Cancer". Med Clin North Am. 104 (6): 1051–1062. doi:10.1016/j.mcna.2020.08.007. PMC 8287565. PMID 33099450.
- Coleman RE, Croucher PI, Padhani AR, Clézardin P, Chow E, Fallon M, et al. (October 2020). "Bone Metastases". Nat Rev Dis Primers. 6 (1): 83. doi:10.1038/s41572-020-00216-3. hdl:20.500.11820/6bac9e59-0afa-4b4a-bebf-4e747b889917. PMID 33060614. S2CID 222350837.
- Costello AJ (March 2020). "Considering the Role of Radical Prostatectomy in 21st Century Prostate Cancer Care". Nat Rev Urol. 17 (3): 177–188. doi:10.1038/s41585-020-0287-y. PMID 32086498. S2CID 211234353.
- Dall'Era MA (2023). "39-17: Prostate Cancer". In Papadakis MA, McPhee SJ, Rabow MW, McQuaid KR (eds.). Current Medical Diagnosis & Treatment 2023 (62 ed.). McGraw Hill. ISBN 978-1-264-68734-3.
- Denmeade SR, Isaacs JT (May 2002). "A history of prostate cancer treatment". Nature Reviews. Cancer. 2 (5): 389–396. doi:10.1038/nrc801. PMC 4124639. PMID 12044015.
- Desai K, McManus JM, Sharifi N (May 2021). "Hormonal Therapy for Prostate Cancer". Endocr Rev. 42 (3): 354–373. doi:10.1210/endrev/bnab002. PMC 8152444. PMID 33480983.
- Duffy MJ (February 2020). "Biomarkers for Prostate Cancer: Prostate-Specific Antigen and Beyond". Clin Chem Lab Med. 58 (3): 326–339. doi:10.1515/cclm-2019-0693. PMID 31714881.
- Epstein JI (January 2018). "Prostate Cancer Grading: a Decade After the 2005 Modified System". Mod Pathol. 31 (S1): S47–63. doi:10.1038/modpathol.2017.133. PMID 29297487.
- Hessen MT, ed. (August 2022). "Prostate Cancer". Clinical Overviews. Point of Care. Elsevier.
- Ittmann M, Huang J, Radaelli E, Martin P, Signoretti S, Sullivan R, et al. (May 2013). "Animal Models of Human Prostate Cancer: the Consensus Report of the New York Meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee". Cancer Res. 73 (9): 2718–36. doi:10.1158/0008-5472.CAN-12-4213. PMC 3644021. PMID 23610450.
- Liu JL, Patel HD, Haney NM, Epstein JI, Partin AW (April 2021). "Advances in the Selection of Patients with Prostate Cancer for Active Surveillance". Nat Rev Urol. 18 (4): 197–208. doi:10.1038/s41585-021-00432-w. PMID 33623103. S2CID 232024016.
- Lytton B (June 2001). "Prostate cancer: a brief history and the discovery of hormonal ablation treatment". The Journal of Urology. 165 (6 Pt 1): 1859–1862. doi:10.1016/S0022-5347(05)66228-3. PMID 11371867.
- Maffei D, Giganti F, Moore CM (February 2023). "Seminar: Revisiting the Value of PSA-Based Prostate Cancer Screening Essay No 5: Should Men Undergo MRI Before Prostate Biopsy? (Pro)". Urol Oncol. 41 (2): 88–91. doi:10.1016/j.urolonc.2022.04.016. PMID 35871993.
- Mai CW, Chin KY, Foong LC, Pang KL, Yu B, Shu Y, et al. (September 2022). "Modeling Prostate Cancer: What Does it Take to Build an Ideal Tumor Model?". Cancer Lett. 543: 215794. doi:10.1016/j.canlet.2022.215794. PMID 35718268. S2CID 249831438.
- McHugh J, Saunders EJ, Dadaev T, McGrowder E, Bancroft E, Kote-Jarai Z, et al. (June 2022). "Prostate Cancer Risk in Men of Differing Genetic Ancestry and Approaches to Disease Screening and Management in these Groups". Br J Cancer. 126 (10): 1366–1373. doi:10.1038/s41416-021-01669-3. PMC 9090767. PMID 34923574.
- McIntosh SA, Alam F, Adams L, Boon IS, Callaghan J, Conti I, et al. (June 2023). "Global Funding for Cancer Research Between 2016 and 2020: a Content Analysis of Public and Philanthropic Investments". Lancet Oncol. 24 (6): 636–645. doi:10.1016/S1470-2045(23)00182-1. PMID 37269844.
- Merriel SW, Funston G, Hamilton W (September 2018). "Prostate Cancer in Primary Care". Adv Ther. 35 (9): 1285–1294. doi:10.1007/s12325-018-0766-1. PMC 6133140. PMID 30097885.
- Mundle R, Afenya E, Agarwal N (September 2021). "The Effectiveness of Psychological Intervention for Depression, Anxiety, and Distress in Prostate Cancer: a Systematic Review of Literature". Prostate Cancer Prostatic Dis. 24 (3): 674–687. doi:10.1038/s41391-021-00342-3. PMID 33750905. S2CID 232325496.
- Patel MS, Halpern JA, Desai AS, Keeter MK, Bennett NE, Brannigan RE (May 2020). "Success of Prostate and Testicular Cancer Awareness Campaigns Compared to Breast Cancer Awareness Month According to Internet Search Volumes: A Google Trends Analysis". Urology. 139: 64–70. doi:10.1016/j.urology.2019.11.062. PMID 32001306. S2CID 210982209.
- Pernar CH, Ebot EM, Wilson KM, Mucci LA (December 2018). "The Epidemiology of Prostate Cancer". Cold Spring Harb Perspect Med. 8 (12): a030361. doi:10.1101/cshperspect.a030361. PMC 6280714. PMID 29311132.
- Pilié P, Viscuse P, Logothetis CJ, Corn PG (2022). "45: Prostate Cancer". In Kantarjian HM, Wolff RA, Rieber AG (eds.). The MD Anderson Manual of Medical Oncology (4 ed.). McGraw Hill. ISBN 978-1-260-46764-2.
- Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, et al. (February 2021). "Prostate cancer". Nat Rev Dis Primers. 7 (1): 9. doi:10.1038/s41572-020-00243-0. PMID 33542230. S2CID 231794303.
- Scher HI, Eastham JA (2022). "87: Benign and Malignant Diseases of the Prostate". In Loscalzo J, Fauci A, Kasper D, et al. (eds.). Harrison's Principles of Internal Medicine (21 ed.). McGraw Hill. ISBN 978-1-264-26850-4.
- Stephenson AJ, Abouassaly R, Klein EA (2021). "Epidemiology, Etiology, and Prevention of Prostate Cancer". In Partin AW, Dmochowski RR, Kavoussi LR, Peters CA (eds.). Cambell-Walsh-Wein Urology (12 ed.). Elsevier. pp. 3457–3477. ISBN 978-0-323-54642-3.
- Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG (September 2021). "Prostate cancer". Lancet. 398 (10305): 1075–1090. doi:10.1016/S0140-6736(21)00950-8. PMID 34370973. S2CID 236941733.
- Teo MY, Rathkopf DE, Kantoff P (January 2019). "Treatment of Advanced Prostate Cancer". Annu Rev Med. 70: 479–499. doi:10.1146/annurev-med-051517-011947. PMC 6441973. PMID 30691365.
- Thompson JC, Wood J, Feuer D (2007). "Prostate Cancer: Palliative Care and Pain Relief". Br Med Bull. 83: 341–354. doi:10.1093/bmb/ldm018. PMID 17628024.
- Valier H (2016). "The Problematic Prehistory of Prostate Cancer". A History of Prostate Cancer. Springer Nature. pp. 15–42. ISBN 978-1-4039-8803-4.
External links
| Classification | D |
|---|---|
| External resources |
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Testicles |
| ||||||||
| Prostate | |||||||||
| Penis | |||||||||