| Revision as of 12:14, 1 December 2010 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report errors or← Previous edit | Latest revision as of 08:17, 19 December 2024 edit undoPygos (talk | contribs)Extended confirmed users, New page reviewers2,304 edits →See Also | ||
| (134 intermediate revisions by 70 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| |Verifiedfields = changed | |||
| | verifiedrevid = 399907716 | |||
| |Watchedfields = changed | |||
| | Name = Dimethylacetamide | |||
| |verifiedrevid = 414427167 | |||
| | ImageFileL1 = Dimethylacetamide.svg | |||
| |ImageFile = Dimethylacetamide.svg | |||
| | ImageSizeL1 = 110px | |||
| |ImageFile_Ref = {{chemboximage|correct|??}} | |||
| | ImageFileR1 = Dimethylacetamide-3D-balls-B.png | |||
| |ImageSize = 100 | |||
| | ImageSizeR1 = 130px | |||
| | |
|ImageName = Skeletal formula of dimethylacetamide | ||
| | |
|ImageFile1 = Dimethylacetamide-3D-balls-B.png | ||
| |ImageFile1_Ref = {{chemboximage|correct|??}} | |||
| | OtherNames = DMAc, DMA, acetic acid-dimethylamide, N,N-dimethylacetamide, acetyldimethylamine | |||
| |ImageSize1 = 160 | |||
| | Section1 = {{Chembox Identifiers | |||
| |ImageName1 = Ball and stick model of dimethylacetamide | |||
| | SMILES = O=C(N(C)C)C | |||
| |PIN = ''N'',''N''-Dimethylacetamide | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| |Section1={{Chembox Identifiers | |||
| | ChemSpiderID = 29107 | |||
| |Abbreviations = DMA, DMAC, DMAc<ref>{{ cite journal |author1=Munro, D. D. |author2=Stoughton, R. B. | title = Dimethylacetamide (DMAC) and Dimethylformamide (DMFA). Effect on Percutaneous Absorption | journal = Archives of Dermatology | year = 1965 | volume = 92 | issue = 5 | pages = 585–586 | doi = 10.1001/archderm.1965.01600170101020 | pmid = 5844405}}</ref> | |||
| | PubChem = 31374 | |||
| |CASNo = 127-19-5 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| |CASNo_Ref = {{cascite|correct|CAS}} | |||
| | UNII = JCV5VDB3HY | |||
| |PubChem = 31374 | |||
| | InChI = 1/C4H9NO/c1-4(6)5(2)3/h1-3H3 | |||
| |ChemSpiderID = 29107 | |||
| | InChIKey = FXHOOIRPVKKKFG-UHFFFAOYAE | |||
| | |
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| |UNII = JCV5VDB3HY | |||
| | StdInChI = 1S/C4H9NO/c1-4(6)5(2)3/h1-3H3 | |||
| | |
|UNII_Ref = {{fdacite|correct|FDA}} | ||
| |EINECS = 204-826-4 | |||
| | StdInChIKey = FXHOOIRPVKKKFG-UHFFFAOYSA-N | |||
| |MeSHName = dimethylacetamide | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| |ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | CASNo = 127-19-5 | |||
| |ChEBI = 84254 | |||
| | RTECS = AB7700000 | |||
| |ChEMBL = 11873 | |||
| }} | |||
| |ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | Section2 = {{Chembox Properties | |||
| |RTECS = AB7700000 | |||
| | C=4|H=9|N=1|O=1 | |||
| |Beilstein = 1737614 | |||
| | Appearance = Colorless liquid with<br />faint ammonia odor | |||
| |SMILES = CN(C)C(C)=O | |||
| | Density = 0.94 g/cm<sup>3</sup> | |||
| |StdInChI = 1S/C4H9NO/c1-4(6)5(2)3/h1-3H3 | |||
| | MeltingPtC = -20 | |||
| |StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | BoilingPt = 164-166 °C | |||
| |StdInChIKey = FXHOOIRPVKKKFG-UHFFFAOYSA-N | |||
| | Refractive index = 1.4375 | |||
| |StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | Viscosity = 1.956 cP @ 25 °C<br/> | |||
| }} | |||
| 1.279 cP @ 50 °C<br/> | |||
| |Section2={{Chembox Properties | |||
| 0.896 cP @ 75 °C<br/> | |||
| |C=4 | H=9 | N=1 | O=1 | |||
| 0.661 cP @ 100 °C}} | |||
| |Appearance = Colorless liquid | |||
| | Section7 = {{Chembox Hazards | |||
| |Odor = Ammoniacal | |||
| | MainHazards = Toxic ('''T''') | |||
| |Density = 0.937 g/mL | |||
| | RPhrases = {{R61}} {{R20/21}} | |||
| |MeltingPtC = −20 | |||
| | SPhrases = {{S53}} {{S45}} | |||
| | |
|BoilingPtK = 438.2 | ||
| |Solubility = Miscible | |||
| | NFPA-F = | |||
| |LogP = −0.253 | |||
| | NFPA-R = | |||
| | |
|VaporPressure = 300 Pa | ||
| |LambdaMax = 270 nm | |||
| | Autoignition = 490 °C | |||
| |RefractIndex = 1.4375 | |||
| }} | |||
| |Viscosity = 0.945 mPa·s <ref>Iloukhani, H., K. Khanlarzadeh. "Densities, viscosities, and refractive indices for binary and ternary mixtures of N, N-dimethylacetamide (1)+ 2-methylbutan-2-ol (2)+ ethyl acetate (3) at 298.15 K for m liquid region and at ambient pressure". Journal of Chemical & Engineering Data, 51.4 (2006): 1226–1231. {{doi|10.1021/je050538q}}.</ref> | |||
| | Section8 = {{Chembox Related | |||
| }} | |||
| | Function = ]s | |||
| |Section3={{Chembox Thermochemistry | |||
| | OtherFunctn = ''N'',''N''-] | |||
| |DeltaHf = −300.1 kJ/mol | |||
| | Function = Compounds | |||
| |DeltaHc = −2.5835–−2.5805 MJ/mol | |||
| | OtherFunctn = ]<br />]<br />] | |||
| |HeatCapacity = 178.2 J/(K·mol) | |||
| }} | |||
| }} | |||
| |Section4={{Chembox Hazards | |||
| |GHSPictograms = {{gHS exclamation mark}} {{gHS health hazard}} | |||
| |GHSSignalWord = '''DANGER''' | |||
| |HPhrases = {{h-phrases|312|319|332|360}} | |||
| |PPhrases = {{p-phrases|280|308+313}} | |||
| |NFPA-H = 2 | |||
| |NFPA-F = 2 | |||
| |NFPA-R = 0 | |||
| |FlashPtC = 63 | |||
| |AutoignitionPtC = 490 | |||
| |ExploLimits = 1.8–11.5% | |||
| |LD50 = 2.24 g/kg <small>(dermal, rabbit)</small><br />4.3 g/kg <small>(oral, rat)</small><br />4.8 g/kg (oral, rat)<br />4.62 g/kg (oral, mouse)<ref name=IDLH/> | |||
| |PEL = TWA 10 ppm (35 mg/m<sup>3</sup>) <ref name=PGCH>{{PGCH|0218}}</ref> | |||
| |IDLH = 300 ppm<ref name=PGCH/> | |||
| |REL = TWA 10 ppm (35 mg/m<sup>3</sup>) <ref name=PGCH/> | |||
| |LC50 = 2475 ppm (rat, 1 ])<ref name=IDLH>{{IDLH|127195|Dimethyl acetamide}}</ref> | |||
| }} | |||
| |Section5={{Chembox Related | |||
| |OtherCompounds = {{Unbulleted list|]|]|]}} | |||
| }} | |||
| }} | }} | ||
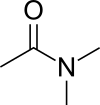
| '''Dimethylacetamide''' is the ] with the ] CH<sub>3</sub>C(O)N(CH<sub>3</sub>)<sub>2</sub>. This colorless, water |
'''Dimethylacetamide''' ('''DMAc''' or '''DMA''') is the ] with the ] CH<sub>3</sub>C(O)N(CH<sub>3</sub>)<sub>2</sub>. This colorless, water-miscible, high-boiling liquid is commonly used as a polar ] in ]. DMA is miscible with most other solvents, although it is poorly soluble in ] ]s. | ||
| ==Synthesis and production== | |||
| The chemical reactions of dimethylacetamide are typical of ''N'',''N''-disubstituted ]s. It will ] in the presence of ]s: | |||
| DMA is prepared commercially by the reaction of ] with ] or ]. Dehydration of the salt of dimethylamine and acetic acid also furnishes this compound:<ref name=Ullmann>{{ Ullmann | author = Cheung, H. | author2 = Tanke, R. S. | author3 = Torrence, G. P. | title = Acetic Acid | doi = 10.1002/14356007.a01_045.pub2}}</ref> | |||
| : CH<sub>3</sub>CO<sub>2</sub>H·HN(CH<sub>3</sub>)<sub>2</sub> → H<sub>2</sub>O + CH<sub>3</sub>CON(CH<sub>3</sub>)<sub>2</sub> | |||
| Dimethylacetamide can also be produced by the reaction of ] with ].<ref name="production">{{cite web |last1=Grafmans |first1=Horst |last2=Maas |first2=Steffen |last3=Weck |first3=Alexander |last4=Rütter |first4=Heinz |last5=Schulz |first5=Michael |last6=Ross |first6=Karl-Heinz |title=Method for the production of n,n-dimethylacetamide (DMAC) |url=https://patents.google.com/patent/EP1828102B1/en |website=Google Patents |publisher=BASF SE |accessdate=18 July 2019}}</ref> | |||
| : CH<sub>3</sub>CON(CH<sub>3</sub>)<sub>2</sub> + H<sub>2</sub>O + HCl → CH<sub>3</sub>COOH + (CH<sub>3</sub>)<sub>2</sub>NH<sub>2</sub><sup>+</sup>Cl<sup>-</sup> | |||
| : ] | |||
| Dimethylacetamide is useful as a medium for strong bases such as ].<ref>{{OrgSynth | author = S. Zen and E. Kaji | title = Dimethyl nitrosuccinate | collvol = 6 | collvolpages = 503 | year = 1988 | prep = CV6P0503}}</ref> Dimethylacetamide is commonly used as a solvent for fibers or in the ] industry. It is also employed in the production of ] and ]s as a reaction medium. | |||
| The separation and purification of the product is carried out by multistage ] in rectification columns. DMA is obtained with essentially quantitive (99%) ] referred to methyl acetate.<ref name=production/> | |||
| ==Reactions and applications== | |||
| The chemical reactions of dimethylacetamide are typical of ''N'',''N''-disubstituted ]s. ] of the acyl-N bond occurs in the presence of ]s: | |||
| : CH<sub>3</sub>CON(CH<sub>3</sub>)<sub>2</sub> + H<sub>2</sub>O + HCl → CH<sub>3</sub>COOH + (CH<sub>3</sub>)<sub>2</sub>NH<sub>2</sub><sup>+</sup>Cl<sup>−</sup> | |||
| However, it is resistant to bases. For this reason DMA is a useful solvent for reactions involving strong bases such as ].<ref>{{OrgSynth | author = Zen, S. | author2 = Kaji, E. | title = Dimethyl nitrosuccinate | volume = 57 | pages = 60 | collvol = 6 | collvolpages = 503 | year = 1977 | prep = CV6P0503}}</ref> | |||
| Dimethylacetamide is commonly used as a solvent for fibers (e.g., ], ]) or in the ] industry.<ref name=Ullmann/> It is also employed in the production of ] and ]s as a reaction medium. | |||
| A solution of ] in DMAc (LiCl/DMAc) can dissolve ]. Unlike many other cellulose solvents, LiCl/DMAc gives a molecular dispersion, i.e. a "true solution". For this reason, it is used in ] to determine the ] of cellulose samples. | |||
| Dimethylacetamide is also used as an ] in drugs, e.g. in Vumon (]), Busulfex (]) or Amsidine (]). | |||
| ==Toxicity== | |||
| Dimethylacetamide, like most simple alkyl amides, is of low acute toxicity. Chronic exposure can cause ].<ref name="cdc.gov">U.S. Department of Health and Human Services & U.S. Department of Labor (1978) . Now: Occupational Health Guideline for Chemical Hazards. . January 1981. The National Institute for Occupational Safety and Health (NIOSH).</ref><ref>{{ cite journal |author1=Baum, S. L. |author2=Suruda, A. J. | title = Toxic Hepatitis from Dimethylacetamide | journal = International Journal of Occupational and Environmental Health | year = 1997 | volume = 3 | issue = 1 | pages = 1–4 | doi = 10.1179/oeh.1997.3.1.1 | pmid = 9891094 }}</ref><ref>{{ cite journal |author1=Lee, C.-Y. |author2=Jung, S.-J. |author3=Kim, S.-A. | author4=Park, K.-S. | author5=Ha, B.-G. | title = Incidence of dimethylacetamide induced hepatic injury among new employees in a cohort of elastane fibre workers | journal = Occupational and Environmental Medicine | year = 2006 | volume = 63 | issue = 10 | pages = 688–693 | doi = 10.1136/oem.2005.023580 | pmid = 16728503 | pmc = 2078052 }}</ref><ref>{{ cite journal |author1=Gong, W. |author2=Liu, X. |author3=Zhu, B. | title = Dimethylacetamide-induced occupational toxic hepatitis with a short term recurrence: a rare case report | journal = Journal of Thoracic Disease | year = 2016 | volume = 8 | issue = 6 | pages = E408–E411 | doi = 10.21037/jtd.2016.04.44 | pmid = 27293868 | pmc = 4885965 |doi-access=free }}</ref> At high doses (400 mg/kg body mass daily), dimethylacetamide causes effects on the ] (e.g. ], ]s and ]).<ref name="cdc.gov"/><ref>{{ cite journal |author1= Weiss, A. J. |author2= Jackson, L. G. |author3= Carabasi, R. A. | author4= Mancall, E. L. | author5= White, J. C. | title = A Phase I Study of Dimethylacetamide | journal = Cancer Chemotherapy Reports | year = 1962 | volume = 16 | issue = February 1962 | pages = 477–485 | pmid = 14005853}}</ref><ref>{{ cite journal |author1= Weiss, A. J. |author2= Mancall, E. L. |author3= Koltes, J. A. | author4= White, J. C. | author5= Jackson, L. G. | title = Dimethylacetamide: A Hitherto Unrecognized Hallucinogenic Agent | journal = Science | year = 1962 | volume = 136 | issue = 3511 | pages = 151–152| doi = 10.1126/science.136.3511.151| pmid = 14005854|bibcode= 1962Sci...136..151W |s2cid= 20098340 }}</ref> | |||
| Dimethylacetamide may be incompatible with ] or ]. Devices (e.g. syringes) that contain polycarbonate or ABS can dissolve when coming into contact with dimethylacetamide.<ref>. 10 March 2015.</ref> | |||
| ==Regulation== | |||
| In 2011, dimethylacetamide was identified in the EU as a ] (SVHC) because of its ].<ref>.</ref> In 2014, the European Commission has started an investigation to restrict the use of dimethylacetamide in the EU according to ].<ref>, Official Journal of the European Union, 19.08.2014.</ref> | |||
| In 2015, the CNESST (Committee on Standards, Equity, Health and Safety at Work in ]) has adopted a tightened classification of dimethylacetamide:<ref>Commission des normes, de l'équité, de la santé et de la sécurité du travail (CNESST), Quebec, Canada: .</ref> | |||
| {| class="wikitable" | |||
| |- | |||
| ! Description !! Category !! ] | |||
| |- | |||
| | Reproductive toxicity || 2|| Suspected of damaging fertility or the unborn child (H361) | |||
| |- | |||
| | Specific target organ toxicity – repeated exposure || 2|| May cause damage to organs through prolonged or repeated exposure (H373) | |||
| |- | |||
| | Serious eye damage/eye irritation || 2|| Causes serious eye irritation (H319) | |||
| |- | |||
| | Acute toxicity – inhalation || 3|| Toxic if inhaled (H331) | |||
| |- | |||
| | Specific target organ toxicity – single exposure – narcotic effects || 3|| May cause drowsiness or dizziness (H336) | |||
| |- | |||
| | Flammable liquid || 4|| Combustible liquid (H227) | |||
| |} | |||
| ==See Also== | |||
| * ] | |||
| * ] | |||
| ==References== | ==References== | ||
| {{ |
{{Reflist}} | ||
| ==External links== | |||
| * | |||
| * | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 08:17, 19 December 2024
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name N,N-Dimethylacetamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | DMA, DMAC, DMAc |
| Beilstein Reference | 1737614 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.389 |
| EC Number |
|
| MeSH | dimethylacetamide |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H9NO |
| Molar mass | 87.122 g·mol |
| Appearance | Colorless liquid |
| Odor | Ammoniacal |
| Density | 0.937 g/mL |
| Melting point | −20 °C (−4 °F; 253 K) |
| Boiling point | 165.1 °C; 329.1 °F; 438.2 K |
| Solubility in water | Miscible |
| log P | −0.253 |
| Vapor pressure | 300 Pa |
| UV-vis (λmax) | 270 nm |
| Refractive index (nD) | 1.4375 |
| Viscosity | 0.945 mPa·s |
| Thermochemistry | |
| Heat capacity (C) | 178.2 J/(K·mol) |
| Std enthalpy of formation (ΔfH298) |
−300.1 kJ/mol |
| Std enthalpy of combustion (ΔcH298) |
−2.5835–−2.5805 MJ/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H312, H319, H332, H360 |
| Precautionary statements | P280, P308+P313 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 63 °C (145 °F; 336 K) |
| Autoignition temperature |
490 °C (914 °F; 763 K) |
| Explosive limits | 1.8–11.5% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 2.24 g/kg (dermal, rabbit) 4.3 g/kg (oral, rat) 4.8 g/kg (oral, rat) 4.62 g/kg (oral, mouse) |
| LC50 (median concentration) | 2475 ppm (rat, 1 h) |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA 10 ppm (35 mg/m) |
| REL (Recommended) | TWA 10 ppm (35 mg/m) |
| IDLH (Immediate danger) | 300 ppm |
| Related compounds | |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Dimethylacetamide (DMAc or DMA) is the organic compound with the formula CH3C(O)N(CH3)2. This colorless, water-miscible, high-boiling liquid is commonly used as a polar solvent in organic synthesis. DMA is miscible with most other solvents, although it is poorly soluble in aliphatic hydrocarbons.
Synthesis and production
DMA is prepared commercially by the reaction of dimethylamine with acetic anhydride or acetic acid. Dehydration of the salt of dimethylamine and acetic acid also furnishes this compound:
- CH3CO2H·HN(CH3)2 → H2O + CH3CON(CH3)2
Dimethylacetamide can also be produced by the reaction of dimethylamine with methyl acetate.
The separation and purification of the product is carried out by multistage distillation in rectification columns. DMA is obtained with essentially quantitive (99%) yield referred to methyl acetate.
Reactions and applications
The chemical reactions of dimethylacetamide are typical of N,N-disubstituted amides. Hydrolysis of the acyl-N bond occurs in the presence of acids:
- CH3CON(CH3)2 + H2O + HCl → CH3COOH + (CH3)2NH2Cl
However, it is resistant to bases. For this reason DMA is a useful solvent for reactions involving strong bases such as sodium hydroxide.
Dimethylacetamide is commonly used as a solvent for fibers (e.g., polyacrylonitrile, spandex) or in the adhesive industry. It is also employed in the production of pharmaceuticals and plasticizers as a reaction medium.
A solution of lithium chloride in DMAc (LiCl/DMAc) can dissolve cellulose. Unlike many other cellulose solvents, LiCl/DMAc gives a molecular dispersion, i.e. a "true solution". For this reason, it is used in gel permeation chromatography to determine the molar mass distribution of cellulose samples.
Dimethylacetamide is also used as an excipient in drugs, e.g. in Vumon (teniposide), Busulfex (busulfan) or Amsidine (amsacrine).
Toxicity
Dimethylacetamide, like most simple alkyl amides, is of low acute toxicity. Chronic exposure can cause hepatotoxicity. At high doses (400 mg/kg body mass daily), dimethylacetamide causes effects on the central nervous system (e.g. depression, hallucinations and delusion).
Dimethylacetamide may be incompatible with polycarbonate or ABS. Devices (e.g. syringes) that contain polycarbonate or ABS can dissolve when coming into contact with dimethylacetamide.
Regulation
In 2011, dimethylacetamide was identified in the EU as a Substance of very high concern (SVHC) because of its reproductive toxicity. In 2014, the European Commission has started an investigation to restrict the use of dimethylacetamide in the EU according to REACH.
In 2015, the CNESST (Committee on Standards, Equity, Health and Safety at Work in Quebec) has adopted a tightened classification of dimethylacetamide:
| Description | Category | GHS hazard statement |
|---|---|---|
| Reproductive toxicity | 2 | Suspected of damaging fertility or the unborn child (H361) |
| Specific target organ toxicity – repeated exposure | 2 | May cause damage to organs through prolonged or repeated exposure (H373) |
| Serious eye damage/eye irritation | 2 | Causes serious eye irritation (H319) |
| Acute toxicity – inhalation | 3 | Toxic if inhaled (H331) |
| Specific target organ toxicity – single exposure – narcotic effects | 3 | May cause drowsiness or dizziness (H336) |
| Flammable liquid | 4 | Combustible liquid (H227) |
See Also
References
- Munro, D. D.; Stoughton, R. B. (1965). "Dimethylacetamide (DMAC) and Dimethylformamide (DMFA). Effect on Percutaneous Absorption". Archives of Dermatology. 92 (5): 585–586. doi:10.1001/archderm.1965.01600170101020. PMID 5844405.
- Iloukhani, H., K. Khanlarzadeh. "Densities, viscosities, and refractive indices for binary and ternary mixtures of N, N-dimethylacetamide (1)+ 2-methylbutan-2-ol (2)+ ethyl acetate (3) at 298.15 K for m liquid region and at ambient pressure". Journal of Chemical & Engineering Data, 51.4 (2006): 1226–1231. doi:10.1021/je050538q.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0218". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Dimethyl acetamide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Cheung, H.; Tanke, R. S.; Torrence, G. P. "Acetic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_045.pub2. ISBN 978-3527306732.
- ^ Grafmans, Horst; Maas, Steffen; Weck, Alexander; Rütter, Heinz; Schulz, Michael; Ross, Karl-Heinz. "Method for the production of n,n-dimethylacetamide (DMAC)". Google Patents. BASF SE. Retrieved 18 July 2019.
- Zen, S.; Kaji, E. (1977). "Dimethyl nitrosuccinate". Organic Syntheses. 57: 60; Collected Volumes, vol. 6, p. 503.
- ^ U.S. Department of Health and Human Services & U.S. Department of Labor (1978) Occupational Health Guideline for Dimethyl Acetamide. Now: Occupational Health Guideline for Chemical Hazards. DHHS (NIOSH) Publication Number 81-123. January 1981. The National Institute for Occupational Safety and Health (NIOSH).
- Baum, S. L.; Suruda, A. J. (1997). "Toxic Hepatitis from Dimethylacetamide". International Journal of Occupational and Environmental Health. 3 (1): 1–4. doi:10.1179/oeh.1997.3.1.1. PMID 9891094.
- Lee, C.-Y.; Jung, S.-J.; Kim, S.-A.; Park, K.-S.; Ha, B.-G. (2006). "Incidence of dimethylacetamide induced hepatic injury among new employees in a cohort of elastane fibre workers". Occupational and Environmental Medicine. 63 (10): 688–693. doi:10.1136/oem.2005.023580. PMC 2078052. PMID 16728503.
- Gong, W.; Liu, X.; Zhu, B. (2016). "Dimethylacetamide-induced occupational toxic hepatitis with a short term recurrence: a rare case report". Journal of Thoracic Disease. 8 (6): E408 – E411. doi:10.21037/jtd.2016.04.44. PMC 4885965. PMID 27293868.
- Weiss, A. J.; Jackson, L. G.; Carabasi, R. A.; Mancall, E. L.; White, J. C. (1962). "A Phase I Study of Dimethylacetamide". Cancer Chemotherapy Reports. 16 (February 1962): 477–485. PMID 14005853.
- Weiss, A. J.; Mancall, E. L.; Koltes, J. A.; White, J. C.; Jackson, L. G. (1962). "Dimethylacetamide: A Hitherto Unrecognized Hallucinogenic Agent". Science. 136 (3511): 151–152. Bibcode:1962Sci...136..151W. doi:10.1126/science.136.3511.151. PMID 14005854. S2CID 20098340.
- FDA warns health care professionals not to use Treanda Injection (solution) with closed system transfer devices, adapters, and syringes containing polycarbonate or acrylonitrile-butadiene-styrene. 10 March 2015.
- Agreement of the Member State Committee on the Identification of N,N-Dimethylacetamide (DMAC) as a Substance of Very High Concern – Adopted on 24 November 2011.
- Commission Regulation (EU) No 895/2014, Official Journal of the European Union, 19.08.2014.
- Commission des normes, de l'équité, de la santé et de la sécurité du travail (CNESST), Quebec, Canada: WHMIS 2015 classification of N,N-Dimethylacetamide.
External links
- Process flowsheet of Dimethylacetamide Production from Acetic Acid and Dimethylamine
- CDC – NIOSH Pocket Guide to Chemical Hazards
