| Revision as of 09:23, 23 February 2011 editAnypodetos (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers39,350 editsm Sufficient refs for the contents of this stub← Previous edit | Latest revision as of 04:47, 27 October 2024 edit undoWhywhenwhohow (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers49,153 edits rank | ||
| (46 intermediate revisions by 22 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Antihypertensive medication}} | |||
| {{Use dmy dates|date=February 2024}} | |||
| {{Drugbox | {{Drugbox | ||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 415479203 | ||
| | type |
| type = combo | ||
| | image |
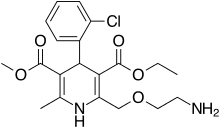
| image = Amlodipine.svg | ||
| | alt = | |||
| | image2 = Benazepril.svg | |||
| | image2 = Benazepril.svg | |||
| | component1 = Amlodipine | |||
| | alt2 = | |||
| | class1 = ] | |||
| | component2 = Benazepril | |||
| <!-- Combo data --> | |||
| | class2 = ] | |||
| | component1 = Amlodipine | |||
| | class1 = ] | |||
| | component2 = Benazepril | |||
| | class2 = ] | |||
| <!-- Clinical data --> | |||
| | tradename = Lotrel | |||
| | Drugs.com = {{drugs.com|ppa|amlodipine-and-benazepril}} | |||
| | DailyMedID = Amlodipine and benazepril | |||
| | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| | pregnancy_category = | |||
| | routes_of_administration = ] | |||
| | ATC_prefix = C09 | |||
| | ATC_suffix = BB13 | |||
| <!-- Legal status --> | |||
| | legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled --> | |||
| | legal_AU_comment = | |||
| | legal_BR = <!-- OTC, A1, A2, A3, B1, B2, C1, C2, C3, C4, C5, D1, D2, E, F --> | |||
| | legal_BR_comment = | |||
| | legal_CA = <!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| | legal_CA_comment = | |||
| | legal_DE = <!-- Anlage I, II, III or Unscheduled --> | |||
| | legal_DE_comment = | |||
| | legal_NZ = <!-- Class A, B, C --> | |||
| | legal_NZ_comment = | |||
| | legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM / Class A, B, C --> | |||
| | legal_UK_comment = | |||
| | legal_US = Rx-only | |||
| | legal_US_comment = <ref name="Lotrel FDA label">{{cite web | title=Lotrel- amlodipine besylate and benazepril hydrochloride capsule | work = DailyMed | publisher = U.S. National Library of Medicine | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94ae6054-b7ae-4212-a567-4f803af8f2c7 | access-date=20 June 2021}}</ref> | |||
| | legal_EU = | |||
| | legal_EU_comment = | |||
| | legal_UN = <!-- N I, II, III, IV / P I, II, III, IV --> | |||
| | legal_UN_comment = | |||
| | legal_status = <!-- For countries not listed above --> | |||
| <!-- Identifiers --> | |||
| | CAS_number_Ref = {{cascite|changed|??}} | |||
| | CAS_number = 357437-90-2 | |||
| | PubChem = 5746247 | |||
| | DrugBank = | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 4676979 | | ChemSpiderID = 4676979 | ||
| | KEGG = D11069 | |||
| | InChI = 1/C24H28N2O5.C20H25ClN2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28;1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28);5-8,17,23H,4,9-11,22H2,1-3H3/t19-,20-;/m0./s1 | |||
| | smiles = Clc1ccccc1C2C(\C(=O)OC)=C(/N\C(=C2\C(=O)OCC)COCCN)C.O=C(OCC)(N2C(=O)N(c1ccccc1CC2)CC(=O)O)CCc3ccccc3 | |||
| | InChIKey = SRTZYSFUFGOMFR-FKLPMGAJBV | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C24H28N2O5.C20H25ClN2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28;1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28);5-8,17,23H,4,9-11,22H2,1-3H3/t19-,20-;/m0./s1 | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = SRTZYSFUFGOMFR-FKLPMGAJSA-N | |||
| | CAS_number = | |||
| | ATC_prefix = None | |||
| | ATC_suffix = | |||
| | PubChem = 5746247 | |||
| | DrugBank = | |||
| | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| | pregnancy_US = D | |||
| | pregnancy_category= | |||
| | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| | legal_US = Rx-only | |||
| | legal_status = | |||
| | routes_of_administration = Oral | |||
| }} | }} | ||
| '''Amlodipine/benazepril''', marketed in the U.S. as '''Lotrel''' by ] and manufactured as a ] by ] and ], is an ] medication which combines a ] (] besilate) with an ] (]).<ref></ref> This drug, like similar combinations, is prescribed when either agent alone is not sufficient to bring a person's blood pressure down to target range. As a combination agent, Lotrel shares the ] profile of both of its individual parts.<ref>Drugs.com: </ref><ref>RxList.com: </ref> | |||
| <!-- Definition and medical uses --> | |||
| == See also == | |||
| '''Amlodipine/benazepril''', sold under the brand name '''Lotrel''' among others, is a ] medication used to treat ].<ref name="Lotrel FDA label" /> It is a combination of ], as the besilate, a ], and ], an ].<ref name="Lotrel FDA label"/> It may be used if a single agent is not sufficient.<ref name="Lotrel FDA label"/> It is taken ].<ref name="Lotrel FDA label"/> | |||
| * ] | |||
| * ] | |||
| <!-- Side effects and mechanisms --> | |||
| ==References== | |||
| Common side effects include cough, dizziness, and swelling.<ref name="Lotrel FDA label"/> Serious side effects may include ], ], ], ], and ].<ref name="Lotrel FDA label"/> Use in ] is not recommended.<ref name="Lotrel FDA label"/> Amlodipine works by ] while benazepril works by decreasing ] activity.<ref name="Lotrel FDA label"/> | |||
| {{Reflist}} | |||
| <!-- Society and culture --> | |||
| ==External links== | |||
| The combination was approved for medical use in the United States in 1995.<ref name=MTM2019>{{cite web | author = Cerner Multum |title=Amlodipine and benazepril Uses, Side Effects & Warnings |url=https://www.drugs.com/mtm/amlodipine-and-benazepril.html |website=Drugs.com |access-date=10 March 2019 |language=en}}</ref> It is available as a ].<ref>{{cite book | vauthors = Bope ET, Kellerman RD |title=Conn's Current Therapy 2017 E-Book |date=2016 |publisher=Elsevier Health Sciences |isbn=978-0-323-44335-7 |page=124 |url=https://books.google.com/books?id=UitFDQAAQBAJ&pg=PT158 }}</ref> In 2022, it was the 170th most commonly prescribed medication in the United States, with more than 3{{nbsp}}million prescriptions.<ref>{{cite web | title=The Top 300 of 2022 | url=https://clincalc.com/DrugStats/Top300Drugs.aspx | website=ClinCalc | access-date=30 August 2024 | archive-date=30 August 2024 | archive-url=https://web.archive.org/web/20240830202410/https://clincalc.com/DrugStats/Top300Drugs.aspx | url-status=live }}</ref><ref>{{cite web | title = Amlodipine; Benazepril Drug Usage Statistics, United States, 2013 - 2022 | website = ClinCalc | url = https://clincalc.com/DrugStats/Drugs/AmlodipineBenazepril | access-date = 30 August 2024 }}</ref> | |||
| * ]: | |||
| ==Medical uses== | |||
| It is used to treat ].<ref name="Lotrel FDA label"/> It is not a first-line treatment.<ref>{{cite journal | vauthors = Faulkner MA, Hilleman DE | title = Amlodipine/benazepril: fixed dose combination therapy for hypertension | journal = Expert Opinion on Pharmacotherapy | volume = 2 | issue = 1 | pages = 165–178 | date = January 2001 | pmid = 11336577 | doi = 10.1517/14656566.2.1.165 | s2cid = 23021242 }}</ref> | |||
| == References == | |||
| {{Reflist}} | |||
| {{Calcium channel blockers}} | |||
| {{ACE inhibitors}} | {{ACE inhibitors}} | ||
| {{Calcium channel blockers}} | |||
| {{Portal bar|Medicine}} | |||
| {{DEFAULTSORT:Amlodipine Benazepril}} | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | |||
Latest revision as of 04:47, 27 October 2024
Antihypertensive medicationPharmaceutical compound
 | |
 | |
| Combination of | |
|---|---|
| Amlodipine | Calcium channel blocker |
| Benazepril | ACE inhibitor |
| Clinical data | |
| Trade names | Lotrel |
| AHFS/Drugs.com | Professional Drug Facts |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| (what is this?) (verify) | |
Amlodipine/benazepril, sold under the brand name Lotrel among others, is a fixed-dose combination medication used to treat high blood pressure. It is a combination of amlodipine, as the besilate, a calcium channel blocker, and benazepril, an angiotensin converting enzyme inhibitor. It may be used if a single agent is not sufficient. It is taken by mouth.
Common side effects include cough, dizziness, and swelling. Serious side effects may include angioedema, myocardial infarction, high blood potassium, liver problems, and low blood pressure. Use in pregnancy is not recommended. Amlodipine works by increasing the size of arteries while benazepril works by decreasing renin-angiotensin-aldosterone system activity.
The combination was approved for medical use in the United States in 1995. It is available as a generic medication. In 2022, it was the 170th most commonly prescribed medication in the United States, with more than 3 million prescriptions.
Medical uses
It is used to treat high blood pressure. It is not a first-line treatment.
References
- ^ "Lotrel- amlodipine besylate and benazepril hydrochloride capsule". DailyMed. U.S. National Library of Medicine. Retrieved 20 June 2021.
- Cerner Multum. "Amlodipine and benazepril Uses, Side Effects & Warnings". Drugs.com. Retrieved 10 March 2019.
- Bope ET, Kellerman RD (2016). Conn's Current Therapy 2017 E-Book. Elsevier Health Sciences. p. 124. ISBN 978-0-323-44335-7.
- "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- "Amlodipine; Benazepril Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- Faulkner MA, Hilleman DE (January 2001). "Amlodipine/benazepril: fixed dose combination therapy for hypertension". Expert Opinion on Pharmacotherapy. 2 (1): 165–178. doi:10.1517/14656566.2.1.165. PMID 11336577. S2CID 23021242.
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |