| Revision as of 21:12, 1 April 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (← Previous edit |
Latest revision as of 15:07, 31 January 2023 edit undoEntranced98 (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers172,777 edits Importing Wikidata short description: "Chemical compound"Tag: Shortdesc helper |
| (19 intermediate revisions by 17 users not shown) |
| Line 1: |
Line 1: |
|
|
{{Short description|Chemical compound}} |
|
{{Drugbox |
|
{{Drugbox |
|
| verifiedrevid = 376111334 |
|
| verifiedrevid = 421882229 |
|
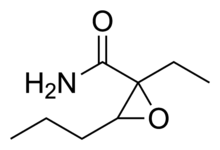
| IUPAC_name = 2-ethyl-3-propyloxirane-2-carboxamide |
|
| IUPAC_name = 2-ethyl-3-propyloxirane-2-carboxamide |
|
| image = Oxanamide.png |
|
| image = Oxanamide.png |
|
|
|
| ⚫ |
| CAS_number = 126-93-2 |
|
|
|
<!--Clinical data--> |
| ⚫ |
| ATC_prefix = none |
|
|
| ATC_suffix = |
|
| tradename = |
|
⚫ |
| pregnancy_category = |
| ⚫ |
| PubChem = 31365 |
|
|
⚫ |
| legal_status = |
| ⚫ |
| C = 8 | H = 15 | N = 1 | O = 2 |
|
|
⚫ |
| routes_of_administration = |
|
| molecular_weight = 157.21 g/mol |
|
|
|
|
| ⚫ |
| bioavailability = |
|
|
|
<!--Pharmacokinetic data--> |
|
| metabolism = |
|
|
⚫ |
| bioavailability = |
| ⚫ |
| elimination_half-life = |
|
|
| excretion = |
|
| metabolism = |
|
⚫ |
| elimination_half-life = |
| ⚫ |
| pregnancy_category= |
|
|
|
| excretion = |
| ⚫ |
| legal_status = |
|
|
|
|
| ⚫ |
| routes_of_administration = |
|
|
|
<!--Identifiers--> |
|
⚫ |
| CAS_number = 126-93-2 |
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
|
| UNII = 050271194T |
|
⚫ |
| ATC_prefix = none |
|
|
| ATC_suffix = |
|
⚫ |
| PubChem = 31365 |
|
|
| ChemSpiderID = 29098 |
|
|
|
|
|
<!--Chemical data--> |
|
⚫ |
| C=8 | H=15 | N=1 | O=2 |
|
|
| smiles = CCCC1C(O1)(CC)C(=O)N |
|
|
| StdInChI = 1S/C8H15NO2/c1-3-5-6-8(4-2,11-6)7(9)10/h6H,3-5H2,1-2H3,(H2,9,10) |
|
|
| StdInChIKey = WBLPIVIXQOFTPQ-UHFFFAOYSA-N |
|
|
|
|
}} |
|
}} |
|
|
|
|
|
'''Oxanamide''' ('''Quiactin''') is an ] and ] which can produce ] and ] effects in sufficiently high doses.<ref>{{cite journal|title=Effects of oxanamide on the central nervous system |journal=Proceedings of the Society for Experimental Biology and Medicine |year=1960 |volume=103 |pages=101–3|pmid=14412594|last1=Kuhn|first1=WL|last2=Ketteler|first2=HJ|last3=Van Maanen|first3=EF}}</ref> An uncontrolled trial on patients treated in a clinical ] practice published in 1959 found that oxanamide was efficacious in the treatment of anxiety resulting from ], ], and various other causes, with minimal sedation or other side effects.<ref>{{cite journal| author=Robert B. Woodhull |title=Oxanamide; adjunctive use of a new tranquilizer in gynecology| journal=California Medicine| year=1959 |volume=90 |issue=4 |pages=275–7 | pmid=13638840| pmc=1577644}}</ref> |
|
'''Oxanamide''' ('''Quiactin''') is an ] and ] which can produce ] and ] effects in sufficiently high doses.<ref>{{cite journal | vauthors = Kuhn WL, Ketteler HJ, Van Maanen EF | s2cid = 40927309 | title = Effects of oxanamide on the central nervous system | journal = Proceedings of the Society for Experimental Biology and Medicine | volume = 103 | pages = 101–3 | date = January 1960 | pmid = 14412594 | doi = 10.3181/00379727-103-25425 }}</ref> An uncontrolled trial on patients treated in a clinical ] practice published in 1959 found that oxanamide was efficacious in the treatment of anxiety resulting from ], ], and various other causes, with minimal sedation or other side effects.<ref>{{cite journal | vauthors = Woodhull RB | title = Oxanamide; adjunctive use of a new tranquilizer in gynecology | journal = California Medicine | volume = 90 | issue = 4 | pages = 275–7 | date = April 1959 | pmid = 13638840 | pmc = 1577644 }}</ref> |
|
|
|
|
|
== References == |
|
== References == |
| Line 29: |
Line 45: |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
|
|
|
|
|
|
|
|
{{musculoskeletal-drug-stub}} |
|
{{musculoskeletal-drug-stub}} |
|
{{nervous-system-drug-stub}} |
|
{{Anxiolytic-stub}} |
|
|
{{Sedative-stub}} |