| Revision as of 08:10, 6 April 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChEMBL.← Previous edit | Latest revision as of 03:43, 22 May 2024 edit undo0dorkmann (talk | contribs)258 edits infobox img do-it-yourself | ||
| (40 intermediate revisions by 30 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | verifiedrevid = 422659402 | |||
| ⚫ | | ImageFile = 3,3'-Dichlorobenzidine.svg | ||
| | ImageFile_Ref = {{chemboximage|correct|??}} | |||
| ⚫ | | ImageFile = 3,3'-Dichlorobenzidine-2D.svg | ||
| | ImageFile2 = 3,3'-Dichlorobenzidine-3D.png | |||
| | ImageSize = 250px | | ImageSize = 250px | ||
| | PIN = 3,3′-Dichloro-4,4′-diamine | |||
| | IUPACName = 4-(4-amino-3-chlorophenyl)-2-chloroaniline | |||
| | Reference = <ref name="pubchem">, ].</ref> | | Reference = <ref name="pubchem">, ].</ref> | ||
| | OtherNames = 4-(4-Amino-3-chlorophenyl)-2-chloroaniline<br />4,4′-Diamino-3,3′-dichlorobiphenyl<br />''o'',''o'''-Dichlorobenzidine<br />3,3′-Dichlorobiphenyl-4,4′-diamine<br />3,3′-Dichloro-4,4′-biphenyldiamine<br/>3,3′-Dichloro-4,4′-diaminobiphenyl | |||
| | OtherNames = (1,1-Biphenyl)-4,4-Diamine,3,3-dichloro; 3,3-dichloro-4,4-biphenyldiamine | |||
| | |
|Section1={{Chembox Identifiers | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 6803 | | ChemSpiderID = 6803 | ||
| | InChI = 1/C12H10Cl2N2/c13-9-5-7(1-3-11(9)15)8-2-4-12(16)10(14)6-8/h1-6H,15-16H2 | | InChI = 1/C12H10Cl2N2/c13-9-5-7(1-3-11(9)15)8-2-4-12(16)10(14)6-8/h1-6H,15-16H2 | ||
| | InChIKey = HUWXDEQWWKGHRV-UHFFFAOYAF | | InChIKey = HUWXDEQWWKGHRV-UHFFFAOYAF | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 314470 | | ChEMBL = 314470 | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C12H10Cl2N2/c13-9-5-7(1-3-11(9)15)8-2-4-12(16)10(14)6-8/h1-6H,15-16H2 | | StdInChI = 1S/C12H10Cl2N2/c13-9-5-7(1-3-11(9)15)8-2-4-12(16)10(14)6-8/h1-6H,15-16H2 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = HUWXDEQWWKGHRV-UHFFFAOYSA-N | | StdInChIKey = HUWXDEQWWKGHRV-UHFFFAOYSA-N | ||
| | CASNo = 91-94-1 | | CASNo = 91-94-1 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 1SDI2328UX | |||
| | PubChem = 7070 | | PubChem = 7070 | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | KEGG = C19225 | |||
| | SMILES = Clc2cc(c1ccc(N)c(Cl)c1)ccc2N | | SMILES = Clc2cc(c1ccc(N)c(Cl)c1)ccc2N | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | Formula = C<sub>12</sub>H<sub>10</sub>Cl<sub>2</sub>N<sub>2</sub> | | Formula = C<sub>12</sub>H<sub>10</sub>Cl<sub>2</sub>N<sub>2</sub> | ||
| | MolarMass = 253.13 g/mol | | MolarMass = 253.13 g/mol | ||
| | Appearance = Gray or purple crystalline solid | | Appearance = Gray or purple crystalline solid | ||
| | MeltingPtC = 132 to 133 | |||
| | Density = | |||
| | MeltingPt = 132-133 °C | |||
| | BoilingPtC = 402 | | BoilingPtC = 402 | ||
| | Solubility = }} | | Solubility = 0.07% (15°C)<ref name=PGCH/>}} | ||
| | |
|Section3={{Chembox Hazards | ||
| | MainHazards = | | MainHazards = Potential carcinogen<ref name=PGCH>{{PGCH|0191}}</ref> | ||
| | PEL = carcinogen<ref name=PGCH/> | |||
| | FlashPt = | |||
| | IDLH = Ca <ref name=PGCH/> | |||
| | Autoignition = }} | |||
| | REL = Ca<ref name=PGCH/> | |||
| }} | |||
| }} | }} | ||
| '''3,3'-Dichlorobenzidine''' is an ] with the formula (C<sub>6</sub>H<sub>3</sub>Cl(NH<sub>2</sub>))<sub>2</sub>. The pure compound is pale yellow, but commercial samples are often colored. It is barely soluble in water and is often supplied as a wet paste. It is widely used in the production of ] pigments used in the production of printing inks.<ref name=Ullmann1>{{cite encyclopedia|author=K. Hunger|author2=W. Herbst |title=Pigments, Organic|encyclopedia=Ullmann's Encyclopedia of Industrial Chemistry|publisher=Wiley-VCH|place=Weinheim|year=2012|doi=10.1002/14356007.a20_371|isbn=9783527303854 }}</ref> Its use in the production of dyes has been largely discontinued because of concerns about carcinogenicity. | |||
| '''3,3'-Dichlorobenzidine''' is used in the production of ]s and is considered a ].<ref name="pubchem"/> It contains two ]s. | |||
| ==Preparation and reactions== | |||
| 3,3'-Dichlorobenzidine is prepared in two steps from ]. The first step involves reduction with zinc in base to afford 2,2'-dichlorodi]. This intermediate undergoes the ] to afford 3,3'-dichlorobenzidine.<ref name=Ullmann>{{cite encyclopedia | author = Schwenecke, H. | author2 = Mayer, D. | title = Benzidine and Benzidine Derivatives | encyclopedia = Ullmann’s Encyclopedia of Industrial Chemistry | year = 2005 | publisher = Wiley-VCH | place = Weinheim | doi = 10.1002/14356007.a03_539| isbn = 9783527303854 }}</ref> | |||
| Aqueous solutions of 3,3'-dichlorobenzidine degrade in light to monochloro derivative. It undergoes chlorination (for example in water treatment plants) to give the tetrachloro derivative. | |||
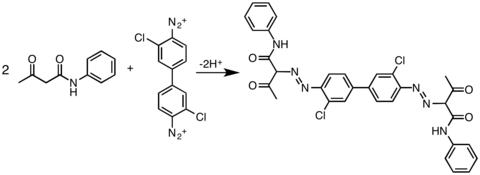
| The most widely practiced reaction of 3,3'-dichlorobenzidine is its double ]. This bis(diazo) intermediate is then coupled to derivatives of acetoacetylaminobenzene (CH<sub>3</sub>C(O)CH<sub>2</sub>C(O)NHAr). In this way, the following commercial yellow pigments are produced: ], ], ], ] and pigment ]<ref name=Ullmann1/> | |||
| ], an industrially significant ], is produced via the diazonium derivative of 3,3'-dichlorobenzidine.]] | |||
| ==Safety== | |||
| 3,3'-Dichlorobenzidine is considered a ].<ref name="pubchem"/> This compound has been shown to increase the incidence of tumors in animals.<ref name=EPA>"". ], Integrated Risk Information System. 7 March 2011. Accessed 3 May 2011.</ref> Because it is structurally similar to ], a known carcinogen, it is believed that it may share a similar mechanism in causing ] in humans.<ref name=EPA/> | |||
| ==References== | ==References== | ||
| Line 36: | Line 60: | ||
| ==External links== | ==External links== | ||
| * | * | ||
| * | |||
| {{DEFAULTSORT:Dichlorobenzidine, 3,3'-}} | {{DEFAULTSORT:Dichlorobenzidine, 3,3'-}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| {{aromatic-stub}} | |||
| ] | |||
| ] | |||
Latest revision as of 03:43, 22 May 2024

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 3,3′-Dichloro-4,4′-diamine | |
| Other names
4-(4-Amino-3-chlorophenyl)-2-chloroaniline 4,4′-Diamino-3,3′-dichlorobiphenyl o,o'-Dichlorobenzidine 3,3′-Dichlorobiphenyl-4,4′-diamine 3,3′-Dichloro-4,4′-biphenyldiamine 3,3′-Dichloro-4,4′-diaminobiphenyl | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.918 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H10Cl2N2 |
| Molar mass | 253.13 g/mol |
| Appearance | Gray or purple crystalline solid |
| Melting point | 132 to 133 °C (270 to 271 °F; 405 to 406 K) |
| Boiling point | 402 °C (756 °F; 675 K) |
| Solubility in water | 0.07% (15°C) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Potential carcinogen |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | carcinogen |
| REL (Recommended) | Ca |
| IDLH (Immediate danger) | Ca |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
3,3'-Dichlorobenzidine is an organic compound with the formula (C6H3Cl(NH2))2. The pure compound is pale yellow, but commercial samples are often colored. It is barely soluble in water and is often supplied as a wet paste. It is widely used in the production of diarylide yellow pigments used in the production of printing inks. Its use in the production of dyes has been largely discontinued because of concerns about carcinogenicity.
Preparation and reactions
3,3'-Dichlorobenzidine is prepared in two steps from 2-nitrochlorobenzene. The first step involves reduction with zinc in base to afford 2,2'-dichlorodiphenylhydrazine. This intermediate undergoes the benzidine rearrangement to afford 3,3'-dichlorobenzidine.
Aqueous solutions of 3,3'-dichlorobenzidine degrade in light to monochloro derivative. It undergoes chlorination (for example in water treatment plants) to give the tetrachloro derivative.
The most widely practiced reaction of 3,3'-dichlorobenzidine is its double diazotization. This bis(diazo) intermediate is then coupled to derivatives of acetoacetylaminobenzene (CH3C(O)CH2C(O)NHAr). In this way, the following commercial yellow pigments are produced: pigment Yellow 12, pigment Yellow 13, pigment Yellow 14, pigment Yellow 17 and pigment Yellow 83.
Safety
3,3'-Dichlorobenzidine is considered a carcinogen. This compound has been shown to increase the incidence of tumors in animals. Because it is structurally similar to benzidine, a known carcinogen, it is believed that it may share a similar mechanism in causing bladder cancer in humans.
References
- ^ Dichlorobenzidine - Compound Summary, PubChem.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0191". National Institute for Occupational Safety and Health (NIOSH).
- ^ K. Hunger; W. Herbst (2012). "Pigments, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a20_371. ISBN 9783527303854.
- Schwenecke, H.; Mayer, D. (2005). "Benzidine and Benzidine Derivatives". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_539. ISBN 9783527303854.
- ^ "3, 3'-Dichlorobenzidine". U.S. Environmental Protection Agency, Integrated Risk Information System. 7 March 2011. Accessed 3 May 2011.